Unveiling Silent Consequences: Impact of Pulmonary Tuberculosis on Lung Health and Functional Wellbeing after Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Definitions
2.2. Chest X-ray Assessment
2.3. Reader Agreement
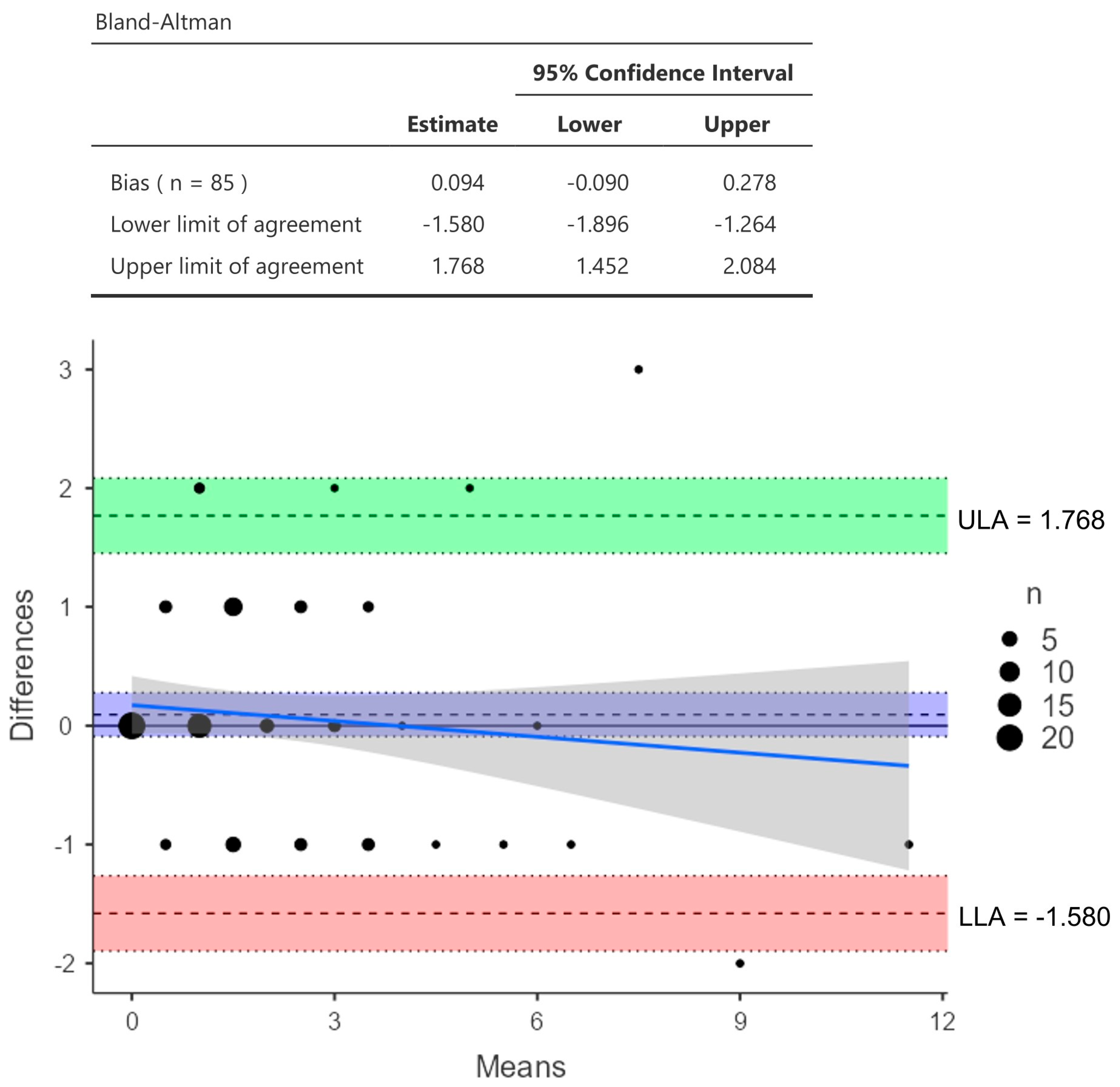
2.4. Spirometry and DLCO
- Normal—an FEV1/FVC ratio of >70% and an FVC >80% of the predicted level;
- Obstructive—airway obstruction was defined as an FEV1/FVC ratio of <70% and an FVC >80% of the predicted level;
- Combined defects—an FVC <80% of the predicted level and an FEV1/FVC ratio of <0.70;
- Restrictive—an FEV1/FVC ratio > 70% and an FVC < 80% of the predicted level [36].
