Decreased Cerebral Creatine and N-Acetyl Aspartate Concentrations after Severe COVID-19 Infection: A Magnetic Resonance Spectroscopy Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Recruitment and Demographic Information
2.2. Magnetic Resonance Imaging and Spectroscopy Procedures
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mir, T.; Almas, T.; Kaur, J.; Faisaluddin, M.; Song, D.; Ullah, W.; Mamtani, S.; Rauf, H.; Yadav, S.; Latchana, S.; et al. Coronavirus disease 2019 (COVID-19): Multisystem review of pathophysiology. Ann. Med. Surg. 2021, 69, 102745. [Google Scholar] [CrossRef] [PubMed]
- Savarraj, J.; Park, E.S.; Colpo, G.D.; Hinds, S.N.; Morales, D.; Ahnstedt, H.; Paz, A.S.; Assing, A.; Liu, F.; Juneja, S.; et al. Brain Injury, Endothelial Injury and Inflammatory Markers Are Elevated and Express Sex-Specific Alterations after COVID-19. J. Neuroinflamm. 2021, 18, 277. [Google Scholar] [CrossRef] [PubMed]
- Zingaropoli, M.A.; Iannetta, M.; Piermatteo, L.; Pasculli, P.; Latronico, T.; Mazzuti, L.; Campogiani, L.; Duca, L.; Ferraguti, G.; De Michele, M.; et al. Neuro-Axonal Damage and Alteration of Blood-Brain Barrier Integrity in COVID-19 Patients. Cells 2022, 11, 2480. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T. Neurological Infection with SARS-CoV-2—The Story so Far. Nat. Rev. Neurol. 2021, 17, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Nordvig, A.S.; Fong, K.T.; Willey, J.Z.; Thakur, K.T.; Boehme, A.K.; Vargas, W.S.; Smith, C.J.; Elkind, M.S.V. Potential Neurologic Manifestations of COVID-19. Neurol. Clin. Pract. 2021, 11, e135–e146. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A First Case of Meningitis/Encephalitis Associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Kremer, S.; Lersy, F.; de Sèze, J.; Ferré, J.C.; Maamar, A.; Carsin-Nicol, B.; Collange, O.; Bonneville, F.; Adam, G.; Martin-Blondel, G.; et al. Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study. Radiology 2020, 297, E242–E251. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.Y.; Huang, C.C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral micro-structural changes in COVID-19 patients—An MRI-based 3-month follow-up study. EClinicalMedicine 2020, 25, 100484. [Google Scholar] [CrossRef]
- Bispo, D.D.C.; Brandão, P.R.P.; Pereira, D.A.; Maluf, F.B.; Dias, B.A.; Paranhos, H.R.; von Glehn, F.; de Oliveira, A.C.P.; Regattieri, N.A.T.; Silva, L.S.; et al. Brain microstructural changes and fatigue after COVID-19. Front. Neurol. 2022, 13, 1029302. [Google Scholar] [CrossRef]
- Chhatbar, C.; Prinz, M. The roles of microglia in viral encephalitis: From sensome to therapeutic targeting. Cell Mol. Immunol. 2021, 18, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Virhammar, J.; Nääs, A.; Fällmar, D.; Cunningham, J.L.; Klang, A.; Ashton, N.J.; Jackmann, S.; Westman, G.; Frithiof, R.; Blennow, K.; et al. Biomarkers for Central Nervous System Injury in Cerebrospinal Fluid Are Elevated in COVID-19 and Associated with Neurological Symptoms and Disease Severity. Eur. J. Neurol. 2021, 28, 3324–3331. [Google Scholar] [CrossRef]
