Different Neurogenic Bladders in Patients with Cervical and Thoracic Myelopathy: Direct Comparison from a Prospective Case Series
Abstract
:1. Introduction
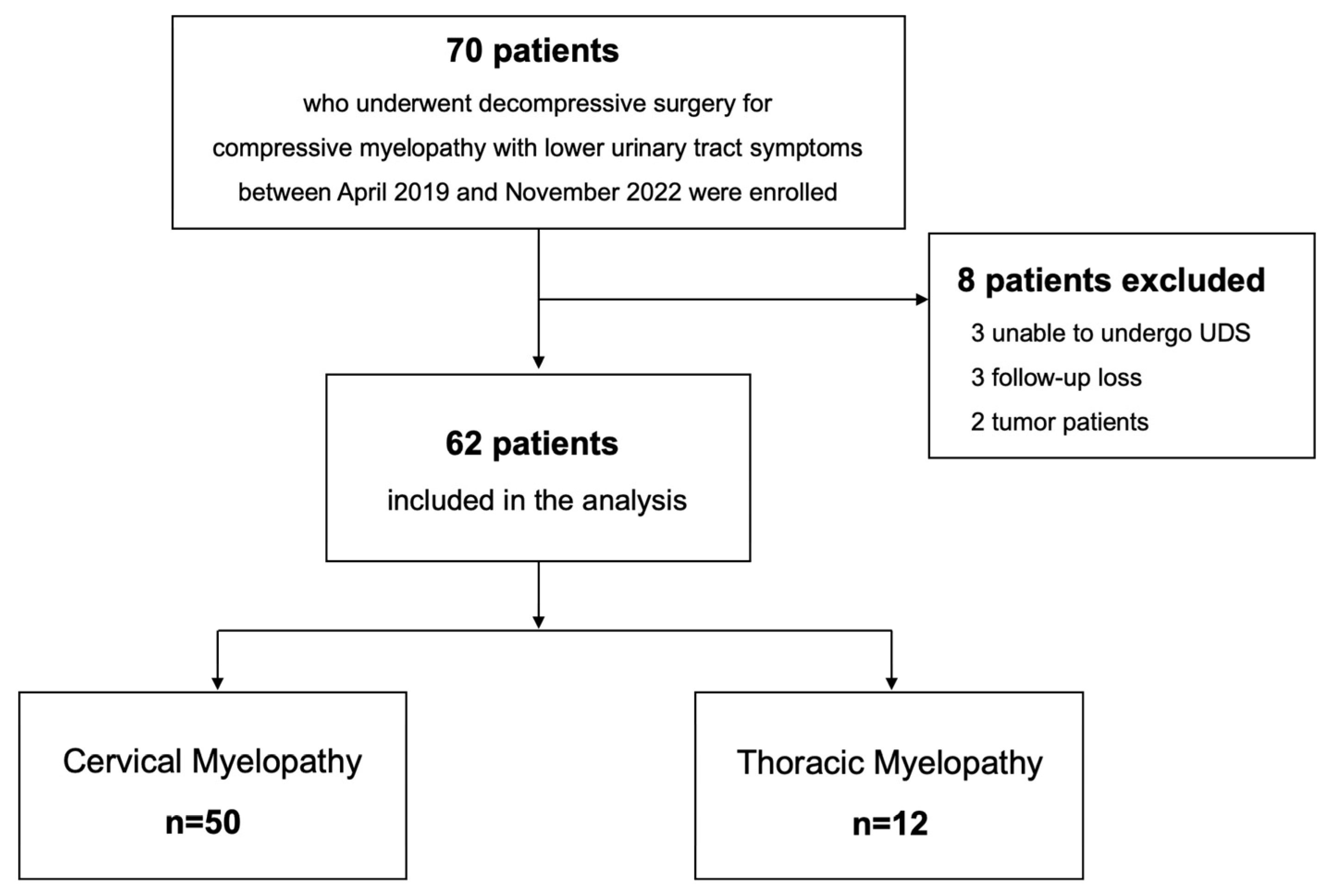
2. Materials and Methods
2.1. Study Design and Cohort
2.2. Urodynamic Study
2.3. Patient-Reported Outcomes
2.4. Data Collection
2.5. Statistical Analysis
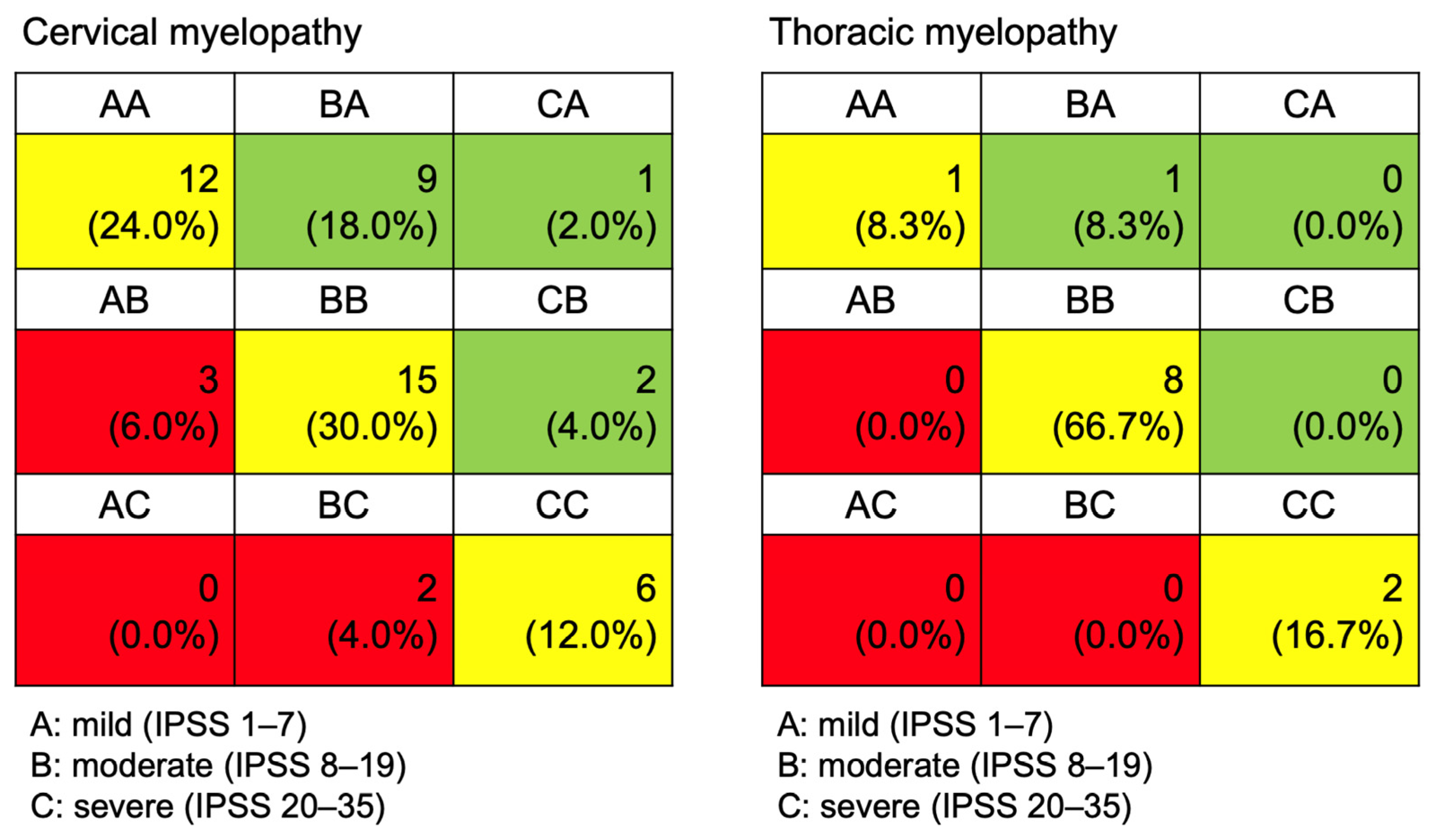
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kimura, S.; Takyu, S.; Kawamorita, N.; Namima, T.; Morozumi, N.; Ito, A. Neurogenic lower urinary tract dysfunction in association with severity of degenerative spinal diseases: Short-term outcomes of decompression surgery. Low. Urin. Tract. Symptoms 2022, 14, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Ozaki, T.; Tsumura, N.; Sengoku, A.; Nomi, M.; Yanagiuchi, A.; Nishida, K.; Kuroda, R.; Iguchi, T. Neurogenic bladder associated with pure cervical spondylotic myelopathy: Clinical characteristics and recovery after surgery. Spine 2013, 38, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, E.G.; Radoslovich, S.; Marshall, L.M.; Yoo, J.U. Lower Urinary Tract Symptoms and Urinary Bother Are Common in Patients Undergoing Elective Cervical Spine Surgery. Clin. Orthop. Relat. Res. 2019, 477, 872–878. [Google Scholar] [CrossRef]
- Kim, M.W.; Kang, C.N.; Choi, S.H. Update of the Natural History, Pathophysiology, and Treatment Strategies of Degenerative Cervical Myelopathy: A Narrative Review. Asian Spine J. 2023, 17, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Kim, Y.I.; Hong, J.T.; Lee, D.S. Rationales for a Urodynamic Study in Patients with Cervical Spondylotic Myelopathy. World Neurosurg. 2018, 124, e147–e155. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M.; Holly, L.T.; Mizuno, J.; Fehlings, M.G. Future Directions and New Technologies for the Management of Degenerative Cervical Myelopathy. Neurosurg. Clin. N. Am. 2018, 29, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Rujeedawa, T.; Mowforth, O.D.; Davies, B.M.; Yang, C.; Nouri, A.; Francis, J.J.; Aarabi, B.; Kwon, B.K.; Harrop, J.; Wilson, J.R.; et al. Degenerative Thoracic Myelopathy: A Scoping Review of Epidemiology, Genetics, and Pathogenesis. Glob. Spine J. 2024, 14, 1664–1677. [Google Scholar] [CrossRef] [PubMed]