2.5. Statistical Analysis
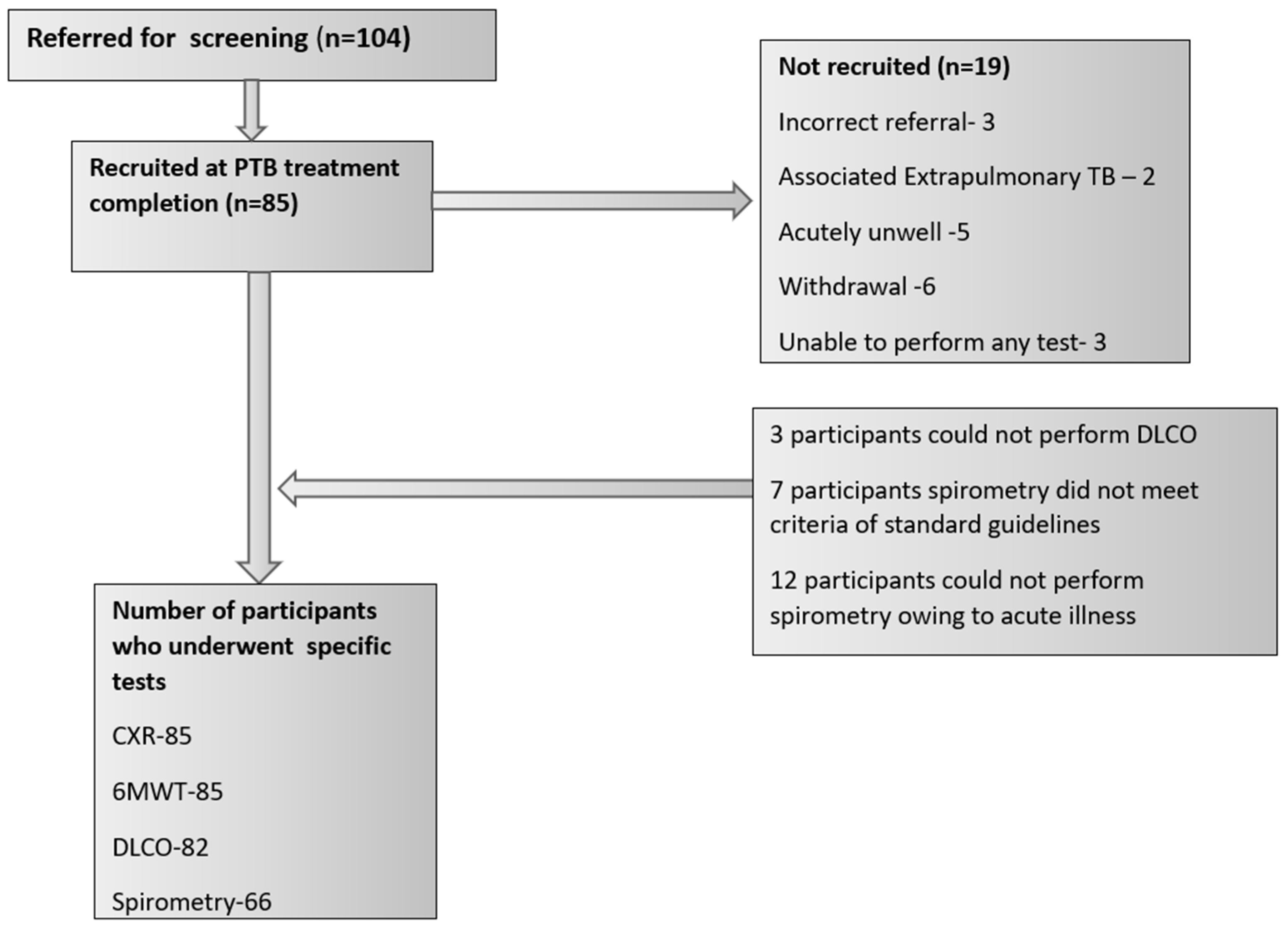
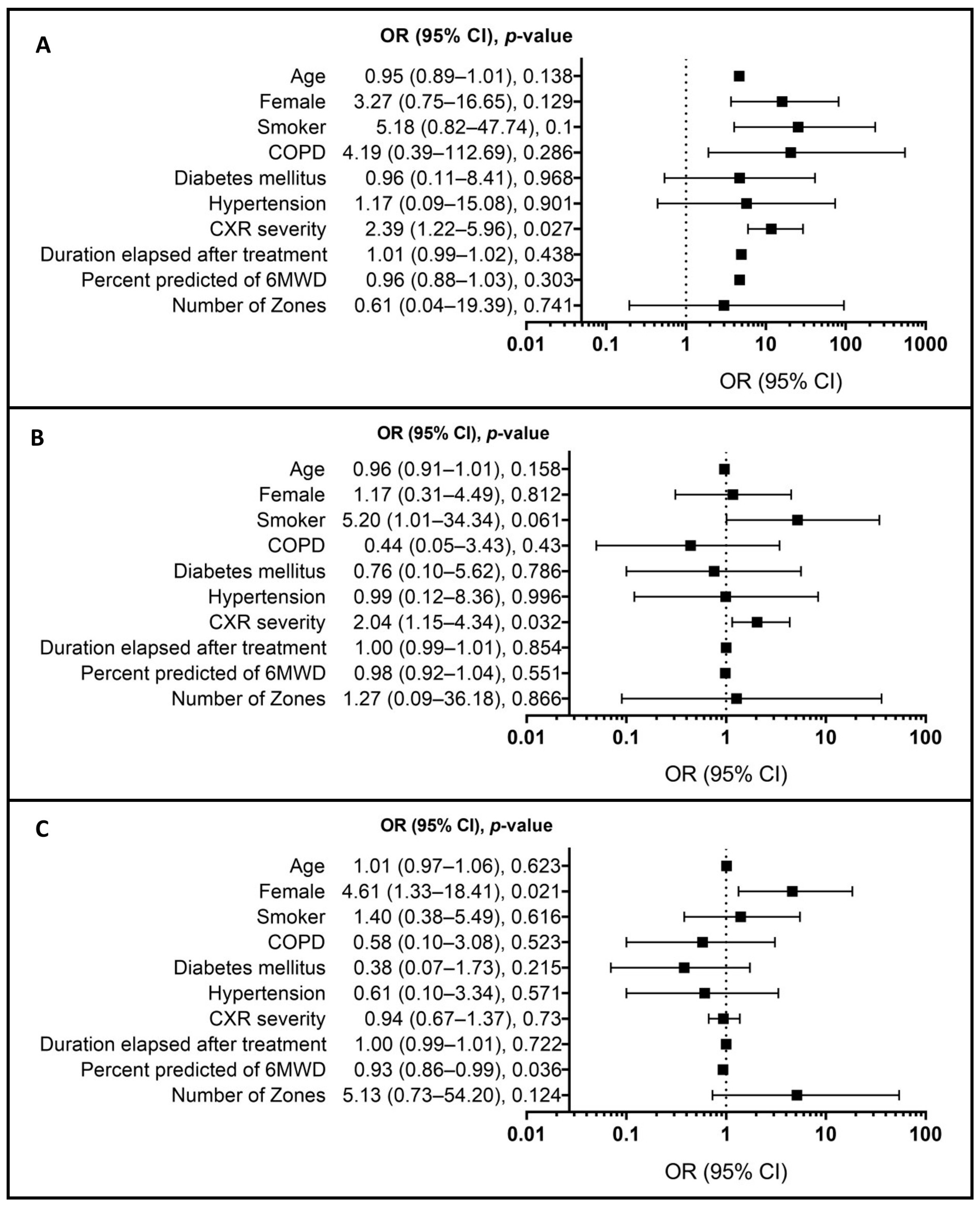
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Tuberculosis Report 2022. Available online: https://www.who.int/publications-detail-redirect/9789240061729 (accessed on 24 April 2023).