- Kanberg, N.; Ashton, N.J.; Andersson, L.M.; Yilmaz, A.; Lindh, M.; Nilsson, S.; Price, R.W.; Blennow, K.; Zetterberg, H.; Gisslén, M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology 2020, 95, e1754–e1759. [Google Scholar] [CrossRef]
- Mohammadhosayni, M.; Sadat Mohammadi, F.; Ezzatifar, F.; Mahdavi Gorabi, A.; Khosrojerdi, A.; Aslani, S.; Hemmatzadeh, M.; Yazdani, S.; Arabi, M.; Marofi, F.; et al. Matrix Metalloproteinases Are Involved in the Development of Neurological Complications in Patients with Coronavirus Disease 2019. Int. Immunopharmacol. 2021, 100, 108076. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Fries, G.R.; Stertz, L.; Badawy, M.; Passos, I.C.; Barichello, T.; Kapczinski, F.; Quevedo, J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015, 300, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Helms, G. Volume correction for edema in single-volume proton MR spectroscopy of contrast-enhancing multiple sclerosis lesions. Magn. Reson. Med. 2001, 46, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Chaganti, J.; Brew, B.J. MR spectroscopy in HIV associated neurocognitive disorder in the era of cART: A review. AIDS Res. Ther. 2021, 18, 65. [Google Scholar] [CrossRef]
- Boban, J.; Kozic, D.; Turkulov, V.; Ostojic, J.; Semnic, R.; Lendak, D.; Brkic, S. HIV-associated neurodegeneration and neuroimmunity: Multivoxel MR spectroscopy study in drug-naïve and treated patients. Eur. Radiol. 2017, 27, 4218–4236. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.K.; Egan, M.; Wallis, F.; Jakeman, P. Repeatability of two-dimensional chemical shift imaging multivoxel proton magnetic resonance spectroscopy for measuring human cerebral choline-containing compounds. World J. Psychiatry 2018, 8, 20–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buonocore, M.H.; Maddock, R.J. Magnetic resonance spectroscopy of the brain: A review of physical principles and technical methods. Rev. Neurosci. 2015, 26, 609–632. [Google Scholar] [CrossRef] [PubMed]
- Gasparovic, C.; Song, T.; Devier, D.; Bockholt, H.J.; Caprihan, A.; Mullins, P.G.; Posse, S.; Jung, R.E.; Morrison, L.A. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med. 2006, 55, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Stefan, D.; Di Cesare, F.; Andrasescu, A.; Popa, E.; Lazariev, A.; Vescovo, E.; Strbak, O.; Williams, S.; Starcuk, Z.; Cabanas, M.; et al. Quantitation of magnetic resonance spectroscopy signals: The jMRUI software package. Meas. Sci. Technol. 2009, 20, 104035. [Google Scholar] [CrossRef]
- Vanhamme, L.; van den Boogaart, A.; Van Huffel, S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J. Magn. Reson. 1997, 129, 35–43. [Google Scholar] [CrossRef]
- Barker, P.B.; Hearshen, D.O.; Boska, M.D. Single-voxel proton MRS of the human brain at 1.5T and 3.0T. Magn. Reson. Med. 2001, 45, 765–769. [Google Scholar] [CrossRef]
- Ethofer, T.; Mader, I.; Seeger, U.; Helms, G.; Erb, M.; Grodd, W.; Ludolph, A.; Klose, U. Comparison of longitudinal metabolite relaxation times in different regions of the human brain at 1.5 and 3 Tesla. Magn. Reson. Med. 2003, 50, 1296–1301. [Google Scholar] [CrossRef]
