Advances in Non-Small Cell Lung Cancer: Current Insights and Future Directions
Abstract
1. Introduction
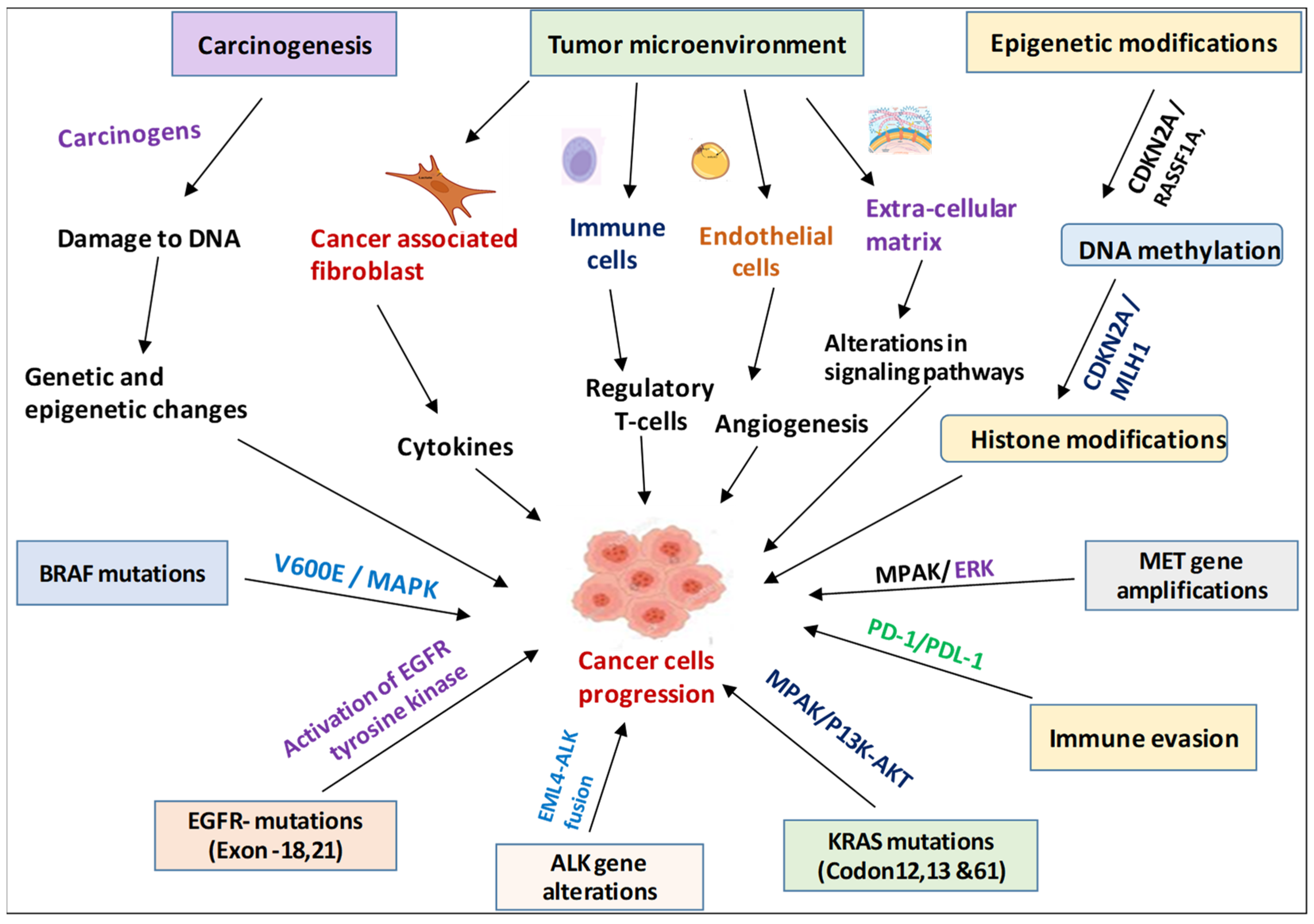
2. Mechanisms Underlying NSCLC Development
2.1. Carcinogenesis
2.2. Tumor Microenvironment
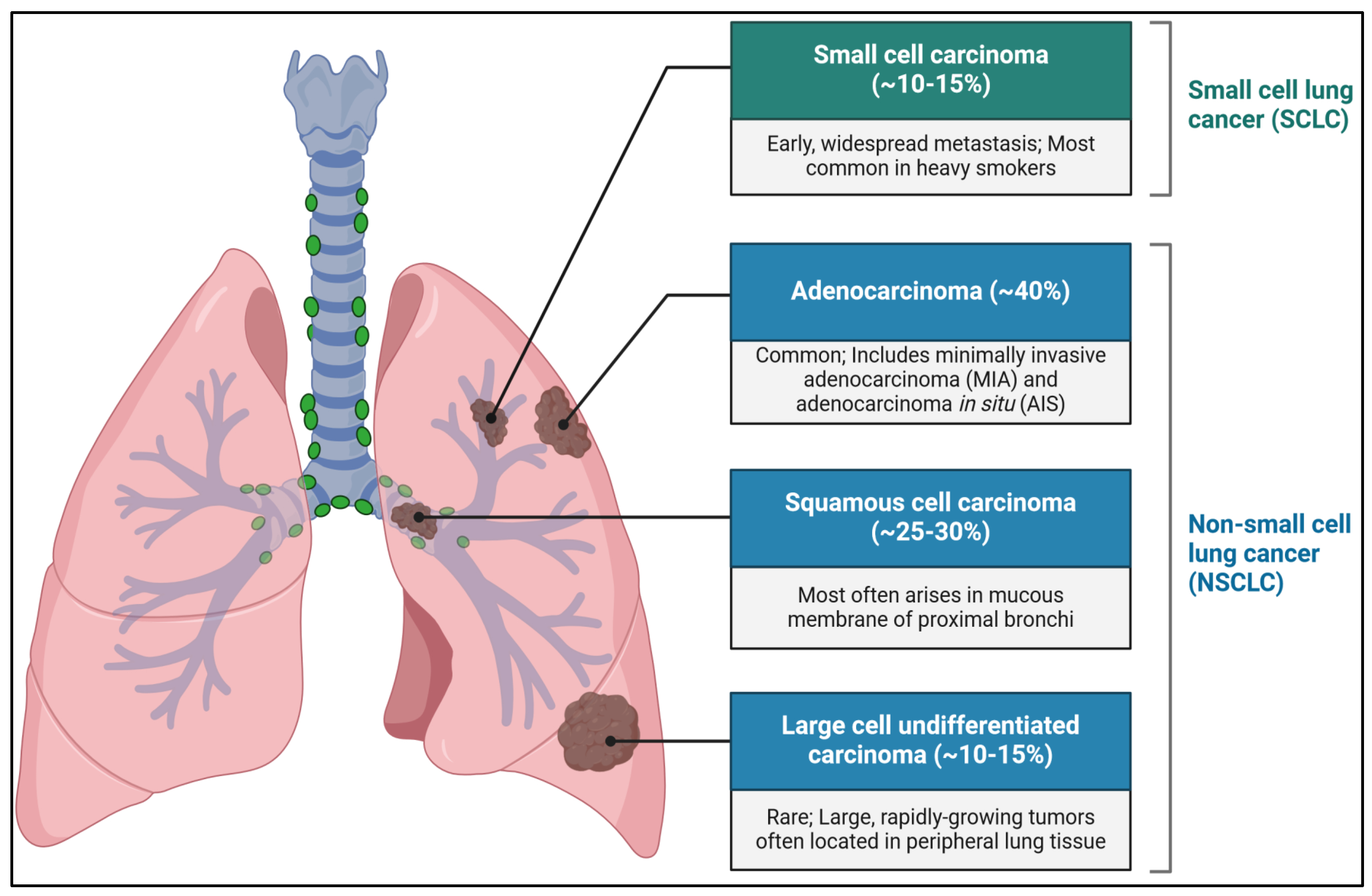
2.3. Genetic and Molecular Alterations in NSCLC
2.3.1. Epidermal Growth Factor Receptor (EGFR)
2.3.2. Anaplastic Lymphoma Kinase (ALK)
2.3.3. KRAS
2.3.4. Other Genetic Alterations
ROS1
BRAF
MET
2.3.5. Epigenetic Changes
DNA Methylation
Histone Modifications
2.3.6. Immune Evasion
PD-1/PD-L1 Pathway
3. Cutting-Edge Imaging Techniques and Molecular Diagnostic Biomarkers for Pioneering NSCLC Diagnosis
3.1. Computed Tomography (CT)
3.1.1. Low-Dose CT (LDCT)
3.1.2. High-Resolution CT (HRCT)
3.1.3. Multi-Detector CT (MDCT)
3.2. Positron Emission Tomography–Computed Tomography (PET-CT)
3.3. Magnetic Resonance Imaging (MRI)
3.4. Magnetic Resonance Spectroscopy (MRS)
3.5. Molecular Diagnostic Biomarkers
3.5.1. Genetic Profiling
3.5.2. Liquid Biopsies
3.5.3. Integration of Imaging and Molecular Diagnostics
4. Innovative Therapies in NSCLC
4.1. Surgical Advances in NSCLC
4.1.1. Minimally Invasive Techniques
Video-Assisted Thoracoscopic Surgery (VATS)
Robotic-Assisted Surgery
Enhanced Recovery after Surgery (ERAS) Protocols
Preoperative Measures
Intraoperative Measures
Postoperative Measures
4.2. Stereotactic Body Radiotherapy (SBRT)
4.3. Intensity-Modulated Radiotherapy (IMRT)
4.4. Innovations in Treatment Planning
4.4.1. Advanced Imaging Techniques
Four-Dimensional CT Imaging
MRI Integration
4.4.2. Adaptive Radiotherapy
Real-Time Monitoring
Adaptive Replanning
5. Systemic Therapy Innovations in NSCLC
5.1. Epidermal Growth Factor Receptor (EGFR) Inhibitors
5.1.1. First-Generation EGFR Inhibitors (Examples: Erlotinib and Gefitinib)
5.1.2. Second-Generation EGFR Inhibitors (Examples: Afatinib and Dacomitinib)
5.1.3. Third-Generation EGFR Inhibitors (Example: Osimertinib)
5.2. Anaplastic Lymphoma Kinase (ALK) Inhibitors
5.2.1. First-Generation ALK Inhibitors (Example: Crizotinib)
5.2.2. Second-Generation ALK Inhibitors (Examples: Ceritinib, Alectinib, and Brigatinib)
5.2.3. Third-Generation ALK Inhibitor (Example: Lorlatinib)
5.3. ROS1 Inhibitors
5.3.1. Crizotinib
5.3.2. Second-Generation ROS1 Inhibitors (Examples: Entrectinib and Lorlatinib)
5.4. Other Targeted Inhibitors
5.4.1. BRAF Inhibitors (Example: Dabrafenib (Often Combined with Trametinib))
5.4.2. MET Inhibitors (Examples: Capmatinib and Tepotinib)
5.4.3. RET Inhibitors (Examples: Selpercatinib and Pralsetinib)
5.5. Combination Therapies
5.5.1. Targeted Therapy and Immunotherapy
5.5.2. Targeted Therapy and Chemotherapy
5.5.3. Precision Medicine and Future Directions
5.5.4. Comprehensive Genomic Profiling
5.5.5. Emerging Targets
6. Challenges in NSCLC Management
6.1. Healthcare Disparities (Access to Diagnostic and Therapeutic Advances)
6.2. Economic and Ethical Issues (Cost-Effectiveness and Ethical Considerations in Treatment)
7. Emerging Research and Future Prospects
7.1. Cutting-Edge Research and Experimental Therapies
