Determinants of Pre- and Post-Procedural Neurological Assessment, and Outcome of Carotid Endarterectomy or Stenting
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Case Selection and Grouping
2.3. Study Outcomes
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Patients
3.2. Perioperative Management and Treatment
3.3. Neurological Assessment by Indication for Treatment
3.4. Hospital and Regional Characteristics (Bavarian Data Only)
3.5. Neurological Assessment by Outcome
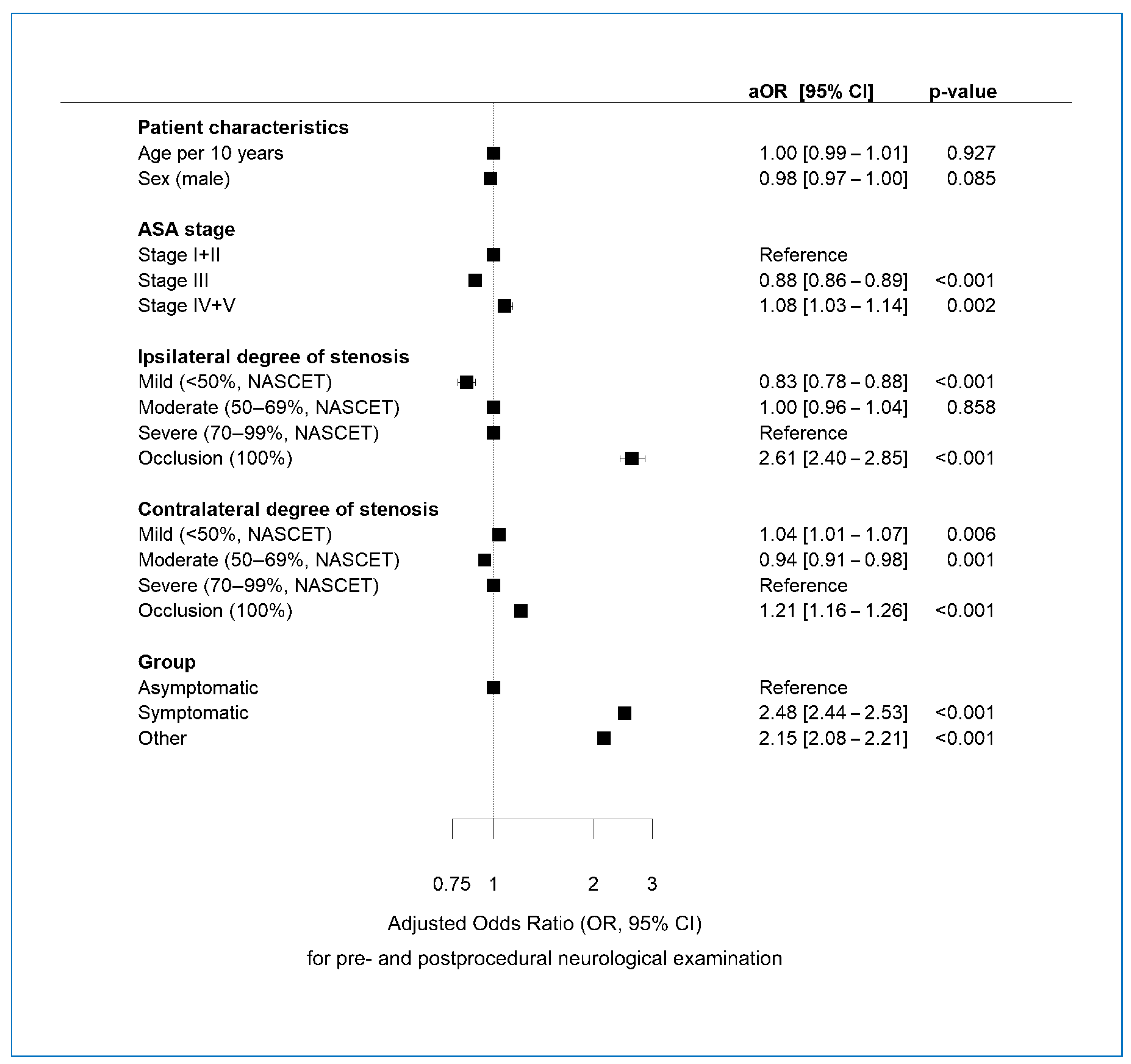
3.6. Multivariable Regression Analysis of Determinants of Pre-NA and Post-NA
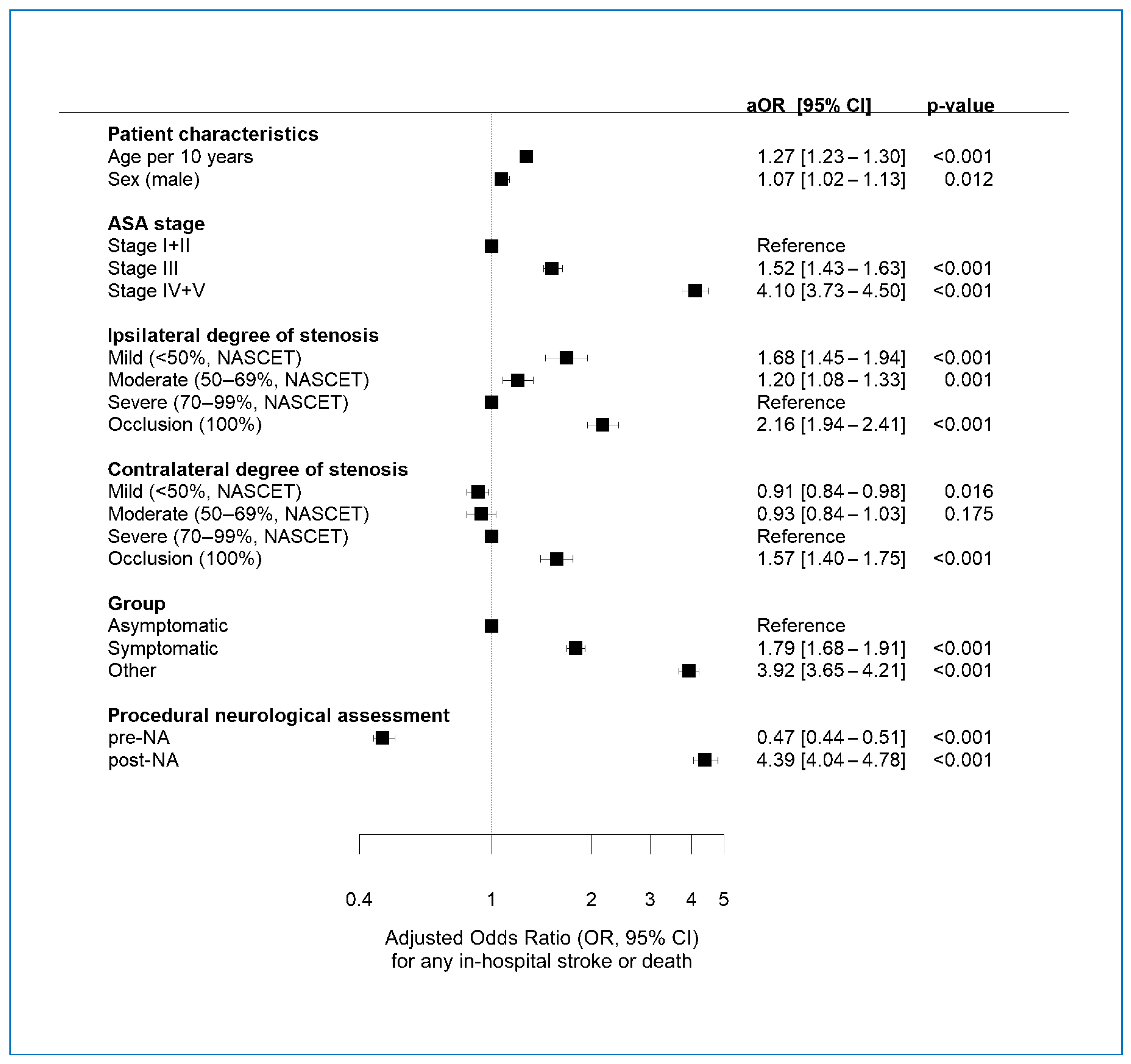
3.7. Multivariate Regression Analysis of Factors Associated with POE
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AbuRahma, A.F.; Avgerinos, E.D.; Chang, R.W.; Darling, R.C., 3rd; Duncan, A.A.; Forbes, T.L.; Malas, M.B.; Murad, M.H.; Perler, B.A.; Powell, R.J.; et al. Society for Vascular Surgery Clinical Practice Guidelines for Management of Extracranial Cerebrovascular Disease. J. Vasc. Surg. 2022, 75, 4S–22S. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, H.-H.; Tsantilas, P.; Kühnl, A.; Haller, B.; Breitkreuz, T.; Zimmermann, A.; Kallmayer, M. Surgical and Endovascular Treatment of Extracranial Carotid Stenosis. Dtsch. Arztebl. Int. 2017, 114, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Kallmayer, M.A.; Tsantilas, P.; Knappich, C.; Haller, B.; Storck, M.; Stadlbauer, T.; Kühnl, A.; Zimmermann, A.; Eckstein, H.H. Patient