- Kreis, R. Quantitative localized 1H MR spectroscopy for clinical use. Prog. Nucl. Magn. Res. Spectrosc. 1997, 31, 155–195. [Google Scholar] [CrossRef]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson: New York, NY, USA, 2010. [Google Scholar]
- Li, B.S.; Wang, H.; Gonen, O. Metabolite ratios to assumed stable creatine level may confound the quantification of proton brain MR spectroscopy. Magn. Reason. Imaging 2003, 21, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and Creatinine Metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Sestili, P.; Martinelli, C.; Bravi, G.; Piccoli, G.; Curci, R.; Battistelli, M.; Falcieri, E.; Agostini, D.; Gioacchini, A.M.; Stocchi, V. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic. Biol. Med. 2006, 40, 837–849. [Google Scholar] [CrossRef]
- Lensman, M.; Korzhevskii, D.E.; Mourovets, V.O.; Kostkin, V.B.; Izvarina, N.; Perasso, L.; Gandolfo, C.; Otellin, V.A.; Polenov, S.A.; Balestrino, M. Intracerebroventricular administration of creatine protects against damage by global cerebral ischemia in rat. Brain Res. 2006, 1114, 187–194. [Google Scholar] [CrossRef]
- Slankamenac, J.; Ranisavljev, M.; Todorovic, N.; Ostojic, J.; Stajer, V.; Ostojic, S.M. Effects of six-month creatine supplementation on patient- and clinician-reported outcomes, and tissue creatine levels in patients with post-COVID-19 fatigue syndrome. Food Sci. Nutr. 2023, 11, 6899–6906. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F. Neuroprotective effects of creatine. Amino Acids 2011, 40, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.L.Y.; Cady, E.B.; Penrice, J.; Wyatt, J.S.; Cox, I.J.; Robertson, N.J. Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury: Metabolite peak-area ratios, relaxation times, and absolute concentrations. Am. J. Neuroradiol. 2006, 27, 1546. [Google Scholar] [PubMed]
- Finer, N.N.; Carlo, W.A.; Walsh, M.C.; Rich, W.; Gantz, M.G.; Laptook, A.R.; SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network; Yoder, B.A.; Faix, R.G.; Das, A.; et al. Early CPAP versus surfactant in extremely preterm infants. N. Engl. J. Med. 2010, 362, 1970–1979. [Google Scholar]
- Barton, S.K.; Moss, T.J.M.; Hooper, S.B.; Crossley, K.J.; Gill, A.W.; Kluckow, M.; Zahra, V.; Wong, F.Y.; Pichler, G.; Galinsky, R.; et al. Protective ventilation of preterm lambs exposed to acute chorioamnionitis does not reduce ventilation-induced lung or brain injury. PLoS ONE 2014, 9, e112402. [Google Scholar] [CrossRef] [PubMed]
- Polglase, G.R.; Miller, S.L.; Barton, S.K.; Baburamani, A.A.; Wong, F.Y.; Aridas, J.D.; Gill, A.W.; Moss, T.J.M.; Tolcos, M.; Kluckow, M.; et al. Initiation of resuscitation with high tidal volumes causes cerebral hemodynamic disturbance, brain inflammation and injury in preterm lambs. PLoS ONE 2012, 7, e39535. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, D.M.; Chan, K.Y.Y.; Stojanovska, V.; LaRosa, D.; Barton, S.K.; Nitsos, I.; Zahra, V.; Barbuto, J.; Farrell, M.; Yamaoka, S.; et al. Diffusion tensor imaging detects ventilation-induced brain injury in preterm lambs. PLoS ONE 2017, 12, e0188737. [Google Scholar] [CrossRef] [PubMed]