7.1.1. CAR-T Cell Therapy
7.1.2. Oncolytic Viruses
7.2. Promising Clinical Trials and Their Potential Impact
7.2.1. Immunotherapy Combinations
7.2.2. Targeted Therapy Advances
7.3. Future Directions in NSCLC Research and Treatment Development
7.3.1. Personalized Medicine
7.3.2. Artificial Intelligence (AI) and Machine Learning
7.3.3. Novel Therapeutic Targets
8. Conclusions
9. Clinical Impact of Advances in NSCLC
10. Significance of Advancement in NSCLC
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global epidemiology of lung cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Chen, J.; Ye, J.; Xu, S.; Lin, B.; Hou, K. Epidemiological analysis of global and regional lung cancer mortality: Based on 30-year data analysis of global burden disease database. Healthcare 2023, 11, 2920. [Google Scholar] [CrossRef] [PubMed]
- Flor, L.S.; Anderson, J.A.; Ahmad, N.; Aravkin, A.; Carr, S.; Dai, X.; Gil, G.F.; Hay, S.I.; Malloy, M.J.; McLaughlin, S.A.; et al. Health effects associated with exposure to secondhand smoke: A burden of proof study. Nat. Med. 2024, 30, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Dubey, A.; Saini, D.; Singh, M.; Prasad, C.P.; Roy, S.; Bharati, S.J.; Rinki, M.; Singh, N.; Seth, T.; et al. Environmental and occupational determinants of lung cancer. Transl. Lung Cancer Res. 2019, 8 (Suppl. S1), S31–S49. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent insights into particulate matter (PM2.5)-mediated toxicity in humans: An overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Bhandare, R.R.; Boddu, S.H.S.; Shaik, A.B.; Saktivel, L.P.; Gupta, G.; Negi, P.; Barakat, M.; Singh, S.K.; Dua, K.; et al. Molecular mechanisms underlying the regulation of tumor suppressor genes in lung cancer. Biomed. Pharmacother. 2024, 173, 116275. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef]
- Grodzka, A.; Knopik-Skrocka, A.; Kowalska, K.; Kurzawa, P.; Krzyzaniak, M.; Stencel, K.; Bryl, M. Molecular alterations of driver genes in non-small cell lung cancer: From diagnostics to targeted therapy. EXCLI J. 2023, 22, 415–432. [Google Scholar] [CrossRef]
- Min, H.Y.; Lee, H.Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Talwar, D.; Meshram, P.; Julka, P.K.; Mehta, A.; Somashekhar, S.P.; Chilukuri, S.; Bansal, A. Navigating patient journey in early diagnosis of lung cancer in India. Lung India 2023, 40, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.Y. Biopsy and mutation detection strategies in non-small cell lung cancer. Tuberc. Respir. Dis. 2013, 75, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Mannani, R.; Heidarnejad Maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.L.; Zhang, A.L.; Bruno, D.S.; Almeida, F.A. NSCLC in the Era of Targeted and Immunotherapy: What Every Pulmonologist Must Know. Diagnostics 2023, 13, 1117. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Concepcion, J.; Uprety, D.; Adjei, A.A. Challenges in the use of targeted therapies in non-small cell lung cancer. Cancer Res. Treat. 