characteristics and outcomes of carotid endarterectomy and carotid artery stenting: Analysis of the German mandatory national quality assurance registry—2003 to 2014. J. Cardiovasc. Surg. 2015, 56, 827–836. [Google Scholar]
- Eckstein, H.-H.; Kühnl, A.; Berkefeld, J.; Lawall, H.; Storck, M.; Sander, D. Diagnosis, Treatment and Follow-up in Extracranial Carotid Stenosis. Dtsch. Arztebl. Int. 2020, 117, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.G.; Hobson, R.W., 2nd; Howard, G.; Roubin, G.S.; Clark, W.M.; Brooks, W.; Mackey, A.; Hill, M.D.; Leimgruber, P.P.; Sheffet, A.J.; et al. Stenting Versus Endarterectomy for Treatment of Carotid-Artery Stenosis. N. Engl. J. Med. 2010, 363, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.; Warlow, C. Is Self-Audit Reliable? Lancet 1995, 346, 1623. [Google Scholar] [CrossRef] [PubMed]
- Theiss, W.; Hermanek, P.; Mathias, K.; Ahmadi, R.; Heuser, L.; Hoffmann, F.J.; Kerner, R.; Leisch, F.; Sievert, H.; von Sommoggy, S. Pro-Cas: A Prospective Registry of Carotid Angioplasty and Stenting. Stroke 2004, 35, 2134–2139. [Google Scholar] [CrossRef] [PubMed]
- Bazan, H.A.; Caton, G.; Talebinejad, S.; Hoffman, R.; Smith, T.A.; Vidal, G.; Gaines, K.; Sternbergh, W.C. A Stroke/Vascular Neurology Service Increases the Volume of Urgent Carotid Endarterectomies Performed in a Tertiary Referral Center. Ann. Vasc. Surg. 2014, 28, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, U.; Krautz, C.; Weber, G.F.; Mansky, T.; Grützmann, R. Nationwide In-hospital Mortality Following Pancreatic Surgery in Germany is Higher than Anticipated. Ann. Surg. 2016, 264, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, U.; Mansk, T. Deaths Following Cholecystectomy and Herniotomy: An Analysis of Nationwide German Hospital Discharge Data from 2009 to 2013. Dtsch. Arztebl. Int. 2015, 112, 535–543. [Google Scholar] [PubMed]
- Nimptsch, U.; Mansky, T. Trends in Acute Inpatient Stroke Care in Germany--an Observational Study Using Administrative Hospital Data from 2005-2010. Dtsch. Arztebl. Int. 2012, 109, 885–892. [Google Scholar] [PubMed]
- Wengler, A.; Nimptsch, U.; Mansky, T. Hip and Knee Replacement in Germany and the USA: Analysis of Individual Inpatient Data from German and Us Hospitals for the Years 2005 to 2011. Dtsch. Arztebl. Int. 2014, 111, 407–416. [Google Scholar] [PubMed]
- Knappich, C.; Kuehnl, A.; Tsantilas, P.; Schmid, S.; Breitkreuz, T.; Kallmayer, M.; Zimmermann, A.; Eckstein, H.-H. The Use of Embolic Protection Devices Is Associated with a Lower Stroke and Death Rate after Carotid Stenting. JACC Cardiovasc. Interv. 2017, 10, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Catalogue of Bias Collaboration; Mahtani, K.; Spencer, E.A.; Brassey, J. Observer Bias. 2017. Available online: https://www.catalogofbias.org/biases/observer-bias (accessed on 19 September 2023).