- Bendella, Z.; Widmann, C.N.; Layer, J.P.; Layer, Y.L.; Haase, R.; Sauer, M.; Bieler, L.; Lehnen, N.C.; Paech, D.; Heneka, M.T.; et al. Brain Volume Changes after COVID-19 Compared to Healthy Controls by Artificial Intelligence-Based MRI Volumetry. Diagnostics 2023, 13, 1716. [Google Scholar] [CrossRef]
- Rothstein, T.L. Cortical Grey matter volume depletion links to neurological sequelae in post COVID-19 “long haulers”. BMC Neurol. 2023, 23, 22. [Google Scholar] [CrossRef]
- Castillo, M.; Kwock, L.; Mukherji, S.K. Clinical applications of proton MR spectroscopy. AJNR Am. J. Neuroradiol. 1996, 17, 1–15. [Google Scholar]
- Ostojic, S.M. Low Tissue Creatine: A Therapeutic Target in Clinical Nutrition. Nutrients 2022, 14, 1230. [Google Scholar] [CrossRef]
- Laakso, M.P.; Hiltunen, Y.; Könönen, M.; Kivipelto, M.; Koivisto, A.; Hallikainen, M.; Soininen, H. Decreased brain creatine levels in elderly apolipoprotein E epsilon 4 carriers. J. Neural Transm. 2003, 110, 267–275. [Google Scholar] [CrossRef]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef] [PubMed]
- Nunn, A.V.W.; Guy, G.W.; Brysch, W.; Botchway, S.W.; Frasch, W.; Calabrese, E.J.; Bell, J.D. SARS-CoV-2 and mitochondrial health: Implications of lifestyle and ageing. Immun. Ageing 2020, 17, 33. [Google Scholar] [CrossRef]
- Taquet, M.; Luciano, S.; Geddes, J.R.; Harrison, P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62,354 COVID-19 cases in the USA. Lancet Psychiatry 2021, 8, 130–140. [Google Scholar] [CrossRef]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar] [CrossRef] [PubMed]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Ramirez, S.H. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef] [PubMed]
- Smith, K. The role of choline in the development and progression of Alzheimer’s disease. Acta Neuropathol. 2019, 11, 1–15. [Google Scholar]
- Gustafsson, M.C.; Dahlqvist, O.; Jaworski, J.; Lundberg, P.; Landtblom, A.M. Low choline concentrations in normal-appearing white matter of patients with multiple sclerosis and normal MR imaging brain scans. AJNR Am. J. Neuroradiol. 2007, 28, 1306–1312. [Google Scholar] [CrossRef]
- Hoch, S.E.; Kirov, I.I.; Tal, A. When are metabolic ratios superior to absolute quantification? A statistical analysis. NMR Biomed. 2017, 30, e3710. [Google Scholar] [CrossRef] [PubMed]
- Vance, H.; Maslach, A.; Stoneman, E.; Harmes, K.; Ransom, A.; Seagly, K.; Furst, W. Addressing Post-COVID Symptoms: A Guide for Primary Care Physicians. J. Am. Board Fam. Med. 2021, 34, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Franke, C.; Berlit, P.; Prüss, H. Neurological manifestations of post-COVID-19 syndrome S1-guideline of the German society of neurology. Neurol. Res. Pract. 2022, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Poletti, S.; Mazza, M.G.; Vai, B.; Lorenzi, C.; Colombo, C.; Benedetti, F. Proinflammatory Cytokines Predict Brain Metabolite Concentrations in the Anterior Cingulate Cortex of Patients with Bipolar Disorder. Front. Psychiatry 2020, 11, 590095. [Google Scholar] [CrossRef]