- Revised National Tuberculosis Control Programme—National Strategic Plan (NSP) for Tuberculosis Elimination 2017–2025. Central TB Division, Directorate General of Health Services, Ministry of Health & Family Welfare. Available online: https://tbcindia.gov.in/WriteReadData/NSP%20Draft%2020.02.2017%201.pdf (accessed on 24 April 2023).
- Pape, S.; Karki, S.J.; Heinsohn, T.; Brandes, I.; Dierks, M.-L.; Lange, B. Tuberculosis Case Fatality Is Higher in Male than Female Patients in Europe: A Systematic Review and Meta-Analysis. Infection 2024. [Google Scholar] [CrossRef] [PubMed]
- Tomeny, E.M.; Nightingale, R.; Chinoko, B.; Nikolaidis, G.F.; Madan, J.J.; Worrall, E.; Ngwira, L.G.; Banda, N.P.; Lönnroth, K.; Evans, D.; et al. TB Morbidity Estimates Overlook the Contribution of Post-TB Disability: Evidence from Urban Malawi. BMJ Glob. Health 2022, 7, e007643. [Google Scholar] [CrossRef] [PubMed]
- Muniyandi, M.; Rajeswari, R.; Balasubramanian, R.; Nirupa, C.; Gopi, P.G.; Jaggarajamma, K.; Sheela, F.; Narayanan, P.R. Evaluation of Post-Treatment Health-Related Quality of Life (HRQoL) among Tuberculosis Patients. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2007, 11, 887–892. [Google Scholar]
- Kim, H.Y.; Song, K.S.; Goo, J.M.; Lee, J.S.; Lee, K.S.; Lim, T.H. Thoracic Sequelae and Complications of Tuberculosis. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc 2001, 21, 839–858; discussion 859–860. [Google Scholar] [CrossRef] [PubMed]
- De La Mora, I.L.; Martínez-Oceguera, D.; Laniado-Laborín, R. Chronic Airway Obstruction after Successful Treatment of Tuberculosis and Its Impact on Quality of Life. Int. J. Tuberc. Lung Dis. 2015, 19, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Post Tuberculosis Sequelae in Patients Treated for Tuberculosis: An Observational Study at a Tertiary Care Center of a High TB Burden Country|European Respiratory Society. Eur. Respir. J. 2018, 52, PA2745. Available online: https://erj.ersjournals.com/content/52/suppl_62/PA2745.abstract (accessed on 25 May 2023).
- Ehrlich, R.I.; Adams, S.; Baatjies, R.; Jeebhay, M.F. Chronic Airflow Obstruction and Respiratory Symptoms Following Tuberculosis: A Review of South African Studies. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2011, 15, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Maycher, B.; Dhar, A.; Manfreda, J.; Hershfield, E.; Anthonisen, N. Pulmonary Tuberculosis Treated with Directly Observed Therapy: Serial Changes in Lung Structure and Function. Chest 1998, 113, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Akkara, S.A.; Shah, A.D.; Adalja, M.; Akkara, A.G.; Rathi, A.; Shah, D.N. Pulmonary Tuberculosis: The Day After. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2013, 17, 810–813. [Google Scholar] [CrossRef]
- Panda, A.; Bhalla, A.S.; Sharma, R.; Mohan, A.; Sreenivas, V.; Kalaimannan, U.; Upadhyay, A.D. Correlation of Chest Computed Tomography Findings with Dyspnea and Lung Functions in Post-Tubercular Sequelae. Lung India Off. Organ Indian Chest Soc. 2016, 33, 592–599. [Google Scholar] [CrossRef]
- Conlan, A.A.; Hurwitz, S.S.; Krige, L.; Nicolaou, N.; Pool, R. Massive Hemoptysis. J. Thorac. Cardiovasc. Surg. 1983, 85, 120–124. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Jiang, G.; Chen, C. Pneumonectomy for Treatment of Destroyed Lung: A Retrospective Study of 137 Patients. Thorac. Cardiovasc. Surg. 2017, 65, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Liu, F.; Han, M.; Gong, C. Incidence and Risk Factors of Postoperative Complications in Patients with Tuberculosis-Destroyed Lung. BMC Pulm. Med. 2021, 21, 273. [Google Scholar] [CrossRef]
- Pefura-Yone, E.W.; Kengne, A.P.; Tagne-Kamdem, P.E.; Afane-Ze, E. Clinical Significance of Low Forced Expiratory Flow between 25% and 75% of Vital Capacity Following Treated Pulmonary Tuberculosis: A Cross-Sectional Study. BMJ Open 2014, 4, e005361. [Google Scholar] [CrossRef] [PubMed]
- Obstructive Airway Disease in Patients with Treated Pulmonary Tuberculosis|American Review of Respiratory Disease. Available online: https://www.atsjournals.org/doi/10.1164/arrd.1971.103.5.625?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 25 May 2023).