- Catalogue of Bias Collaboration; Banerjee, A.; O’Sullivan, J.; Pluddemann, A.; Spencer, E.A. Diagnostic Access Bias. 2017. Available online: https://catalogofbias.org/biases/diagnostic-access-bias/ (accessed on 19 September 2023).
- Group, OCEBM Levels of Evidence Working. The Oxford 2009 Levels of Evidence. 2009. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed on 19 September 2023).
- Halliday, A.; Bulbulia, R.; Bonati, L.H.; Chester, J.; Cradduck-Bamford, A.; Peto, R.; Pan, H.; Potter, J.; Eckstein, H.H.; Farrell, B.; et al. Second asymptomatic carotid surgery trial (ACST-2): A randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet 2021, 398, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K.S.; Mehrabadi, A.; Lisonkova, S. Confounding by Indication and Related Concepts. Curr. Epidemiol. Rep. 2014, 1, 1–8. [Google Scholar] [CrossRef]
- Catalogue of Bias Collabaration; Banerjee, A.; Pluddemann, A.; O’Sullivan, J. Diagnostic Suspicion Bias. 2017. Available online: https://catalogofbias.org/biases/diagnostic-suspicion-bias/ (accessed on 19 September 2023).
- Knappich, C.; Bohmann, B.B.; Kirchhoff, F.; Lohe, V.B.; Naher, S.M.; Kallmayer, M.; Eckstein, H.-H.; Kuehnl, A.M. Intraoperative Completion Studies and their Associations with Carotid Endarterectomy Outcomes. Ann. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Kuehnl, A.; Kallmayer, M.; Bohmann, B.; Lohe, V.; Moser, R.; Naher, S.; Kirchhoff, F.; Eckstein, H.H.; Knappich, C. Association between Hospital Ownership and Patient Selection, Management, and Outcomes after Carotid Endarterectomy or Carotid Artery Stenting: Secondary Data Analysis of the Bavarian Statutory Quality Assurance Database. BMC Surg. 2024, 24, 158. [Google Scholar] [CrossRef] [PubMed]
- Touzé, E.; Trinquart, L.; Chatellier, G.; Mas, J.-L. Systematic Review of the Perioperative Risks of Stroke or Death After Carotid Angioplasty and Stenting. Stroke 2009, 40, E683–E693. [Google Scholar] [CrossRef] [PubMed]
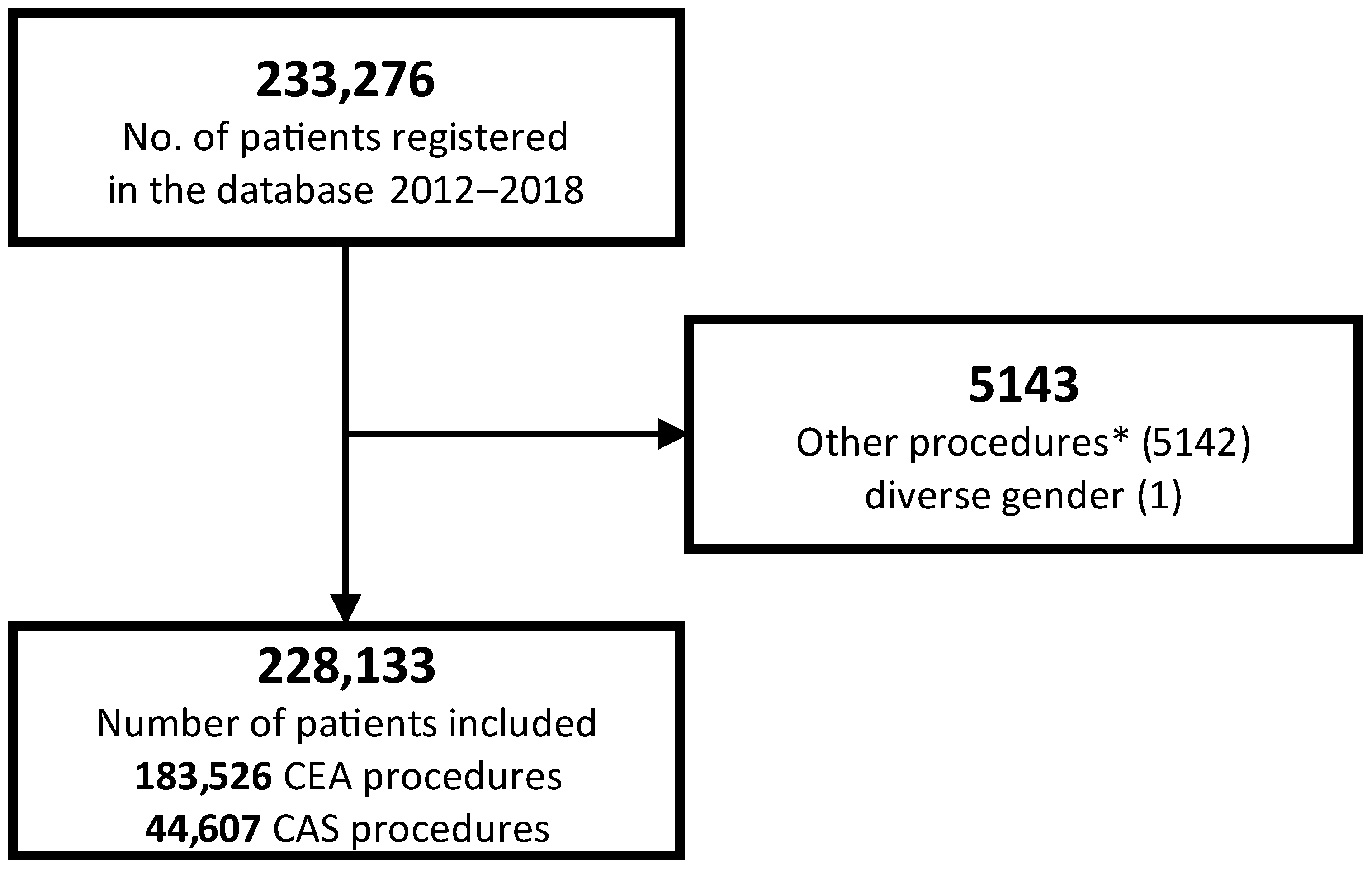
| Overall (Column-%) | Pre-Operative Neurological Assessment | Post-Operative Neurological Assessment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||||||