- Kalita, J.; Misra, U.K.; Kumar, G.; Kapoor, R. Bladder dysfunction in spinal tuberculosis: Clinical, urodynamic and MRI study. Spinal Cord. 2010, 48, 697–703. [Google Scholar] [CrossRef]
- Choi, J.H.; Birring, P.S.; Lee, J.; Hashmi, S.Z.; Bhatia, N.N.; Lee, Y.P. A Comparison of Short-Term Outcomes after Surgical Treatment of Multilevel Degenerative Cervical Myelopathy in the Geriatric Patient Population: An Analysis of the National Surgical Quality Improvement Program Database 2010–2020. Asian Spine J. 2024, 18, 190–199. [Google Scholar] [CrossRef]
- Schizas, C.; Theumann, N.; Burn, A.; Tansey, R.; Wardlaw, D.; Smith, F.W.; Kulik, G. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine 2010, 35, 1919–1924. [Google Scholar] [CrossRef]
- Kwon, J.W.; Moon, S.H.; Park, S.Y.; Park, S.J.; Park, S.R.; Suk, K.S.; Kim, H.S.; Lee, B.H. Lumbar Spinal Stenosis: Review Update 2022. Asian Spine J. 2022, 16, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, J.B.; Schurch, B.; Hamid, R.; Averbeck, M.; Sakakibara, R.; Agrò, E.F.; Dickinson, T.; Payne, C.K.; Drake, M.J.; Haylen, B.T. An International Continence Society (ICS) report on the terminology for adult neurogenic lower urinary tract dysfunction (ANLUTD). Neurourol. Urodyn. 2018, 37, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- McVary, K.T.; Roehrborn, C.G.; Avins, A.L.; Barry, M.J.; Bruskewitz, R.C.; Donnell, R.F.; Foster, H.E., Jr.; Gonzalez, C.M.; Kaplan, S.A.; Penson, D.F.; et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J. Urol. 2011, 185, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Chiba, K.; Kawakami, M.; Kikuchi, S.; Konno, S.; Miyamoto, M.; Seichi, A.; Shimamura, T.; Shirado, O.; Taguchi, T.; et al. An outcome measure for patients with cervical myelopathy: Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ): Part 1. J. Orthop. Sci. 2007, 12, 227–240. [Google Scholar] [CrossRef]