- Pasipanodya, J.G.; Miller, T.L.; Vecino, M.; Munguia, G.; Garmon, R.; Bae, S.; Drewyer, G.; Weis, S.E. Pulmonary Impairment after Tuberculosis. Chest 2007, 131, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, R. Gas Exchange in Pulmonary Tuberculosis. II. Review of Literature, Clinical Significance and Conclusions. Scand. J. Respir. Dis. 1966, 47, 277–305. [Google Scholar]
- Maguire, G.P.; Anstey, N.M.; Ardian, M.; Waramori, G.; Tjitra, E.; Kenangalem, E.; Handojo, T.; Kelly, P.M. Pulmonary Tuberculosis, Impaired Lung Function, Disability and Quality of Life in a High-Burden Setting. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2009, 13, 1500–1506. [Google Scholar]
- Cruz, R.d.C.S.; De Albuquerque, M.d.F.P.M.; Campelo, A.R.L.; Costa e Silva, E.J.d.; Mazza, E.; Menezes, R.C.; Kosminsky, S. Pulmonary tuberculosis: Association between extent of the residual pulmonary lesion and alteration in the lung function. Rev. Assoc. Medica Bras. 1992 2008, 54, 406–410. [Google Scholar] [CrossRef]
- Báez-Saldaña, R.; López-Arteaga, Y.; Bizarrón-Muro, A.; Ferreira-Guerrero, E.; Ferreyra-Reyes, L.; Delgado-Sánchez, G.; Cruz-Hervert, L.P.; Mongua-Rodríguez, N.; García-García, L. A Novel Scoring System to Measure Radiographic Abnormalities and Related Spirometric Values in Cured Pulmonary Tuberculosis. PLoS ONE 2013, 8, e78926. [Google Scholar] [CrossRef]
- Nishi, M.P.; Mancuzo, E.V.; Sulmonett, N.; de Almeida, I.N.; César, A.L.A.; de Miranda, S.S. Pulmonary Functional Assessment: Longitudinal Study after Treatment of Pulmonary Tuberculosis. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e65. [Google Scholar] [CrossRef]
- Kiryukhina, L.D.; Kokorina, E.V.; Gavrilov, P.V.; Denisova, N.V.; Archakova, L.I.; Yablonskiy, P.K. Gas Exchange in Patients with Pulmonary Tuberculosis: Relationships with Pulmonary Poorly Communicating Fraction and Alveolar Volume. J. Respir. 2023, 3, 107–117. Available online: https://www.mdpi.com/2673-527X/3/2/11 (accessed on 26 July 2023). [CrossRef]
- Lingam, L.; Pitre, A. Gender Inequities in Health Access and Outcomes. A report on health inequities in Maharashtra, SATHI (Support for Advocacy and Training into Health Initiatives), in collaboration with CEHAT and TISS, Pune, Maharashtra. 2008, pp. 75–94. Available online: https://catalog.ihsn.org/citations/10408 (accessed on 10 July 2024).
- Singh, A.L.; Jamal, S. Unhealthy Cooking and Prevalence of Tuberculosis in Indian Women: A Case Study. J. Environ. Prot. 2012, 3, 648–656. [Google Scholar] [CrossRef]
- Revised National TB Control Programme. Technical and Operational Guidelines for TB Control in India 2016. Central TB Division, Directorate General of Health Services, Ministry of Health & Family Welfare. Available online: https://tbcindia.gov.in/WriteReadData/Revised%20Technical%20and%20Operational%20Guidelines/files/assets/basic-html/page-1.html (accessed on 20 November 2023).
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Solsona Peiró, J.; De Souza Galvão, M.L.; Altet Gómez, M.N. Inactive Fibrotic Lesions versus Pulmonary Tuberculosis with Negative Bacteriology. Arch. Bronconeumol. Engl. Ed. 2014, 50, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.R. Felson’s Principles of Chest Roentgenology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780323625678. Available online: https://www.asia.elsevierhealth.com/felsons-principles-of-chest-roentgenology-a-programmed-text-9780323625678.html (accessed on 20 November 2023).