- Miller, B.L.; Moats, R.A.; Shonk, T.; Ernst, T.; Woolley, S.; Ross, B.D. Alzheimer disease: Depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology 1993, 187, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Yanez Lopez, M.; Pardon, M.C.; Baiker, K.; Prior, M.; Yuchun, D.; Agostini, A.; Bai, L.; Auer, D.P.; Faas, H.M. Myoinositol CEST signal in animals with increased Iba-1 levels in response to an inflammatory challenge-Preliminary findings. PLoS ONE 2019, 14, e0212002. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, D.; Kumar, V.; Vyas, S.; Das, B.K.; Srivastava, A.K.; Pandey, R.M.; Sharma, S.K.; Jagannathan, N.R.; Sinha, S. Case control study: Magnetic resonance spectroscopy of brain in HIV infected patients. BMC Neurol. 2016, 16, 99. [Google Scholar] [CrossRef]
- Mahboubisarighieh, A.; Shahverdi, H.; Jafarpoor Nesheli, S.; Alipoor Kermani, M.; Niknam, M.; Torkashvand, M.; Rezaeijo, S.M. Assessing the efficacy of 3D Dual-CycleGAN model for multi-contrast MRI synthesis. Egypt. J. Radiol. Nucl. Med. 2024, 55, 118. [Google Scholar] [CrossRef]
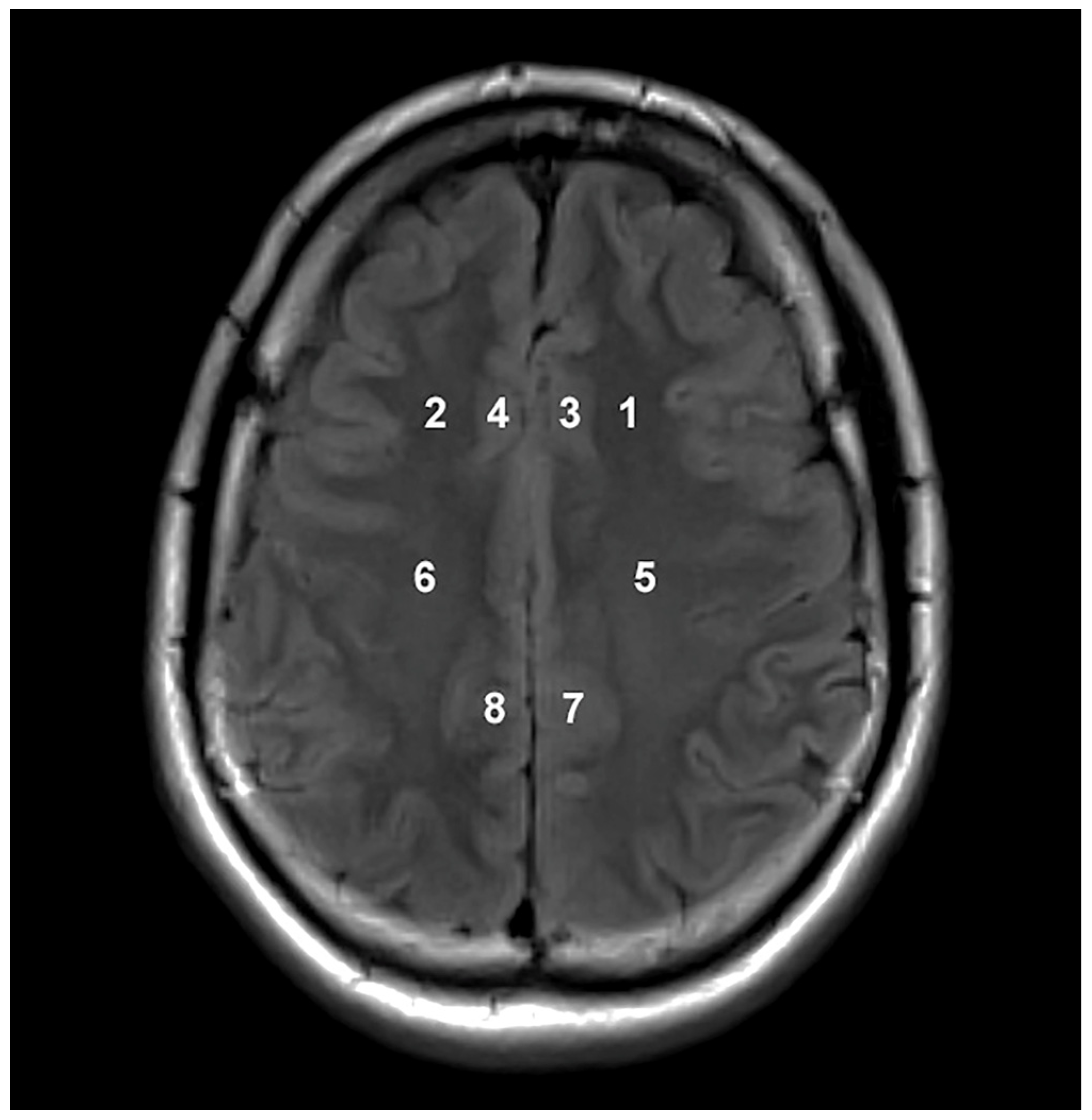
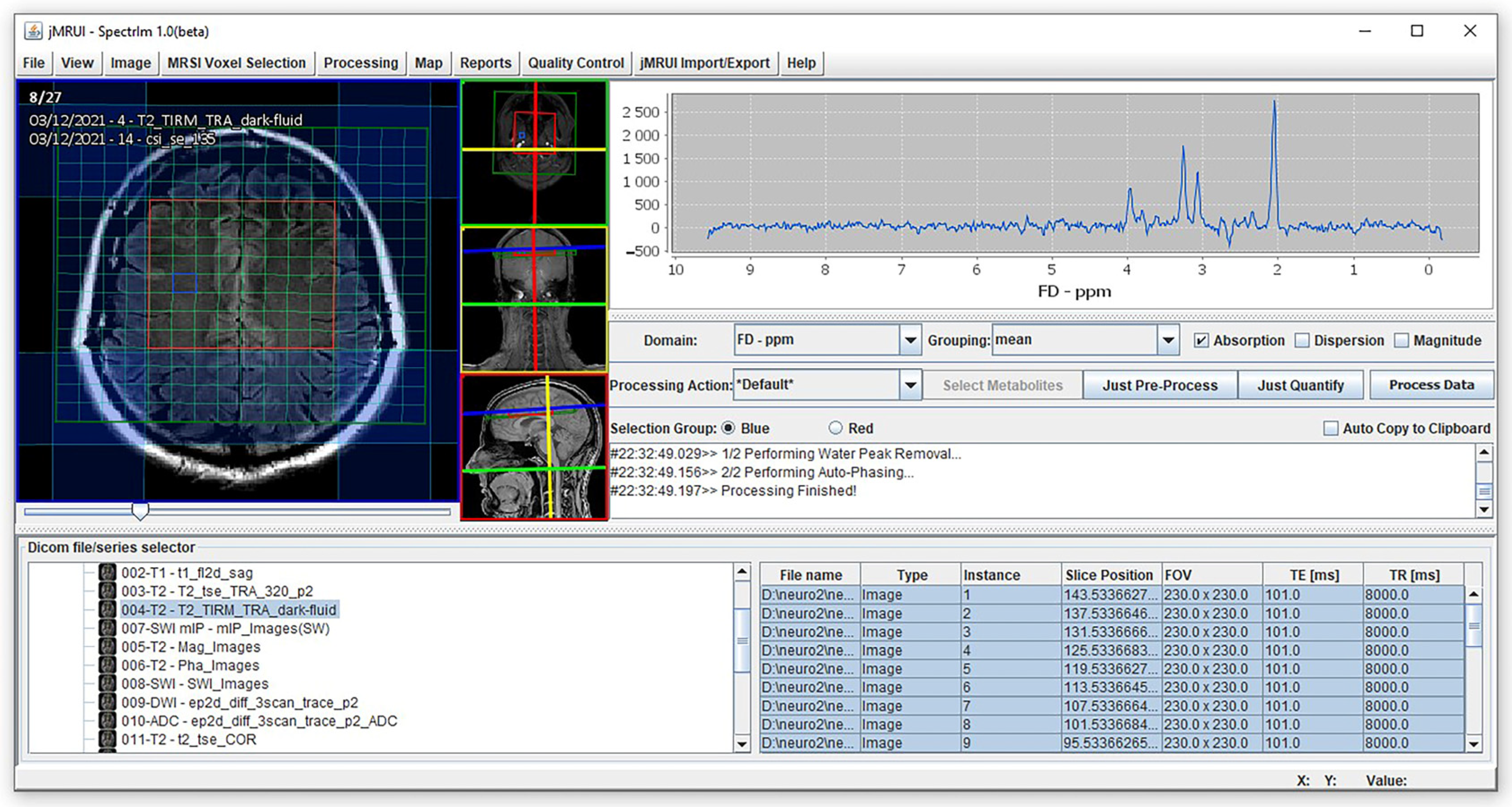
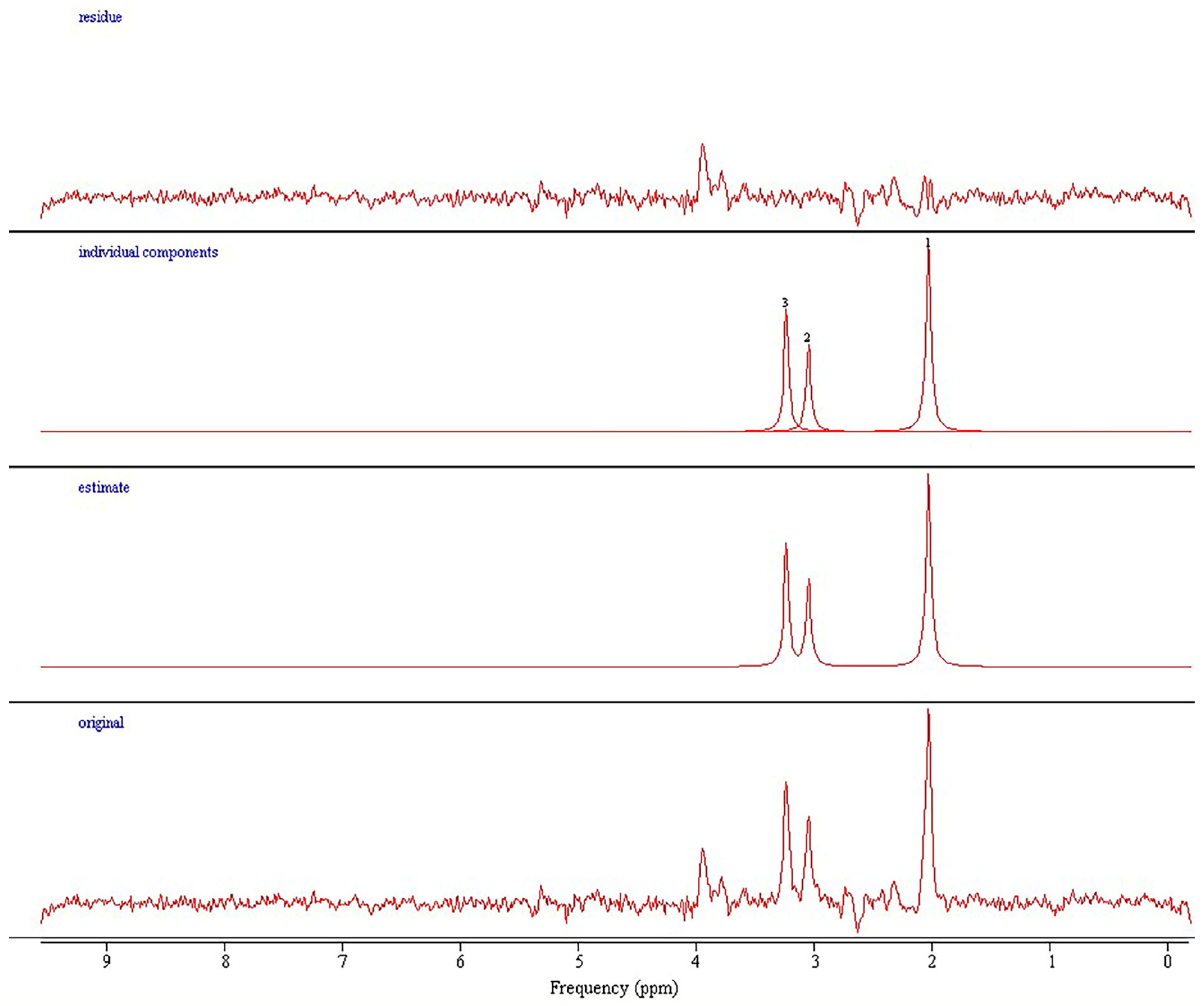

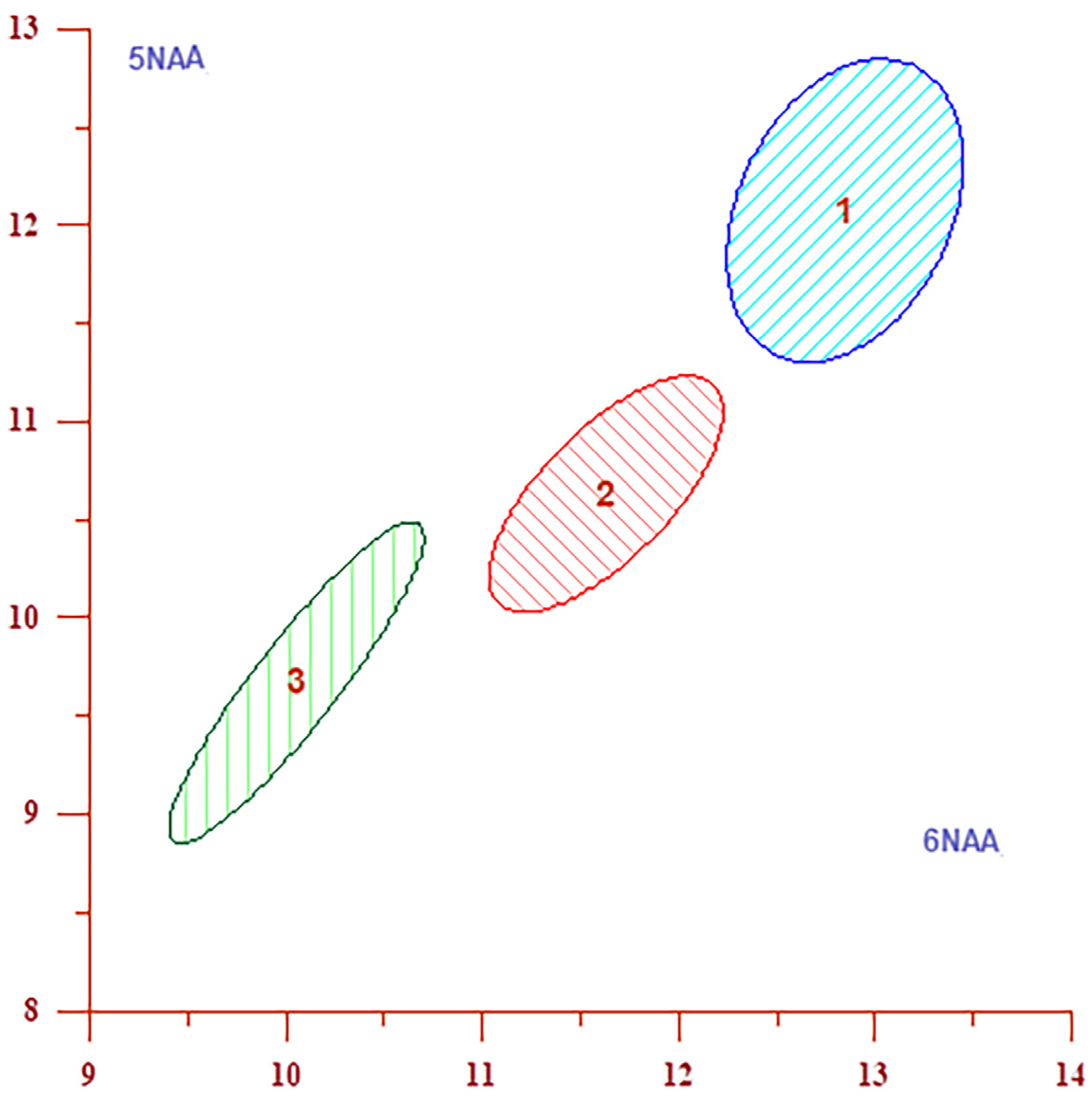
| Description | Mild (n = 17) | Moderate (n = 36) | Severe (n = 28) |
|---|---|---|---|
| Median Age (range) | 49 (40–58) | 51 (42–60) | 52.5 (45–60) |
| Gender | |||
| Male | 7 | 19 | 14 |
| Female | 10 | 17 | 14 |
| Weight average (range)—kg | 76.27 (53–105) | 92 (60–125) | 88 (51–120) |
| BMI kg/m2 average (range) | 25.36 (19.2–33.9) | 29.8 (20.7–40.8) | 29.6 (18.7–45.7) |
| Acute phase symptoms N (average duration—days, duration range—days) | |||
| Fever Malaise | 16 (4.8; 1–4) 15 (12.1; 1–30) | 26 (8.6; 3–16) 24 (23.5; 3–180) | 25 (15.48; 3–60) 21 (25.2; 2–90) |
| Myalgia | 15 (9.5; 1–40) | 13 (34.5; 3–180) | 15 (19.8; 5–60) |
| Arthralgia | 13 (10.6; 2–30) | 9 (36.6; 3–180) | 11 (23.6; 5–60) |
| Nausea | 8 (10.5; 1–30) | 8 (5.7; 2–14) | 11 (8.1; 2–15) |
| Vomiting | 3 (5.3; 1–10) | 5 (3; 1–7) | 3 (7; 2–14) |
| Diarrhea | 6 (5.7; 1–14) | 10 (4.6; 2–9) | 12 (8.1; 2–20) |
| Sore throat | 6 (5.3; 1–10) | 8 (5.6; 3–10) | 10 (9.9; 3–15) |
| Cough | 6 (13.6; 2–21) | 16 (10.8; 3–30) | 14 (14.7; 7–60) |
| Headache | 15 (9.1; 2–21) | 9 (8.1; 2–15) | 13 (14.8; 7–60) |
| Dizziness | 7 (4.8; 1–10) | 9 (8.5; 3–30) | 6 (9.3; 4–15) |
| Altered taste | 12 (33.6; 5–120) | 11 (15.6; 4–30) | 8 (35.5; 7–210) |
| Altered sense of smell | 10 (25.1; 5–90) | 13 (14; 3–30) | 8 (38.2; 7–210) |
| Loss of Appetite | 5 (6.6; 2–14) | 3 (10; 10–10) | 10 (9.9; 3–15) |
| Limb tingling | 2 (25; 10–40) | / | 9 (77.1; 10–240) |
| CRP in Acute phase (average range) | / | 86.3 (8.2–239.2) | 145.6 (3.4–350) |
| Comorbidities | |||
| Arterial hypertension | 3 | 12 | 3 |
| Diabetes | 1 | 9 | 2 |
| Lung disease (Asthma, CODP) | / | / | 1 |
| Hyperlipidemia | 1 | 5 | 1 |
| Hypothyroidism | 3 | 3 | / |
| Psychiatric disorder (depression) | / | 1 | / |
| Deep venous thrombosis | / | 1 | 2 |
| AC (mM/kg)/Ratio | Groups | Mean | SD | t | p | |||