2022, 54, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.; Akter, Z.; Modareszadeh, P.; Modareszadeh, P.; Berisha, E.; Alemi, P.S.; Chacon Castro, M.d.C.; Deese, A.R.; Zhang, L. Current landscape of therapeutic resistance in lung cancer and promising strategies to overcome resistance. Cancers 2022, 14, 4562. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.E.; Minna, J.D. Molecular biology of lung cancer: Clinical implications. Clin. Chest Med. 2011, 32, 703–740. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tian, L.; Qi, W.; Lv, Q.; Wang, T. Advancements in NSCLC: From pathophysiological insights to targeted treatments. Am. J. Clin. Oncol. 2024, 47, 291–303. [Google Scholar] [CrossRef]
- Ansari, J.; Shackelford, R.E.; El-Osta, H. Epigenetics in non-small cell lung cancer: From basics to therapeutics. Transl. Lung Cancer Res. 2016, 5, 155–171. [Google Scholar] [CrossRef]
- Barnes, J.L.; Zubair, M.; John, K.; Poirier, M.C.; Martin, F.L. Carcinogens and DNA damage. Biochem. Soc. Trans. 2018, 46, 1213–1224. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair. 2019, 83, 102673. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The role of tumor microenvironment in cancer metastasis: Molecular mechanisms and therapeutic opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Matsueda, S.; Saya, H. Significance of cancer-associated fibroblasts in the interactions of cancer cells with the tumor microenvironment of heterogeneous tumor tissue. Cancers 2023, 15, 2536. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Suster, D.I.; Mino-Kenudson, M. Molecular pathology of primary non-small cell lung cancer. Arch. Med. Res. 2020, 51, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, A.; Perol, M.; Banna, G.L.; Parikh, K.; Addeo, A. Oncogenic alterations in advanced NSCLC: A molecular super-highway. Biomark. Res. 2024, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, A.; Banna, G.; Malapelle, U.; Pisapia, P.; Addeo, A. Next generation sequencing and genetic alterations in squamous cell lung carcinoma: Where are we today? Front. Oncol. 2019, 9, 166. [Google Scholar] [CrossRef]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar]
- Gazdar, A.F. Activating and resistance mutations of EGFR in non-small-cell lung cancer: Role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene 2009, 28 (Suppl. S1), S24–S31. [Google Scholar] [CrossRef]
- Arbour, K.C.; Riely, G.J. Diagnosis and treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer. Hematol. Oncol. Clin. N. Am. 2017, 31, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, R.E.; Vora, M.; Mayhall, K.; Cotelingam, J. ALK-rearrangements and testing methods in non-small cell lung cancer: A review. Genes Cancer 2014, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Reita, D.; Pabst, L.; Pencreach, E.; Guerin, E.; Dano, L.; Rimelen, V.; Voegeli, A.-C.; Vallat, L.; Mascaux, C.; Beau-Faller, M. Direct targeting KRAS mutation in non-small cell lung cancer: Focus on resistance. Cancers 2022, 14, 1321. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, M.; Ciappina, G.; Spagnolo, C.C.; Squeri, A.; Passalacqua, M.I.; Aguilar, A.; Gonzalez-Cao, M.; Giovannetti, E.; Silvestris, N.; Rosell, R. Targeted therapies for KRAS-mutant non-small cell lung cancer: From preclinical studies to clinical development-a narrative review. Transl. Lung Cancer Res. 2023, 12, 346–368. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, A.; Sobhani, N.; Chapman, R.; Bagby, S.; Bortoletti, C.; Traversini, M.; Ferrari, K.; Voltolini, L.; Darlow, J.; Roviello, G. Focus on ROS1-positive non-small cell lung cancer (NSCLC): Crizotinib, resistance mechanisms and the newer generation of targeted therapies. Cancers 2020, 12, 3293. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Guo, S.; Zhang, H.; Zhang, Z.; Shen, S.; Li, X. BRAF-mutated non-small cell lung cancer: Current treatment status and future perspective. Front. Oncol. 2022, 12, 863043. [Google Scholar] [CrossRef] [PubMed]
- Spitaleri, G.; Trillo Aliaga, P.; Attili, I.; Signore, E.D.; Corvaja, C.; Corti, C.; Uliano, J.; Passaro, A.; de Marinis, F. MET in non-small-cell lung cancer (NSCLC): Cross ‘a long and winding road’ looking for a target. Cancers 2023, 15, 4779. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Esteller, M.; Rountree, M.R.; Bachman, K.E.; Schuebel, K.; Herman, J.G. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum. Mol. Genet. 2001, 10, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Audia, J.E.; Campbell, R.M. Histone modifications and cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019521. [Google Scholar] [CrossRef]
- Delcuve, G.P.; Khan, D.H.; Davie, J.R. Roles of histone deacetylases in epigenetic regulation: Emerging paradigms from studies with inhibitors. Clin. Epigenetics 2012, 4, 5. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Li, B.; Jin, J.; Guo, D.; Tao, Z.; Hu, X. Immune checkpoint inhibitors combined with targeted therapy: The recent advances and future potentials. Cancers 2023, 15, 2858. [Google Scholar] [CrossRef] [PubMed]
- Nooreldeen, R.; Bach, H. Current and future development in lung cancer diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Pulumati, A.; Pulumati, A.; Dwarakanath, B.S.; Verma, A.; Papineni, R.V.L. Technological advancements in cancer diagnostics: Improvements and limitations. Cancer Rep. 2023, 6, e1764. [Google Scholar] [CrossRef]
- Purandare, N.C.; Rangarajan, V. Imaging of lung cancer: Implications on staging and management. Indian J. Radiol. Imaging 2015, 25, 109–120. [Google Scholar] [CrossRef]
- Bonney, A.; Malouf, R.; Marchal, C.; Manners, D.; Fong, K.M.; Marshall, H.M.; Irving, L.B.; Manser, R. Impact of low-dose computed tomography (LDCT) screening on lung cancer-related mortality. Cochrane Database Syst. Rev. 2022, 8, CD013829. [Google Scholar] [CrossRef]
- Kang, M.; Deoghuria, D.; Varma, S.; Gupta, D.; Bhatia, A.; Khandelwal, N. Role of HRCT in detection and characterization of pulmonary abnormalities in patients with febrile neutropenia. Lung India 2013, 30, 124–130. [Google Scholar] [CrossRef]