- Lindberg, A.; Jonsson, A.-C.; Rönmark, E.; Lundgren, R.; Larsson, L.-G.; Lundbäck, B. Ten-Year Cumulative Incidence of COPD and Risk Factors for Incident Disease in a Symptomatic Cohort. Chest 2005, 127, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Chakraborthy, A.; Shivananjaiah, A.; Ramaswamy, S.; Chikkavenkatappa, N. Chest X Ray Score (Timika Score): An Useful Adjunct to Predict Treatment Outcome in Tuberculosis. Adv. Respir. Med. 2018, 86, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Thiel, B.A.; Bark, C.M.; Nakibali, J.G.; Van Der Kuyp, F.; Johnson, J.L. Reader Variability and Validation of the Timika X-Ray Score during Treatment of Pulmonary Tuberculosis. Int. J. Tuberc. Lung Dis. 2016, 20, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, A.; Maroldi, R. COVID-19 Outbreak in Italy: Experimental Chest X-ray Scoring System for Quantifying and Monitoring Disease Progression. Radiol. Med. 2020, 125, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Measures of Interrater Agreement. J. Thorac. Oncol. 2011, 6, 6–7. [Google Scholar] [CrossRef]
- Patil, S.; Patil, R.; Jadhav, A. Pulmonary Functions’ Assessment in Post-Tuberculosis Cases by Spirometry: Obstructive Pattern Is Predominant and Needs Cautious Evaluation in All Treated Cases Irrespective of Symptoms. Int. J. Mycobacteriol. 2018, 7, 128–133. [Google Scholar] [CrossRef]
- ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [CrossRef] [PubMed]
- Silveyra, P.; Fuentes, N.; Rodriguez Bauza, D. Sex and Gender Differences in Lung Disease. Adv. Exp. Med. Biol. 2021, 1304, 227–258. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Diaz-Guzman, E.; Mannino, D.M. Influence of Sex on Chronic Obstructive Pulmonary Disease Risk and Treatment Outcomes. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 1145–1154. [Google Scholar] [CrossRef]
- Patterson, K.C.; Strek, M.E. Pulmonary Fibrosis in Sarcoidosis. Clinical Features and Outcomes. Ann. Am. Thorac. Soc. 2013, 10, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Kawano-Dourado, L.; Glassberg, M.K.; Assayag, D.; Borie, R.; Johannson, K.A. Sex and Gender in Interstitial Lung Diseases. Eur. Respir. Rev. 2021, 30, 210105. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, A.D.; Rifkin, L.M. Sarcoidosis: Sex-Dependent Variations in Presentation and Management. J. Ophthalmol. 2014, 2014, e236905. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Torrico, M.; Cid-Juárez, S.; Gochicoa-Rangel, L.; Torre-Bouscolet, L.; Salazar-Lezama, M.A.; Villarreal-Velarde, H.; Pérez-Padilla, R.; Visca, D.; Centis, R.; D’Ambrosio, L.; et al. Functional Impact of Sequelae in Drug-Susceptible and Multidrug-Resistant Tuberculosis. Int. J. Tuberc. Lung Dis. 2020, 24, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, O.; Hoffmann, V.S.; Lange, C.; Hoelscher, M.; Rachow, A. Post-Tuberculosis Lung Impairment: Systematic Review and Meta-Analysis of Spirometry Data from 14621 People. Eur. Respir. Rev. 2023, 32, 220221. [Google Scholar] [CrossRef]
- Maleche-Obimbo, E.; Odhiambo, M.A.; Njeri, L.; Mburu, M.; Jaoko, W.; Were, F.; Graham, S.M. Magnitude and Factors Associated with Post-Tuberculosis Lung Disease in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. PLoS Glob. Public Health 2022, 2, e0000805. [Google Scholar] [CrossRef]
- Taylor, J.; Bastos, M.L.; Lachapelle-Chisholm, S.; Mayo, N.E.; Johnston, J.; Menzies, D. Residual Respiratory Disability after Successful Treatment of Pulmonary Tuberculosis: A Systematic Review and Meta-Analysis. eClinicalMedicine 2023, 59, 101979. [Google Scholar] [CrossRef]
- Banu Rekha, V.V.; Ramachandran, R.; Kuppurao, K.V.; Rahman, F.; Adhilakshmi, A.R.; Kalaiselvi, D.; Murugesan, P.; Sundaram, V.; Narayanan, P.R. Assessment of Long Term Status of Sputum Positive Pulmonary TB Patients Successfully Treated with Short Course Chemotherapy. Indian J. Tuberc. 2009, 56, 132–140. [Google Scholar] [PubMed]