|---|---|---|---|---|---|---|---|---|
| Cho/Cr | mild | moderate | 1.154 | 1.271 | 0.13 | 0.16 | 2.673 | 0.010 |
| Cho/Cr | mild | severe | 1.154 | 1.377 | 0.13 | 0.20 | 4.573 | 0.001 |
| Cho/Cr | moderate | severe | 1.271 | 1.377 | 0.16 | 0.20 | 2.417 | 0.019 |
| Cho/NAA | mild | severe | 0.666 | 0.739 | 0.11 | 0.12 | 2.077 | 0.044 |
| Cr | mild | moderate | 6.469 | 5.827 | 0.86 | 0.59 | 3.187 | 0.022 |
| Cr | mild | severe | 6.469 | 5.233 | 0.86 | 0.74 | 5.133 | 0.001 |
| Cr | moderate | severe | 5.827 | 5.233 | 0.59 | 0.74 | 3.590 | 0.009 |
| NAA | mild | moderate | 9.535 | 8.902 | 1.08 | 1.02 | 2.071 | 0.043 |
| NAA | mild | severe | 9.535 | 8.291 | 1.08 | 1.35 | 3.218 | 0.002 |
| NAA | moderate | severe | 8.902 | 8.291 | 1.02 | 1.35 | 2.063 | 0.043 |
| AC (mM/kg)/Ratio | Groups | Mean | SD | t | p | |||
|---|---|---|---|---|---|---|---|---|
| Cho/Cr | mild | moderate | 1.181 | 1.284 | 0.16 | 0.17 | 2.078 | 0.043 |
| Cho/Cr | mild | severe | 1.181 | 1.390 | 0.16 | 0.16 | 4.304 | 0.012 |
| Cho/Cr | moderate | severe | 1.284 | 1.390 | 0.17 | 0.16 | 2.533 | 0.014 |
| Cho/NAA | mild | severe | 0.648 | 0.736 | 0.11 | 0.10 | 2.720 | 0.009 |
| Cr | mild | moderate | 6.517 | 5.973 | 0.87 | 0.52 | 2.838 | 0.046 |
| Cr | mild | severe | 6.517 | 5.321 | 0.87 | 0.54 | 5.733 | 0.001 |
| Cr | moderate | severe | 5.973 | 5.321 | 0.52 | 0.54 | 4.903 | 0.039 |
| NAA | mild | severe | 10.075 | 8.502 | 1.34 | 1.29 | 3.903 | 0.025 |
| NAA | moderate | severe | 9.384 | 8.502 | 1.19 | 1.29 | 2.829 | 0.026 |
| AC (mM/kg)/Ratio | Groups | Mean | SD | t | p | |||
|---|---|---|---|---|---|---|---|---|
| Cho/Cr | mild | severe | 1.155 | 1.322 | 0.13 | 0.14 | 4.052 | 0.001 |
| Cho/Cr | moderate | severe | 1.225 | 1.322 | 0.17 | 0.14 | 2.412 | 0.019 |
| Cho/NAA | mild | severe | 0.756 | 0.846 | 0.08 | 0.14 | 2.688 | 0.010 |
| Cho | mild | severe | 1.783 | 1.550 | 0.21 | 0.31 | 2.995 | 0.005 |
| Cr | mild | moderate | 6.743 | 5.891 | 0.88 | 1.05 | 2.891 | 0.008 |
| Cr | mild | severe | 6.743 | 5.133 | 0.88 | 1.04 | 5.312 | 0.001 |
| Cr | moderate | severe | 5.891 | 5.133 | 1.05 | 1.04 | 2.872 | 0.016 |
| NAA | mild | moderate | 7.547 | 6.635 | 0.96 | 1.05 | 2.654 | 0.011 |
| NAA | mild | severe | 7.547 | 5.996 | 0.96 | 1.57 | 4.110 | 0.001 |
| AC (mM/kg)/Ratio | Groups | Mean | SD | t | p | |||
|---|---|---|---|---|---|---|---|---|
| Cho/Cr | mild | severe | 1.152 | 1.286 | 0.11 | 0.13 | 3.540 | 0.001 |
| Cho/NAA | mild | severe | 0.760 | 0.839 | 0.09 | 0.16 | 2.126 | 0.040 |
| Cho | mild | severe | 1.829 | 1.560 | 0.30 | 0.33 | 2.726 | 0.009 |
| Cr | mild | moderate | 6.910 | 5.833 | 1.01 | 1.10 | 3.424 | 0.001 |
| Cr | mild | severe | 6.910 | 5.274 | 1.01 | 0.96 | 5.453 | 0.018 |
| Cr | moderate | severe | 5.833 | 5.274 | 1.10 | 0.96 | 2.135 | 0.037 |
| NAA | mild | moderate | 7.700 | 6.716 | 1.27 | 1.25 | 2.663 | 0.010 |
| NAA | mild | severe | 7.700 | 6.099 | 1.27 | 1.61 | 3.487 | 0.001 |
| NAA | moderate | severe | 6.716 | 6.099 | 1.25 | 1.61 | 1.725 | 0.090 |
| AC (mM/kg)/Ratio | Groups | Mean | SD | t | p | |||
|---|---|---|---|---|---|---|---|---|