- Hirai, T.; Otani, K.; Sekiguchi, M.; Kikuchi, S.I.; Konno, S.I. Epidemiological study of cervical cord compression and its clinical symptoms in community-dwelling residents. PLoS ONE 2021, 16, e0256732. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Misawa, T.; Kamimura, M.; Kinoshita, T.; Itoh, H.; Yuzawa, Y.; Kitahara, J. Neurogenic bladder in patients with cervical compressive myelopathy. J. Spinal Disord. Tech. 2005, 18, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Goldmark, E.; Niver, B.; Ginsberg, D.A. Neurogenic bladder: From diagnosis to management. Curr. Urol. Rep. 2014, 15, 448. [Google Scholar] [CrossRef]
- Ando, M. Neurogenic bladder in patients with cervical cord compression disorders. Nihon Hinyokika Gakkai Zasshi 1990, 81, 243–250. [Google Scholar] [CrossRef]
- Sakakibara, R.; Hattori, T.; Tojo, M.; Yamanishi, T.; Yasuda, K.; Hirayama, K. The location of the paths subserving micturition: Studies in patients with cervical myelopathy. J. Auton. Nerv. Syst. 1995, 55, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, E.; Lindbo, L.; Seiger, Å. The Stockholm Spinal Cord Uro Study: 3. Urodynamic characteristics in a regional prevalence group of persons with spinal cord injury and indications for improved follow-up. Scand. J. Urol. 2021, 55, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Poublon, C.G.; Scholten, E.W.M.; Wyndaele, M.I.A.; Post, M.W.M.; Stolwijk-Swüste, J.M. Changes in bladder emptying during inpatient rehabilitation after spinal cord injury and predicting factors: Data from the Dutch Spinal Cord Injury Database. Spinal Cord. 2023, 61, 624–631. [Google Scholar] [CrossRef] [PubMed]
| Cervical Myelopathy (n = 50) | Thoracic Myelopathy (n = 12) | |
|---|---|---|
| Age (mean, SD) | 62.9 ± 10.9 | 64.6 ± 9.9 |
| Sex (M:F) | 27:23 | 3:9 |