| No. of cases (%) | 228,133 | (100) | 165,459 | (72.5) | 62,674 | (27.5) | 14,2619 | (62.5) | 85,514 | (37.5) |
| Age (M, Q1/3) | 72 | (65–78) | 72 | (64–78) | 72 | (65–77) | 72 | (64–78) | 72 | (65–77) |
| Male sex | 155,532 | (68) | 113,045 | (72.7) | 42,487 | (27.3) | 97,280 | (62.5) | 58,252 | (37.5) |
| Side of treatment (right) | 114,427 | (50) | 82,454 | (72.1) | 31,973 | (27.9) | 70,950 | (62.0) | 43,477 | (38.0) |
| ASA stage | ||||||||||
| Stage I+II | 73,744 | (33) | 53,269 | (72.2) | 20,475 | (27.8) | 46,521 | (63.1) | 27,223 | (36.9) |
| Stage III | 144,104 | (64) | 104,015 | (72.2) | 40,089 | (27.8) | 88,751 | (61.6) | 55,353 | (38.4) |
| Stage IV+V | 8161 | (4) | 6563 | (80.4) | 1598 | (19.6) | 5932 | (72.7) | 2229 | (27.3) |
| Ipsilateral degree of stenosis ° | ||||||||||
| Mild (<50%) | 4076 | (2) | 2723 | (66.8) | 1353 | (33.2) | 2488 | (61.0) | 1588 | (39.0) |
| Moderate (50–69%) | 11,715 | (5) | 9543 | (81.5) | 2172 | (18.5) | 7803 | (66.6) | 3912 | (33.4) |
| Severe (70–99%) | 208,265 | (91) | 149,515 | (71.8) | 58,750 | (28.2) | 128,794 | (61.8) | 79,471 | (38.2) |
| Occlusion (100%) | 4077 | (2) | 3678 | (90.2) | 399 | (9.8) | 3534 | (86.7) | 543 | (13.3) |
| Contralateral degree of stenosis ° | ||||||||||
| Mild (<50%) | 155,231 | (68) | 112,612 | (72.5) | 42,619 | (27.5) | 97,587 | (62.9) | 57,644 | (37.1) |
| Moderate (50–69%) | 31,375 | (14) | 22,370 | (71.3) | 9005 | (28.7) | 18,815 | (60.0) | 12,560 | (40.0) |
| Severe (70–99%) | 27,159 | (12) | 19,593 | (72.1) | 7566 | (27.9) | 16,851 | (62.0) | 10,308 | (38.0) |
| Occlusion (100%) | 14,368 | (6) | 10,884 | (75.8) | 3484 | (24.2) | 9366 | (65.2) | 5002 | (34.8) |
| Neurological symptoms & | ||||||||||
| Asymptomatic (A) | 122,363 | (59) | 73,425 | (60.0) | 48,938 | (40.0) | 66,580 | (54.4) | 55,783 | (45.6) |
| Amaurosis fugax (B) | 12,375 | (6) | 9854 | (79.6) | 2521 | (20.4) | 7937 | (64.1) | 4438 | (35.9) |
| Transitory ischemic attack (B) | 23,257 | (11) | 20,422 | (87.8) | 2835 | (12.2) | 15,933 | (68.5) | 7324 | (31.5) |
| Any stroke (B) | 18,787 | (9) | 17,659 | (94.0) | 1128 | (6.0) | 13,910 | (74.0) | 4877 | (26.0) |
| SIE or crescendo-TIA (C1) | 9962 | (5) | 9206 | (92.4) | 756 | (7.6) | 8289 | (83.2) | 1673 | (16.8) |