- Anderson, P.J.; Yong, R.; Surman, T.L.; Rajion, Z.A.; Ranjitkar, S. Application of three-dimensional computed tomography in craniofacial clinical practice and research. Aust. Dent. J. 2014, 59 (Suppl. S1), 174–185. [Google Scholar] [CrossRef] [PubMed]
- Salminen, E.; Mac Manus, M. FDG-PET imaging in the management of non-small-cell lung cancer. Ann. Oncol. 2002, 13, 357–360. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, K.S.; Ohno, Y.; van Beek, E.J.; Biederer, J. PET/CT versus MRI for diagnosis, staging, and follow-up of lung cancer. J. Magn. Reson. Imaging 2015, 42, 247–260. [Google Scholar] [CrossRef]
- Sim, A.J.; Kaza, E.; Singer, L.; Rosenberg, S.A. A review of the role of MRI in diagnosis and treatment of early-stage lung cancer. Clin. Transl. Radiat. Oncol. 2020, 24, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Winfield, J.M.; Payne, G.S.; Weller, A.; de Souza, N.M. DCE-MRI, DW-MRI, and MRS in cancer: Challenges and advantages of implementing qualitative and quantitative multi-parametric imaging in the clinic. Top. Magn. Reson. Imaging 2016, 25, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.L.; Lim, R.; Huang, C.; Chebib, I.A.; El Fakhri, G. Synergistic role of simultaneous PET/MRI-MRS in soft tissue sarcoma metabolism imaging. Magn. Reson. Imaging 2016, 34, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Mubeen, I.; Ullah, N.; Shah, S.S.U.D.; Khan, B.A.; Zahoor, M.; Ullah, R.; Khan, F.A.; Sultan, M.A. Modern Diagnostic Imaging Technique Applications and Risk Factors in the Medical Field: A Review. Biomed Res. Int. 2022, 2022, 5164970. [Google Scholar] [CrossRef] [PubMed]
- Dalurzo, M.L.; Avilés-Salas, A.; Soares, F.A.; Hou, Y.; Li, Y.; Stroganova, A.; Oz, B.; Abdillah, A.; Wan, H.; Choi, Y.-L. Testing for EGFR mutations and ALK rearrangements in advanced non-small-cell lung cancer: Considerations for countries in emerging markets. Onco Targets Ther. 2021, 14, 4671–4692. [Google Scholar] [CrossRef]
- Orlov-Slavu, M.C.; Popa, A.M.; Tulin, A.; Stoian, A.P.; Poiana, C.; Paleru, C.; Calu, V.; Nitipir, C. The utility of next-generation sequencing in the treatment decision-making for metastatic non-small-cell lung cancer. Cureus 2021, 13, e16919. [Google Scholar] [CrossRef] [PubMed]
- Myint, K.Z.Y.; Shimabuku, M.; Horio, R.; Kaneda, M.; Shimizu, Y.; Taguchi, J. Identification of circulating tumour DNA (ctDNA) from the liquid biopsy results: Findings from an observational cohort study. Cancer Treat. Res. Commun. 2023, 35, 100701. [Google Scholar] [CrossRef] [PubMed]
- Patz, E.F., Jr. Integration of biomarkers and imaging. J. Thorac. Oncol. 2006, 1, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.S.; Bridgewater, J.; Normanno, N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann. Oncol. 2021, 32, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Gheisari, F.; Vali, R. Advancements in molecular imaging for the diagnosis and management of hepatocellular carcinoma. Med. Res. Arch. 2024, 12. [Google Scholar] [CrossRef]
- Joy Mathew, C.; David, A.M.; Joy Mathew, C.M. Artificial intelligence and its future potential in lung cancer screening. EXCLI J. 2020, 19, 1552–1562. [Google Scholar] [CrossRef]