| Cho/Cr | mild | severe | 1.272 | 1.381 | 0.14 | 0.19 | 2.076 | 0.044 |
| Cho/NAA | mild | severe | 0.534 | 0.599 | 0.08 | 0.09 | 2.423 | 0.020 |
| Cho | mild | severe | 1.982 | 1.754 | 0.31 | 0.31 | 2.404 | 0.021 |
| Cr | mild | severe | 6.028 | 4.894 | 1.17 | 0.81 | 3.862 | 0.009 |
| Cr | moderate | severe | 5.482 | 4.894 | 1.02 | 0.81 | 2.496 | 0.015 |
| NAA | mild | moderate | 12.075 | 10.631 | 1.55 | 1.80 | 2.843 | 0.006 |
| NAA | mild | severe | 12.075 | 9.671 | 1.55 | 2.01 | 4.010 | 0.007 |
| AC (mM/kg)/Ratio | Groups | Mean | SD | t | p | |||
|---|---|---|---|---|---|---|---|---|
| Cho/NAA | mild | severe | 0.523 | 0.575 | 0.07 | 0.07 | 2.328 | 0.025 |
| Cho | mid | severe | 2.082 | 1.773 | 0.35 | 0.29 | 3.216 | 0.002 |
| Cho | moderate | severe | 1.979 | 1.773 | 0.34 | 0.29 | 2.569 | 0.013 |
| Cr | mild | moderate | 6.398 | 5.822 | 0.88 | 0.97 | 2.078 | 0.043 |
| Cr | mild | severe | 6.398 | 5.133 | 0.88 | 0.66 | 5.472 | 0.019 |
| Cr | moderate | severe | 5.822 | 5.133 | 0.97 | 0.66 | 3.221 | 0.022 |
| NAA | mild | moderate | 12.848 | 11.631 | 1.21 | 1.79 | 2.910 | 0.006 |
| NAA | mild | severe | 12.848 | 10.054 | 1.21 | 1.71 | 5.896 | 0.002 |
| NAA | moderate | severe | 11.631 | 10.054 | 1.79 | 1.71 | 3.568 | 0.001 |
| AC (mM/kg)/Ratio | Groups | Mean | SD | t | p | |||
|---|---|---|---|---|---|---|---|---|
| Cho/Cr | moderate | severe | 0.981 | 1.050 | 0.15 | 0.12 | 2.032 | 0.046 |
| Cho/NAA | mild | severe | 0.487 | 0.586 | 0.07 | 0.10 | 4.028 | 0.009 |
| Cho/NAA | moderate | severe | 0.528 | 0.586 | 0.08 | 0.10 | 2.621 | 0.011 |
| Cr | mild | severe | 7.199 | 6.141 | 1.47 | 1.10 | 2.764 | 0.028 |
| Cr | moderate | severe | 7.054 | 6.141 | 1.40 | 1.10 | 2.838 | 0.016 |
| NAA | mild | severe | 11.706 | 9.303 | 1.52 | 2.06 | 4.161 | 0.001 |
| NAA | moderate | severe | 10.895 | 9.303 | 2.07 | 2.06 | 3.053 | 0.007 |
| AC (mM/kg)/Ratio | Groups | Mean | SD | t | p | |||
|---|---|---|---|---|---|---|---|---|
| Cho/NAA | mild | severe | 0.487 | 0.571 | 0.07 | 0.11 | 3.077 | 0.004 |
| Cr | mild | moderate | 7.248 | 6.600 | 0.79 | 1.05 | 2.253 | 0.029 |
| Cr | mild | severe | 7.248 | 6.017 | 0.79 | 1.00 | 4.300 | 0.001 |
| Cr | moderate | severe | 6.600 | 6.017 | 1.05 | 1.00 | 2.246 | 0.028 |
| NAA | mild | severe | 11.411 | 8.920 | 1.50 | 1.70 | 4.970 | 0.001 |
| NAA | moderate | severe | 10.454 | 8.920 | 2.38 | 1.70 | 2.878 | 0.005 |
| Location | Metabolite Concentrations and Ratios | Groups Categorized According to Disease Severity | Contribution % | ||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||
| 1 | Cr | 6.469 *2 | 5.827 *1 | 5.233 | 50.333 |
| NAA | 9.535 *2 | 8.902 *1 | 8.291 | 29.333 | |
| Cho/NAA | 0.666 | 0.701 | 0.739 *1 | 8.000 | |
| 2 | Cr | 6.517 *2 | 5.973 *1 | 5.321 | 37.652 |
| NAA | 10.075 *2 | 9.384 *1 | 8.502 | 28.340 | |
| Cho/Cr | 1.181 | 1.284 *1 | 1.390 *2 | 17.814 | |
| 3 | Cho | 1.783 *1 | 1.651 | 1.550 | 42.105 |
| Cho/NAA | 0.756 | 0.801 | 0.846 *1 | 24.342 | |
| Cr | 6.743 *2 | 5.891 *1 | 5.133 | 11.842 | |
| 4 | Cho/NAA | 0.760 | 0.784 | 0.839 *1 | 38.767 |
| Cr | 6.910 *2 | 5.833 *1 | 5.274 | 26.872 | |