| Simultaneous operations (C2) | 6851 | (3) | 4390 | (64.1) | 2461 | (35.9) | 4063 | (59.3) | 2788 | (40.7) |
| Other symptoms (B + C3) | 13,754 | (7) | 10,812 | (78.6) | 2942 | (21.4) | 9317 | (67.7) | 4437 | (32.3) |
| Morphological characteristics | ||||||||||
| Ulcerated plaque | 22,630 | (10) | 17,732 | (78.4) | 4898 | (21.6) | 14,654 | (64.8) | 7976 | (35.2) |
| Aneurysm | 1418 | (1) | 1023 | (72.1) | 395 | (27.9) | 927 | (65.4) | 491 | (34.6) |
| Coiling | 1648 | (1) | 1333 | (80.9) | 315 | (19.1) | 1094 | (66.4) | 554 | (33.6) |
| Multiple lesions | 6354 | (3) | 5213 | (82.0) | 1141 | (18.0) | 4638 | (73.0) | 1716 | (27.0) |
| Others | 7770 | (3) | 5761 | (74.1) | 2009 | (25.9) | 5147 | (66.2) | 2623 | (33.8) |
| Overall (Column-%) | Pre-Operative Neurological Assessment | Post-Operative Neurological Assessment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||||||
| Time interval from symptom onset | ||||||||||
| 0–2 days | 8273 | (11) | 7453 | (90.1) | 820 | (9.9) | 6456 | (78.0) | 1817 | (22.0) |
| 3–7 days | 27,597 | (38) | 25,465 | (92.3) | 2132 | (7.7) | 20,555 | (74.5) | 7042 | (25.5) |
| 8–14 days | 15,333 | (21) | 13,909 | (90.7) | 1424 | (9.3) | 11,017 | (71.9) | 4316 | (28.1) |
| 15–180 days | 22,316 | (30) | 18,228 | (81.7) | 4088 | (18.3) | 13,903 | (62.3) | 8413 | (37.7) |
| Day of week (admission) | ||||||||||
| Monday | 58,066 | (25) | 40,450 | (69.7) | 17,616 | (30.3) | 35,355 | (60.9) | 22,711 | (39.1) |
| Tuesday | 48,287 | (21) | 34,656 | (71.8) | 13,631 | (28.2) | 30,047 | (62.2) | 18,240 | (37.8) |
| Wednesday | 45,356 | (20) | 31,797 | (70.1) | 13,559 | (29.9) | 27,334 | (60.3) | 18,022 | (39.7) |
| Thursday | 35,653 | (16) | 25,773 | (72.3) | 9880 | (27.7) | 21,872 | (61.3) | 13,781 | (38.7) |
| Friday | 17,575 | (8) | 14,470 | (82.3) | 3105 | (17.7) | 12,228 | (69.6) | 5347 | (30.4) |
| Saturday | 7125 | (3) | 6512 | (91.4) | 613 | (8.6) | 5672 | (79.6) | 1453 | (20.4) |
| Sunday | 16,071 | (7) | 11,801 | (73.4) | 4270 | (26.6) | 10,111 | (62.9) | 5960 | (37.1) |
| Preoperative diagnostics | ||||||||||
| Duplex ultrasound | 156,306 | (69) | 110,954 | (71.0) | 45,352 | (29.0) | 93,828 | (60.0) | 62,478 | (40.0) |