- de Jong, D.; Das, J.P.; Ma, H.; Valiplackal, J.P.; Prendergast, C.; Roa, T.; Braumuller, B.; Deng, A.; Dercle, L.; Yeh, R.; et al. Novel targets, novel treatments: The changing landscape of non-small cell lung cancer. Cancers 2023, 15, 2855. [Google Scholar] [CrossRef]
- Montagne, F.; Guisier, F.; Venissac, N.; Baste, J.M. The role of surgery in lung cancer treatment: Present indications and future perspectives-state of the art. Cancers 2021, 13, 3711. [Google Scholar] [CrossRef] [PubMed]
- Berzenji, L.; Wen, W.; Verleden, S.; Claes, E.; Yogeswaran, S.K.; Lauwers, P.; Van Schil, P.; Hendriks, J.M.H. Minimally invasive surgery in non-small cell lung cancer: Where do we stand? Cancers 2023, 15, 4281. [Google Scholar] [CrossRef]
- Dziedzic, D.; Orlowski, T. The role of VATS in lung cancer surgery: Current status and prospects for development. Minim. Invasive Surg. 2015, 2015, 938430. [Google Scholar] [CrossRef][Green Version]
- Sahai, D.; Nayak, R. The evolution of vats and minimally invasive techniques in the treatment of lung cancer: A narrative review. Video-Assist. Thoracoscopic Surg. 2023, 8, 40. [Google Scholar] [CrossRef]
- Reddy, K.; Gharde, P.; Tayade, H.; Patil, M.; Reddy, L.S.; Surya, D. Advancements in robotic surgery: A comprehensive overview of current utilizations and upcoming frontiers. Cureus 2023, 15, e50415. [Google Scholar] [CrossRef] [PubMed]
- Gallina, F.T.; Forcella, D.; Melis, E.; Facciolo, F. Robotic lobectomy without complete fissure for non-small cell lung cancer: Technical aspects and perioperative outcomes of the tunnel technique. Curr. Oncol. 2023, 30, 5898–5905. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.N.; Lamture, Y.; Krishna, M. Enhanced recovery after surgery: Exploring the advances and strategies. Cureus 2023, 15, e47237. [Google Scholar] [CrossRef]
- Baker, S.; Dahele, M.; Lagerwaard, F.J.; Senan, S. A critical review of recent developments in radiotherapy for non-small cell lung cancer. Radiat. Oncol. 2016, 11, 115. [Google Scholar] [CrossRef]
- Buchberger, D.S.; Videtic, G.M.M. Stereotactic body radiotherapy for the management of early-stage non-small-cell lung cancer: A clinical overview. JCO Oncol. Pract. 2023, 19, 239–249. [Google Scholar] [CrossRef]
- Aznar, M.C.; Warren, S.; Hoogeman, M.; Josipovic, M. The impact of technology on the changing practice of lung SBRT. Phys. Med. 2018, 47, 129–138. [Google Scholar] [CrossRef]
- Nutting, C.; Dearnaley, D.P.; Webb, S. Intensity modulated radiation therapy: A clinical review. Br. J. Radiol. 2000, 73, 459–469. [Google Scholar] [CrossRef]
- Dona Lemus, O.M.; Cao, M.; Cai, B.; Cummings, M.; Zheng, D. Adaptive radiotherapy: Next-generation radiotherapy. Cancers 2024, 16, 1206. [Google Scholar] [CrossRef]