- Di Naso, F.C.; Pereira, J.S.; Schuh, S.J.; Unis, G. Functional Evaluation in Patients with Pulmonary Tuberculosis Sequelae. Rev. Port. Pneumol. Engl. Ed. 2011, 17, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Mbatchou Ngahane, B.H.; Nouyep, J.; Nganda Motto, M.; Mapoure Njankouo, Y.; Wandji, A.; Endale, M.; Afane Ze, E. Post-Tuberculous Lung Function Impairment in a Tuberculosis Reference Clinic in Cameroon. Respir. Med. 2016, 114, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Tavares, J.L.; Wangoo, A.; Dilworth, P.; Marshall, B.; Kotecha, S.; Shaw, R.J. Thalidomide Reduces Tumour Necrosis Factor-Alpha Production by Human Alveolar Macrophages. Respir. Med. 1997, 91, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Tramontana, J.M.; Utaipat, U.; Molloy, A.; Akarasewi, P.; Burroughs, M.; Makonkawkeyoon, S.; Johnson, B.; Klausner, J.D.; Rom, W.; Kaplan, G. Thalidomide Treatment Reduces Tumor Necrosis Factor Alpha Production and Enhances Weight Gain in Patients with Pulmonary Tuberculosis. Mol. Med. Camb. Mass 1995, 1, 384–397. [Google Scholar]
| Female (N = 26) | Male (N = 59) | Total (N = 85) | p Value | ||
|---|---|---|---|---|---|
| Age in years | Median (IQR) | 35.5 (25.9–45.1) | 52.0 (36.2–61.8) | 45.0 (30.0–59.0) | <0.001 * |
| CXR severity scoring | Median (IQR) | 1.0 (0.0–2.0) | 1.0 (0.0–2.8) | 1.0 (0.0–2.0) | 0.501 * |
| Duration elapsed after treatment completion (in months) | Median (IQR) | 14.5 (6.0–26.0) | 12.0 (5.2–46.0) | 12.0 (6.0–36.0) | 0.50 * |
| Treatment duration | Median (IQR) | 6 (6.0–6.0) | 6 (6.0–6.0) | 6 (6.0–6.0) | 0.58 * |
| BMI | Median (IQR) | 20.0 (17.0–24.3) | 21.0 (18.0–24.0) | 21.0 (18.0–24.0) | 0.46 * |
| Smoking (m)/biomass exposure (f) | n (%) | 7.0 (26.9%) | 25.0 (42.4%) | 32.0 (37.6%) | 0.18 † |
| Hypertension | n (%) | 0.0 (0.0%) | 13.0 (22.0%) | 13.0 (15.5%) | 0.01 † |
| Diabetes mellitus | n (%) | 1.0 (3.8%) | 11.0 (19.0%) | 12.0 (14.3%) | 0.07 † |
| COPD | n (%) | 1 (3.8) | 18 (35.0) | 19 (22.4) | 0.007 † |
| IHD | n (%) | 0 (0) | 2 (3.4) | 2 (2.4) | 0.342 † |
| Persistence of symptoms | n (%) | 7.0 (26.9%) | 19.0 (32.2%) | 26.0 (30.6%) | 0.63 † |
| Chest X-ray | 0.30 † | ||||
| Normal | n (%) | 10 (11.8%) | 16 (18.8%) | 26 (30.5%) | |
| Abnormal | n (%) | 16 (18.8) | 43 (50.6%) | 59 (69.4%) | |
| No. of zones affected | 0.19 † | ||||
| 0 | n (%) | 10.0 (38.5%) | 16.0 (27.8%) | 26.0 (30.8%) | |
| 1 | n (%) | 4.0 (15.4%) | 20.0 (33.9%) | 24.0 (28.2%) | |
| 2 | n (%) | 9.0 (34.6%) | 10.0 (16.9%) | 19.0 (22.4%) | |
| 3 | n (%) | 2.0 (7.7%) | 3.0 (5.1%) | 5.0 (5.9%) | |
| 4 | n (%) | 1.0 (3.8%) | 8.0 (13.6%) | 9.0 (10.6%) | |
| 5 | n (%) | 0.0 (0.0%) | 1.0 (1.7%) | 1.0 (1.2%) | |
| Spirometry (N = 66) | Female (N = 22) | Male (N = 45) | Total (N = 66) | ||
| Restrictive | n (%) | 6.0 (27.3%) | 13 (30.2%) | 19.0 (29.2%) | 0.89 † |
| Obstructive | n (%) | 6.0 (27.3%) | 17.0 (37.8%) | 22 (33.8%) | 0.40 † |
| Mixed | n (%) | 00 (0) | 04 (9.3%) | 04 (6.2%) | 0.80 † |
| Normal | n (%) | 10 (45.5%) | 10 (23.3%) | 20 (30.8%) | 0.64 † |
| Reversibility | n (%) | 3 (11%) | 6 (10%) | 9 (13.6%) | 0.90 † |
| DLCO (N = 82) | 0.04 † | ||||
| >80% predicted | n (%) | 05 (20.8%) | 26 (44.8%) | 31 (37.8%) | |
| <80% predicted | n (%) | 19 (79.2%) | 32 (55.2%) | 51 (62.2%) |
| Variables | X-ray Findings | Total | p Value | |
|---|---|---|---|---|
| Normal (N = 26) | Abnormal (N = 59) | Mean ± SD (N = 85) | ||
| Age in years (mean ± SD) | 40.27 ± 15.05 | 48.00 ± 16.36 | 45.64 ± 16.28 | 0.043 * |
| Gender | 0.2962 † | |||
| Female (n, %) | 10 (38.5%) | 16 (27.1%) | 26 (30.6%) | |
| Male (n, %) | 16 (61.5%) | 43 (72.9%) | 59 (69.4%) | |
| Treatment duration (mean ± SD) | 6.1 ± 0.6 | 6.5 ± 1.9 | 6.4 ± 1.6 | 0.3761 * |
| Duration elapsed after treatment (mean ± SD) | 39.4 ± 58.5 | 36.2 ± 57.0 | 37.2 ± 57.2 | 0.8141 * |
| DLCO% pred (mean ± SD) | 80.69 ± 23.72 | 74.86 ± 23.35 | 76.71 ± 23.48 | 0.268 * |