| Transcranial Doppler | 46,925 | (21) | 43,789 | (93.3) | 3136 | (6.7) | 36,454 | (77.7) | 10,471 | (22.3) |
| CTA | 86,184 | (38) | 68,688 | (79.7) | 17,496 | (20.3) | 56,993 | (66.1) | 29,191 | (33.9) |
| MRA | 76,551 | (34) | 56,274 | (73.5) | 20,277 | (26.5) | 47,351 | (61.9) | 29,200 | (38.1) |
| Overall (Column-%) | Pre-Operative Neurological Assessment | Post-Operative Neurological Assessment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||||||
| Asymptomatic (A) | ||||||||||
| CEA | 100,915 | (82) | 58,163 | (57.6) | 42,752 | (42.4) | 53,286 | (52.8) | 47,629 | (47.2) |
| CAS | 21,448 | (18) | 15,262 | (71.2) | 6186 | (28.2) | 13,294 | (62.0) | 8154 | (38.0) |
| Symptomatic (elective, B) | ||||||||||
| CEA | 65,852 | (83) | 58,356 | (88.6) | 7496 | (11.4) | 45,526 | (69.1) | 20,326 | (30.9) |
| CAS | 13,115 | (17) | 12,053 | (91.9) | 1062 | (8.1) | 11,125 | (84.8) | 1990 | (15.2) |
| Symptomatic (emergency, C1) | ||||||||||
| CEA | 6076 | (61) | 5461 | (89.9) | 615 | (10.1) | 4631 | (76.2) | 1445 | (23.8) |
| CAS | 3886 | (39) | 3745 | (96.4) | 141 | (3.6) | 3658 | (94.1) | 228 | (5.9) |
| Simultaneous procedure (C2) | ||||||||||
| CEA | 3802 | (55) | 1734 | (45.6) | 2068 | (54.4) | 1482 | (39.0) | 2320 | (61.0) |
| CAS | 3049 | (45) | 2656 | (87.1) | 393 | (12.9) | 2581 | (84.7) | 468 | (15.3) |
| Others (C3) | ||||||||||
| CEA | 6881 | (69) | 5319 | (77.3) | 1562 | (22.7) | 4445 | (64.6) | 2436 | (35.4) |
| CAS | 3109 | (31) | 2710 | (87.2) | 399 | (12.8) | 2591 | (83.3) | 518 | (16.7) |
| Overall | Pre-Operative Neurological Assessment | Post-Operative Neurological Assessment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p | Yes | No | p | |||||||
| Any stroke or death | ||||||||||||
| Overall | 6254 | (2.74) | 4978 | (3.01) | 1276 | (2.04) | <0.001 | 5331 | (3.74) | 923 | (1.08) | <0.001 |
| Asymptomatic (A) | ||||||||||||
| CEA | 1461 | (1.45) | 883 | (1.52) | 578 | (1.35) | 0.031 | 1169 | (2.19) | 292 | (0.61) | <0.001 |
| CAS | 374 | (1.74) | 278 | (1.82) | 96 | (1.55) | 0.191 | 315 | (2.37) | 59 | (0.72) | <0.001 |
| Symptomatic (elective, B) | ||||||||||||