| BMI (mean, SD) | 26.3 ± 3.7 | 26.5 ± 2.6 |
| Symptom duration (months) | 14.8 ± 12.5 | 13.8 ± 8.8 |
| Spinal cord compression ratio | 0.58 ± 0.11 | 0.60 ± 0.09 |
| Preoperative diagnosis | Cervical spondylotic myelopathy (n = 23) Cervical OPLL (n = 16) Cervical HIVD (n = 9) Atlantoaxial instability (n = 2) | Thoracic OYL (n = 6) Thoracic OPLL (n = 4) Thoracic HIVD (n = 2) |
| Surgical treatment | Cervical laminoplasty (n = 27) Anterior cervical discectomy and fusion (n = 17) Cervical laminectomy and fusion (n = 3) Atlantoaxial fusion (n = 2) Anterior cervical corpectomy and fusion (n = 1) | Posterior decompression and fusion (n = 10) Posterior decompression only (n = 2) |
| Total (n, %) | Cervical Myelopathy (n, %) | Thoracic Myelopathy (n, %) | p-Value | |||
|---|---|---|---|---|---|---|
| Storage phase | Bladder function | Overactive | 34 (54.8%) | 30 (60.0%) | 4 (33.3%) | 0.354 |
| Bladder sensation | Hypersensitive | 30 (48.4%) | 24 (48.0%) | 6 (50.0%) | 0.539 | |
| Hyposensitive | 15 (24.2%) | 11 (22.0%) | 4 (33.3%) | 0.258 | ||
| Bladder capacity | Above normal | 19 (30.6%) | 14 (28.0%) | 5 (41.7%) | 0.280 | |
| Below normal | 25 (40.3%) | 22 (44.0%) | 3 (25.0%) | 0.524 | ||
| Urethral function | Incompetent | 7 (11.3%) | 5 (10.0%) | 2 (16.7%) | 0.612 | |
| Voiding phase | Bladder function | Underactive | 14 (22.6%) | 9 (18.0%) | 5 (41.7%) | 0.152 |
| Acontractile | 4 (6.5%) | 0 (0.0%) | 4 (33.3%) | 0.001 | ||
| Urethral function | Abnormal | 13 (21.0%) | 12 (24.0%) | 1 (8.3%) | 0.431 |
| Preoperative | 12 Months Postoperatively | ||||||
|---|---|---|---|---|---|---|---|
| Cervical | Thoracic | p-Value | Cervical | Thoracic | p-Value | ||
| IPSS | Storage symptom | 5.2 ± 3.2 | 5.7 ± 4.0 | 0.912 | 4.3 ± 3.5 | 5.6 ± 3.9 | 0.288 |
| Voiding symptom | 5.0 ± 4.4 | 8.7 ± 4.5 | 0.013 | 4.6 ± 4.7 | 6.5 ± 4.5 | 0.190 | |
| Postmicturition symptom | 1.8 ± 1.6 | 2.8 ± 1.9 | 0.108 | 1.8 ± 1.7 | 2.8 ± 1.9 | 0.120 | |
| Total IPSS | 12.0 ± 8.0 | 17.1 ± 8.7 | 0.057 | 10.8 ± 8.0 | 15.0 ± 7.3 | 0.086 | |