- Boyle, J.; Ackerson, B.; Gu, L.; Kelsey, C.R. Dosimetric advantages of intensity modulated radiation therapy in locally advanced lung cancer. Adv. Radiat. Oncol. 2017, 2, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Filice, A.; Casali, M.; Ciammella, P.; Galaverni, M.; Fioroni, F.; Lotti, C.; Versari, A. Radiotherapy planning and molecular imaging in lung cancer. Curr. Radiopharm. 2020, 13, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jabbour, S.K.; Xiao, Z.; Yue, N.; Wang, X.; Cao, H.; Kuang, Y.; Zhang, Y.; Nie, K. Dosimetric feasibility of 4DCT-ventilation imaging guided proton therapy for locally advanced non-small-cell lung cancer. Radiat. Oncol. 2018, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Owrangi, A.M.; Greer, P.B.; Glide-Hurst, C.K. MRI-only treatment planning: Benefits and challenges. Phys. Med. Biol. 2018, 63, 05TR01. [Google Scholar] [CrossRef]
- Piperdi, H.; Portal, D.; Neibart, S.S.; Yue, N.J.; Jabbour, S.K.; Reyhan, M. Adaptive radiation therapy in the treatment of lung cancer: An overview of the current state of the field. Front. Oncol. 2021, 11, 770382. [Google Scholar] [CrossRef]
- Gálffy, G.; Morócz, É.; Korompay, R.; Hecz, R.; Bujdoso, R.; Puskas, R.; Lovas, T.; Gaspar, E.; Yahya, K.; Kiraly, P.; et al. Targeted therapeutic options in early and metastatic NSCLC-overview. Pathol. Oncol. Res. 2024, 30, 1611715. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Z.; Li, Y.; Zhou, Q. Development of epidermal growth factor receptor tyrosine kinase inhibitors against EGFR T790M. Mutation in non-small-cell lung carcinoma. Open Med. 2016, 11, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, N.; Fernandez-Bruno, M.; Bracht, J.W.P.; Rosell, R. EGFR first- and second-generation TKIs-there is still place for them in EGFR-mutant NSCLC patients. Transl. Cancer Res. 2019, 8 (Suppl. S1), S23–S47. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Kato, T.; Dong, X.; Ahn, M.-J.; Quang, L.-V.; Soparattanapaisarn, N.; Inoue, T.; Wang, C.-L.; Huang, M.; Yang, J.C.-H.; et al. Osimertinib after chemoradiotherapy in stage III EGFR-mutated NSCLC. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef]
- Maraqa, B.; Al-Ashhab, M.; Sughayer, M.A. Anaplastic lymphoma kinase rearrangements in patients with non-small cell lung cancer in Jordan. J. Int. Med. Res. 2022, 50, 3000605221104181. [Google Scholar] [CrossRef]
- Desai, A.; Lovly, C.M. Strategies to overcome resistance to ALK inhibitors in non-small cell lung cancer: A narrative review. Transl. Lung Cancer Res. 2023, 12, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Manabe, R.; Kishino, Y.; Kusumoto, S.; Yamaoka, T.; Tanaka, A.; Ohmori, T.; Sagara, H. Comparative efficacy of ALK inhibitors for treatment-naïve ALK-positive advanced non-small cell lung cancer with central nervous system metastasis: A network meta-analysis. Int. J. Mol. Sci. 2023, 24, 2242. [Google Scholar] [CrossRef]
- Gristina, V.; La Mantia, M.; Iacono, F.; Galvano, A.; Russo, A.; Bazan, V. The emerging therapeutic landscape of ALK inhibitors in non-small cell lung cancer. Pharmaceuticals 2020, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tang, Z.; Li, J.; Jiang, J.; Liu, Y. Progress of non-small-cell lung cancer with ROS1 rearrangement. Front. Mol. Biosci. 2023, 10, 1238093. [Google Scholar] [CrossRef]