| CEA | 1838 | (2.79) | 1660 | (2.84) | 178 | (2.37) | 0.022 | 1608 | (3.53) | 230 | (1.13) | <0.001 |
| CAS | 485 | (3.70) | 438 | (3.63) | 47 | (4.43) | 0.220 | 458 | (4.12) | 27 | (1.36) | <0.001 |
| Symptomatic (emergency, C1) | ||||||||||||
| CEA | 414 | (6.81) | 357 | (6.54) | 57 | (9.27) | 0.014 | 364 | (7.86) | 50 | (3.46) | <0.001 |
| CAS | 417 | (10.7) | 401 | (10.7) | 16 | (11.3) | 0.918 | 393 | (10.7) | 24 | (10.5) | 1.000 |
| Simultaneous procedure (C2) | ||||||||||||
| CEA | 313 | (8.23) | 139 | (8.02) | 174 | (8.41) | 0.700 | 188 | (12.7) | 125 | (5.39) | <0.001 |
| CAS | 318 | (10.4) | 287 | (10.8) | 31 | (7.89) | 0.093 | 281 | (10.9) | 37 | (7.91) | 0.063 |
| Others (C3) | ||||||||||||
| CEA | 386 | (5.61) | 311 | (5.85) | 75 | (4.80) | 0.130 | 328 | (7.38) | 58 | (2.38) | <0.001 |
| CAS | 248 | (7.98) | 224 | (8.27) | 24 | (6.02) | 0.147 | 227 | (8.76) | 21 | (4.05) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallmayer, M.; Knappich, C.; Kirchhoff, F.; Bohmann, B.; Lohe, V.; Naher, S.; Eckstein, H.-H.; Kuehnl, A. Determinants of Pre- and Post-Procedural Neurological Assessment, and Outcome of Carotid Endarterectomy or Stenting. J. Clin. Med. 2024, 13, 4177. https://doi.org/10.3390/jcm13144177
Kallmayer M, Knappich C, Kirchhoff F, Bohmann B, Lohe V, Naher S, Eckstein H-H, Kuehnl A. Determinants of Pre- and Post-Procedural Neurological Assessment, and Outcome of Carotid Endarterectomy or Stenting. Journal of Clinical Medicine. 2024; 13(14):4177. https://doi.org/10.3390/jcm13144177
Chicago/Turabian StyleKallmayer, Michael, Christoph Knappich, Felix Kirchhoff, Bianca Bohmann, Vanessa Lohe, Shamsun Naher, Hans-Henning Eckstein, and Andreas Kuehnl. 2024. "Determinants of Pre- and Post-Procedural Neurological Assessment, and Outcome of Carotid Endarterectomy or Stenting" Journal of Clinical Medicine 13, no. 14: 4177. https://doi.org/10.3390/jcm13144177
APA StyleKallmayer, M., Knappich, C., Kirchhoff, F., Bohmann, B., Lohe, V., Naher, S., Eckstein, H.-H., & Kuehnl, A. (2024). Determinants of Pre- and Post-Procedural Neurological Assessment, and Outcome of Carotid Endarterectomy or Stenting. Journal of Clinical Medicine, 13(14), 4177. https://doi.org/10.3390/jcm13144177