| JOA | Bladder function | 68.4 ± 21.7 | 59.4 ± 19.1 | 0.133 | 76.6 ± 18.7 * | 65.6 ± 16.3 | 0.051 |
| Lower-extremity function | 52.7 ± 27.1 | 36.4 ± 25.4 | 0.060 | 66.3 ± 29.4 * | 49.6 ± 30.0 | 0.099 | |
| Quality of life | 43.6 ± 15.5 | 35.6 ± 25.1 | 0.164 | 58.9 ± 22.9 * | 43.2 ± 21.5 | 0.031 | |
| Overactive Bladder (n = 34) | Underactive Bladder (n = 18) | ||||||
|---|---|---|---|---|---|---|---|
| Preop | 12 Months Postop | p-Value * | Preop | 12 Months Postop | p-Value * | ||
| IPSS | Storage | 5.1 ± 3.3 | 4.1 ± 3.6 | 0.072 | 5.3 ± 3.4 | 6.3 ± 4.3 | 0.442 |
| Voiding | 5.1 ± 4.4 | 5.1 ± 4.5 | 0.969 | 6.7 ± 4.6 | 5.8 ± 4.8 | 0.181 | |
| Postmicturition | 1.8 ± 1.6 | 1.6 ± 1.5 | 0.476 | 1.8 ± 1.8 | 2.8 ± 1.9 | 0.122 | |
| Total score | 11.9 ± 8.4 | 10.8 ± 7.5 | 0.303 | 13.8 ± 8.9 | 14.8 ± 8.5 | 0.669 | |
| JOA | Bladder function | 67.7 ± 21.4 | 75.4 ± 20.0 | 0.034 | 69.4 ± 23.9 | 71.2 ± 19.4 | 0.307 |
| Lower extremity | 46.7 ± 23.6 | 61.1 ± 28.1 | 0.001 | 53.8 ± 31.1 | 63.6 ± 35.1 | 0.148 | |
| Quality of life | 41.4 ± 17.4 | 55.0 ± 24.2 | 0.003 | 42.5 ± 23.8 | 55.3 ± 25.5 | 0.098 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Chang, B.-S.; Park, S.; Nam, Y.; Chang, S.Y. Different Neurogenic Bladders in Patients with Cervical and Thoracic Myelopathy: Direct Comparison from a Prospective Case Series. J. Clin. Med. 2024, 13, 4155. https://doi.org/10.3390/jcm13144155
Kim H, Chang B-S, Park S, Nam Y, Chang SY. Different Neurogenic Bladders in Patients with Cervical and Thoracic Myelopathy: Direct Comparison from a Prospective Case Series. Journal of Clinical Medicine. 2024; 13(14):4155. https://doi.org/10.3390/jcm13144155
Chicago/Turabian StyleKim, Hyoungmin, Bong-Soon Chang, Sanghyun Park, Yunjin Nam, and Sam Yeol Chang. 2024. "Different Neurogenic Bladders in Patients with Cervical and Thoracic Myelopathy: Direct Comparison from a Prospective Case Series" Journal of Clinical Medicine 13, no. 14: 4155. https://doi.org/10.3390/jcm13144155