| TLC % pred (mean ± SD) | 88.04 ± 21.67 | 80.02 ± 18.91 | 82.56 ± 20.05 | 0.092 * |
| Distance in 6MWT % pred (mean ± SD) | 90.96 ± 6.18 | 85.07 ± 12.27 | 86.87 ± 11.08 | 0.023 * |
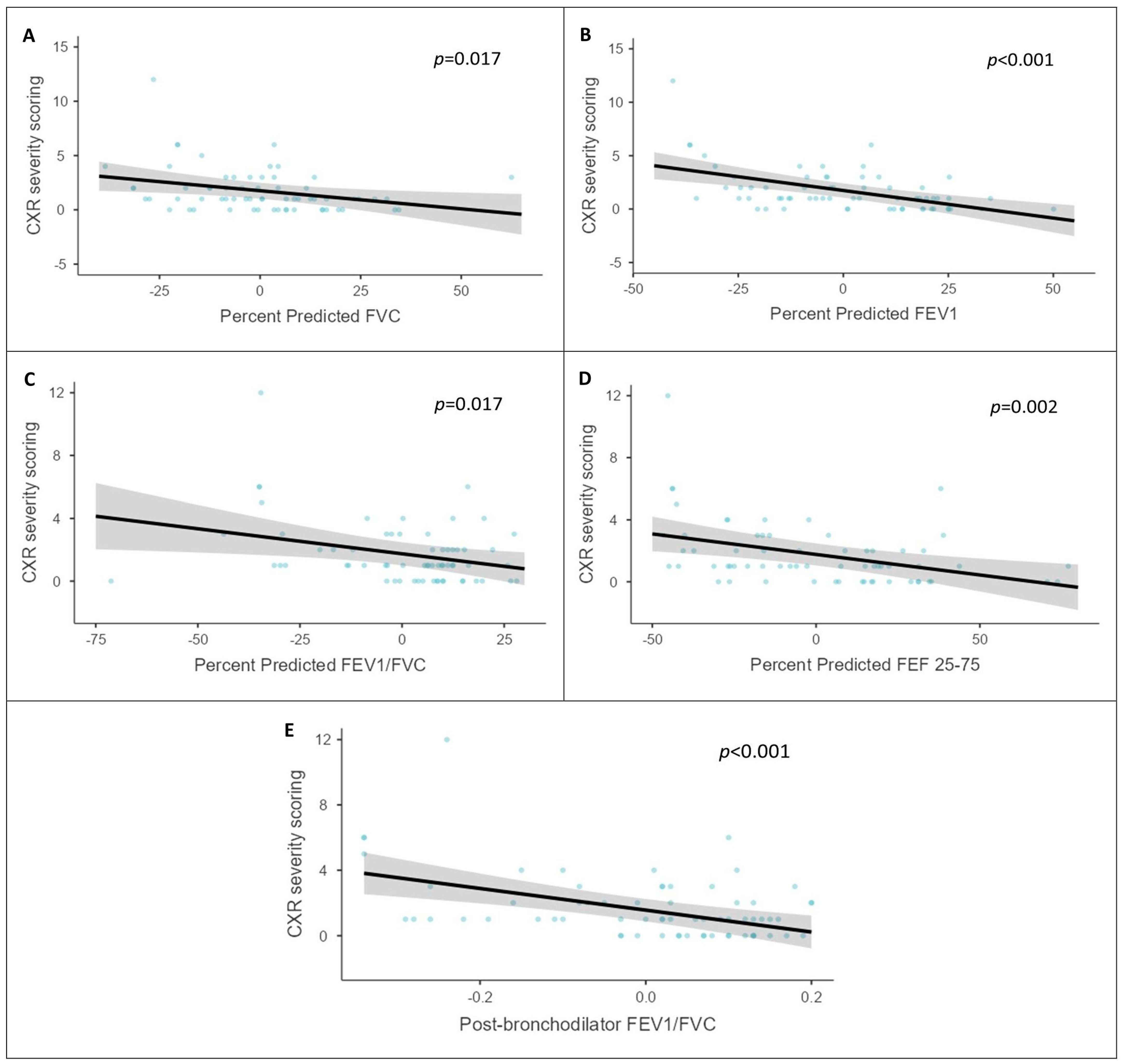
| % Pred FVC (mean ± SD) | 80.11 ± 16.11 | 71.21 ± 19.05 | 73.64 ± 18.60 | 0.083 * |
| % Pred FEV1 (mean ± SD) | 79.3 ± 18.1 | 61.1 ± 19.0 | 66.1 ± 20.3 | <0.001 * |
| % Pred FEV1/FVC Ratio (mean ± SD) | 94.56 ± 21.09 | 87.00 ± 18.03 | 89.06 ± 19.05 | 0.153 * |
| % Pred FEF 25-75 (mean ± SD) | 74.11 ± 27.54 | 48.33 ± 29.19 | 55.36 ± 30.80 | 0.002 * |
| % Pred PEF (mean ± SD) | 92.61 ± 23.74 | 61.83 ± 25.23 | 70.23 ± 28.26 | <0.001 * |
| FEV1/FVC ratio PBD (mean ± SD) | 0.82 ± 0.09 | 0.75 ± 0.15 | 0.77 ± 0.14 | 0.021 * |
| FEV1 % change (mean ± SD) | 2.84 ± 11.20 | 5.67 ± 10.59 | 4.28 ± 10.79 | 0.275 * |
| SPO2% decrease | Total | 0.004 † | ||
| 1 (n, %) | 7 (26.9%) | 17 (28.8%) | 24 (28.2%) | |
| 2 (n, %) | 0 (0%) | 16 (27.1%) | 16 (18.8%) | |
| 3 (n, %) | 0 (0%) | 5 (8.5%) | 5 (5.9%) | |
| 4 (n, %) | 0 (0%) | 2 (3.4%) | 2 (2.4%) | |
| 5 (n, %) | 0 (0%) | 1 (1.7%) | 1 (1.2%) | |
| SPIROMETRY | Total | |||
| Obstruction (n, %) | 1 (4.3%) | 22 (95.7%) | 23 (100%) | 0.003 † |
| Restriction (n, %) | 7 (36.8%) | 12 (63.2%) | 19 (100%) | 0.246 † |
| CXR severity score | <0.001 † | |||
| =/<2 (n, %) | 0 (0) | 37 (62.7%) | 37 (43.5%) | |
| >2 (n, %) | 0 (0) | 14 (23.7%) | 14 (16.47%) | |
| TLC % pred (mean ± SD) | 88 ± 21.7 | 80 ± 18.9 | 82.6 ± 20.0 | 0.0921 * |
| DLCO % pred (mean ± SD) | 80.7 ± 23.7 | 74.9 ± 23.4 | 76.7 ± 23.5 | 0.2981 * |
| BMI (mean ± SD) | 22.7 ± 4.7 | 21.0 ± 5.1 | 21.5 ± 5.0 | 0.1481 * |
| HTN (n, %) | 4 (15.4%) | 9 (15.5%) | 13 (15.5%) | 0.9882 † |
| COPD (n, %) | 1 (3.8%) | 18 (30.5%) | 19 (22.4%) | 0.0072 † |
| DM (n, %) | 2 (7.7%) | 10 (17.2%) | 12 (14.3%) | 0.2482 † |
| Smoking (n, %) | 5 (19.2%) | 27 (45.8%) | 32 (37.6%) | 0.0202 † |
| FVC | FEV1 | DLCO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥80 (N = 16) | <80 (N = 10) | p Value | ≥80 (N = 19) | <80 (N = 7) | p Value | ≥80 (N = 11) | <80 (N = 15) | p Value | |
| Age in years (mean ± SD) | 40.9 ± 13.1 | 39.3 ± 18.5 | 0.8011 * | 40.7 ± 14.2 | 39.1 ± 18.5 | 0.8221 * | 38.4 ± 14.9 | 41.7 ± 15.5 | 0.5911 * |
| Gender | |||||||||
| Female (n, %) | 5.0 (31.2%) | 5.0 (50.0%) | 0.3392 † | 5.0 (26.3%) | 5.0 (71.4%) | 0.0362 † | 2.0 (18.2%) | 8.0 (53.3%) | 0.0692 † |
| Male (n, %) | 11.0 (68.8%) | 5.0 (50.0%) | 14.0 (73.7%) | 2.0 (28.6%) | 9.0 (81.8%) | 7.0 (46.7%) | |||
| Treatment duration (mean ± SD) | 6.0 ± 0.0 | 6.3 ± 0.9 | 0.2121 * | 6.2 ± 0.7 | 6.0 ± 0.0 | 0.5551 * | 6.3 ± 0.9 | 6.0 ± 0.0 | 0.2511 * |
| Duration elapsed after treatment (mean ± SD) | 47.2 ± 61.4 | 26.9 ± 54.1 | 0.4001 * | 40.8 ± 58.1 | 35.4 ± 64.1 | 0.8391 * | 43.5 ± 68.0 | 36.4 ± 52.7 | 0.7681 * |
| Distance in 6MWT % pred (mean ± SD) | 91.8 ± 5.8 | 89.6 ± 6.8 | 0.3851 * | 91.7 ± 6.3 | 88.9 ± 5.9 | 0.3011 * | 93.2 ± 7.5 | 89.3 ± 4.7 | 0.1191 * |
| SPO2% decrease | |||||||||
| 1 (n, %) | 5.0 (31.2%) | 2.0 (20.0%) | 0.5292 † | 5.0 (26.3%) | 2.0 (28.6%) | 0.9082 † | 4.0 (36.4%) | 3.0 (20.0%) | 0.3532 † |
| % Pred FEV1 (mean ± SD) | 92.1 ± 11.1 | 69.0 ± 16.1 | 0.0031 * | 89.6 ± 10.8 | 68.9 ± 18.4 | 0.0101 * | |||
| % Pred FVC (mean ± SD) | 88.2 ± 12.9 | 67.4 ± 12.3 | 0.0041 * | 85.6 ± 12.4 | 74.7 ± 18.2 | 0.1571 * | |||
| % Pred FEV1/FVC (mean ± SD) | 94.8 ± 30.1 | 94.1 ± 7.0 | 0.9441 * | ||||||
| % Pred FEF 25-75 (mean ± SD) | 80.7 ± 20.8 | 68.9 ± 32.1 | 0.3841 * | 85.0 ± 23.2 | 57.0 ± 26.4 | 0.0301 * | 88.4 ± 24.2 | 59.8 ± 23.9 | 0.0231 * |
| % Pred PEF (mean ± SD) | 106.9 ± 25.5 | 81.0 ± 15.1 | 0.0161 * | 101.4 ± 23.6 | 78.7 ± 17.4 | 0.0441 * | 103.9 ± 25.6 | 81.2 ± 15.9 | 0.0381 * |
| Spirometry | |||||||||