| NAA | 7.700 *2 | 6.716 *1 | 6.099 | 14.978 | |
| 5 | Cho/NAA | 0.534 | 0.571 | 0.599 *1 | 22.131 |
| NAA | 12.075 *2 | 10.631 *1 | 9.671 | 20.765 | |
| Cr | 6.028 *2 | 5.482 *1 | 4.894 | 18.852 | |
| 6 | Cr | 6.398 *2 | 5.822 *1 | 5.133 | 36.842 |
| NAA | 12.848 *2 | 11.631 *1 | 10.054 | 22.601 | |
| Cho | 2.082 *1 | 1.979 *1 | 1.773 | 18.576 | |
| 7 | Cr | 7.199 *2 | 7.054 *1 | 6.141 | 42.246 |
| Cho | 2.033 | 2.051 | 1.982 | 26.738 | |
| NAA | 11.706 *1 | 10.895 *1 | 9.303 | 14.973 | |
| 8 | Cr | 7.248 *2 | 6.600 *1 | 6.017 | 35.821 |
| Cho | 2.069 | 2.055 | 1.887 | 32.537 | |
| NAA | 11.411 *1 | 10.454 *1 | 8.920 | 21.493 | |
| Location | Mild | Moderate | Severe | Contribution % |
|---|---|---|---|---|
| 2 | 6.517 *2 | 5.973 *1 | 5.321 | 30.618 |
| 8 | 7.248 *2 | 6.600 *1 | 6.017 | 26.697 |
| 7 | 7.199 *1 | 7.054 *1 | 6.141 | 16.440 |
| 1 | 6.469 *2 | 5.827 *1 | 5.233 | 9.955 |
| 6 | 6.398 *2 | 5.822 *1 | 5.133 | 9.050 |
| 4 | 6.910 *2 | 5.833 *1 | 5.274 | 5.732 |
| 3 | 6.743 *2 | 5.891 *1 | 5.133 | 1.207 |
| 5 | 6.028 *1 | 5.482 *1 | 4.894 | 0.302 |
| Homogeneity | 15/17 | 23/36 | 20/28 | |
| % | 88.24 | 63.89 | 71.43 |
| Location | Mild | Moderate | Severe | Contribution % |
|---|---|---|---|---|
| 6 | 12.848 *2 | 11.631 *1 | 10.054 | 50.368 |
| 5 | 12.075 *2 | 10.631 *1 | 9.671 | 16.544 |
| 2 | 10.075 *2 | 9.384 *1 | 8.502 | 13.971 |
| 1 | 9.535 *2 | 8.902 *1 | 8.291 | 5.882 |
| 4 | 7.700 *2 | 6.716 *1 | 6.099 | 5.515 |
| 8 | 11.411 *1 | 10.454 *1 | 8.920 | 4.779 |
| 3 | 7.547 *2 | 6.635 | 5.996 | 2.941 |
| 7 | 11.706 *1 | 10.895 *1 | 9.303 | 0.000 |
| Homogeneity | 13/17 | 19/36 | 20/28 | |
| % | 76.47 | 52.78 | 71.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostojic, J.; Kozic, D.; Ostojic, S.; Ilic, A.D.; Galic, V.; Matijasevic, J.; Dragicevic, D.; Barak, O.; Boban, J. Decreased Cerebral Creatine and N-Acetyl Aspartate Concentrations after Severe COVID-19 Infection: A Magnetic Resonance Spectroscopy Study. J. Clin. Med. 2024, 13, 4128. https://doi.org/10.3390/jcm13144128
Ostojic J, Kozic D, Ostojic S, Ilic AD, Galic V, Matijasevic J, Dragicevic D, Barak O, Boban J. Decreased Cerebral Creatine and N-Acetyl Aspartate Concentrations after Severe COVID-19 Infection: A Magnetic Resonance Spectroscopy Study. Journal of Clinical Medicine. 2024; 13(14):4128. https://doi.org/10.3390/jcm13144128
Chicago/Turabian StyleOstojic, Jelena, Dusko Kozic, Sergej Ostojic, Aleksandra DJ Ilic, Vladimir Galic, Jovan Matijasevic, Dusan Dragicevic, Otto Barak, and Jasmina Boban. 2024. "Decreased Cerebral Creatine and N-Acetyl Aspartate Concentrations after Severe COVID-19 Infection: A Magnetic Resonance Spectroscopy Study" Journal of Clinical Medicine 13, no. 14: 4128. https://doi.org/10.3390/jcm13144128
APA StyleOstojic, J., Kozic, D., Ostojic, S., Ilic, A. D., Galic, V., Matijasevic, J., Dragicevic, D., Barak, O., & Boban, J. (2024). Decreased Cerebral Creatine and N-Acetyl Aspartate Concentrations after Severe COVID-19 Infection: A Magnetic Resonance Spectroscopy Study. Journal of Clinical Medicine, 13(14), 4128. https://doi.org/10.3390/jcm13144128