| Obstruction (n, %) | 0.0 (0.0%) | 1.0 (10.0%) | 0.3572 † | 0.0 (0.0%) | 1.0 (14.3%) | 0.1972 † | 0.0 (0.0%) | 1.0 (11.1%) | 0.3032 † |
| Restriction (n, %) | 0.0 (0.0%) | 7.0 (70.0%) | 0.0042 † | 2.0 (20.0%) | 5.0 (71.4%) | 0.0342 † | 2.0 (22.2%) | 5.0 (62.5%) | 0.0922 † |
| TLC % Pred (mean ± SD) | 97.6 ± 22.0 | 72.7 ± 8.5 | 0.0021 * | 94.1 ± 21.8 | 71.7 ± 10.2 | 0.0161 * | 102.1 ± 23.1 | 77.7 ± 13.8 | 0.0031 * |
| DLCO % Pred (mean ± SD) | 88.0 ± 25.8 | 69.0 ± 14.5 | 0.0441 * | 87.6 ± 23.6 | 62.0 ± 10.9 | 0.0111 * | 101.5 ± 21.4 | 65.4 ± 9.3 | < 0.001 * |
| BMI (mean ± SD) | 22.4 ± 3.6 | 23.3 ± 6.3 | 0.6371 * | 22.6 ± 3.7 | 23.1 ± 7.2 | 0.7931 * | 23.0 ± 2.8 | 22.5 ± 5.8 | 0.8091 * |
| HTN (n, %) | 2.0 (12.5%) | 2.0 (20.0%) | 0.6062 † | 3.0 (15.8%) | 1.0 (14.3%) | 0.9252 † | 1.0 (9.1%) | 3.0 (20.0%) | 0.4462 † |
| COPD (n, %) | 1.0 (6.2%) | 0.0 (0.0%) | 0.4202 † | 1.0 (5.3%) | 0.0 (0.0%) | 0.5362 † | 0.0 (0.0%) | 1.0 (6.7%) | 0.3822 † |
| DM (n, %) | 1.0 (6.2%) | 1.0 (10.0%) | 0.7272 † | 2.0 (10.5%) | 0.0 (0.0%) | 0.3722 † | 2.0 (18.2%) | 0.0 (0.0%) | 0.0862 † |
| Smoking (n, %) | 2.0 (12.5%) | 3.0 (30.0%) | 0.2712 † | 2.0 (10.5%) | 3.0 (42.9%) | 0.0642 † | 0.0 (0.0%) | 5.0 (33.3%) | 0.0332 † |
| FVC % Pred | FEV1 % Pred | FEF25-75% Pred | FEV1/FVC Ratio | DLCO % Pred | CXR Severity Scoring | Number of Zones | |
|---|---|---|---|---|---|---|---|
| Estimate (CI) | Estimate (CI) | Estimate (CI) | Estimate (CI) | Estimate (CI) | Estimate (CI) | Estimate (CI) | |
| Smoker | −8.7 (−19–1.6) | −10.71 (−20.7–−0.67) | −13.97 (−29.83–1.89) | −0.053 (−0.11–0.009) | −11.45 (−23.74–0.82) | −0.08 (−1.08–0.92) | 0.233 (−0.2–0.182) |
| Diabetes mellitus | 4.96 (−7.96–17.9) | 10.08 (−2.45–22.6) | 17.66 (−2.17–37.5) | 0.055 (−0.023–0.134) | 6.12 (−9.29–21.54) | −0.32 (−1.6–0.96) | −0.024 (−0.575–0.527) |
| Hypertension | 8.23 (−5.9–22.3) | −4.19 (−17.92–9.54) | −15.02 (−36.8–6.68) | −0.14 (−0.23–−0.062) *** | −1.75 (−17.31–13.80) | −0.93 (−2.22–0.34) | 0.219 (−0.336–0.773) |
| CXR severity scoring | −2.5 (−4.9–−0.16) * | −4.45 (−6.75–−2.14) *** | −5.59 (−9.24–−1.95) ** | −0.027 (−0.42–−0.012) *** | −2.23 (−4.94–0.48) | - | 0.473 (0.376–0.570) *** |
| Duration elapsed after treatment (months) | −0.03 (−0.11–0.046) | −0.30 (−0.104–0.044) | −0.005 (−0.12–0.11) | −3.54 (−5.07–4.37) | 0.041 (−0.05–0.134) | 0.006 (−0.002–0.013) | −0 (−0004–0.003) |
| SPO2 drop post-6MWT | 0.58 (−3.7–4.9) | −0.85 (−5.02–3.32) | −2.5 (−9.11–4.07) | −0.029 (−0.055–−0.0026) * | 1.23 (−4.14–6.61) | 0.85 (0.45–1.25) *** | −0.009 (−0.2–0.182) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bansal, N.; Arunachala, S.; Kaleem Ullah, M.; Kulkarni, S.; Ravindran, S.; ShankaraSetty, R.V.; Malamardi, S.; Chaya, S.K.; Lokesh, K.S.; Parthasarathi, A.; et al. Unveiling Silent Consequences: Impact of Pulmonary Tuberculosis on Lung Health and Functional Wellbeing after Treatment. J. Clin. Med. 2024, 13, 4115. https://doi.org/10.3390/jcm13144115
Bansal N, Arunachala S, Kaleem Ullah M, Kulkarni S, Ravindran S, ShankaraSetty RV, Malamardi S, Chaya SK, Lokesh KS, Parthasarathi A, et al. Unveiling Silent Consequences: Impact of Pulmonary Tuberculosis on Lung Health and Functional Wellbeing after Treatment. Journal of Clinical Medicine. 2024; 13(14):4115. https://doi.org/10.3390/jcm13144115
Chicago/Turabian StyleBansal, Nidhi, Sumalatha Arunachala, Mohammed Kaleem Ullah, Shreedhar Kulkarni, Sukanya Ravindran, Rekha Vaddarahalli ShankaraSetty, Sowmya Malamardi, Sindaghatta Krishnarao Chaya, Komarla Sundararaja Lokesh, Ashwaghosha Parthasarathi, and et al. 2024. "Unveiling Silent Consequences: Impact of Pulmonary Tuberculosis on Lung Health and Functional Wellbeing after Treatment" Journal of Clinical Medicine 13, no. 14: 4115. https://doi.org/10.3390/jcm13144115