- Muminovic, M.; Carracedo Uribe, C.R.; Alvarez-Pinzon, A.; Shan, K.; Raez, L.E. Importance of ROS1 gene fusions in non-small cell lung cancer. Cancer Drug Resist. 2023, 6, 332. [Google Scholar] [CrossRef]
- Khunger, A.; Khunger, M.; Velcheti, V. Dabrafenib in combination with trametinib in the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer: Clinical evidence and experience. Ther. Adv. Respir. Dis. 2018, 12, 1753466618767611. [Google Scholar] [CrossRef]
- Santarpia, M.; Massafra, M.; Gebbia, V.; D’Aquino, A.; Garipoli, C.; Altavilla, G.; Rosell, R. A narrative review of MET inhibitors in non-small cell lung cancer with MET exon 14 skipping mutations. Transl. Lung Cancer Res. 2021, 10, 1536–1556. [Google Scholar] [CrossRef]
- Cascetta, P.; Sforza, V.; Manzo, A.; Carillio, G.; Palumbo, G.; Esposito, G.; Montanino, A.; Costanzo, R.; Sendomenico, C.; De Cecio, R.; et al. RET inhibitors in non-small-cell lung cancer. Cancers 2021, 13, 4415. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Singh, S.K.; Mishra, M.K.; Singh, S.; Singh, R. Promising combinatorial therapeutic strategies against non-small cell lung cancer. Cancers 2024, 16, 2205. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Jiang, T.; Liu, X.; Mao, S.; Zhou, F.; Li, X.; Zhao, C.; Chen, X.; Su, C.; Ren, S.; et al. Immune checkpoint inhibitors in EGFR-mutated NSCLC: Dusk or dawn? J. Thorac. Oncol. 2021, 16, 1267–1288. [Google Scholar] [CrossRef] [PubMed]
- Parvaresh, H.; Roozitalab, G.; Golandam, F.; Behzadi, P.; Jabbarzadeh Kaboli, P. Unraveling the potential of ALK-targeted therapies in non-small cell lung cancer: Comprehensive insights and future directions. Biomedicines 2024, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, W.; Li, A.; Kansal, R.; Chen, Y.; Chen, H.; Li, X. Clinical next generation sequencing for precision medicine in cancer. Curr. Genom. 2015, 16, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-M.; Lin, C.-H.; Chou, Y.-Y.; Jen, H.-Y.; Jain, S. Clinical applications of comprehensive genomic profiling in advanced non-small-cell lung cancer—A case series. Curr. Oncol. 2024, 31, 3161–3176. [Google Scholar] [CrossRef] [PubMed]
- Rebuzzi, S.E.; Zullo, L.; Rossi, G.; Grassi, M.; Murianni, V.; Tagliamento, M.; Prelaj, A.; Coco, S.; Longo, L.; Dal Bello, M.G.; et al. Novel Emerging molecular targets in non-small cell lung cancer. Int. J. Mol. Sci. 2021, 22, 2625. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; de Camargo Correia, G.S.; Wang, J.; Manochakian, R.; Zhao, Y.; Lou, Y. Emerging targeted therapies in advanced non-small-cell lung cancer. Cancers 2023, 15, 2899. [Google Scholar] [CrossRef] [PubMed]
- Houda, I.; Dickhoff, C.; Uyl-de Groot, C.A.; Damhuis, R.A.M.; Reguart, N.; Provencio, M.; Levy, A.; Dziadziuszko, R.; Pompili, C.; Di Maio, M.; et al. Challenges and controversies in resectable non-small cell lung cancer: A clinician’s perspective. Lancet Reg. Health Eur. 2024, 38, 100841. [Google Scholar] [CrossRef]
- Stein, J.N.; Rivera, M.P.; Weiner, A.; Duma, N.; Henderson, L.; Mody, G.; Charlot, M. Sociodemographic disparities in the management of advanced lung cancer: A narrative review. J. Thorac. Dis. 2021, 13, 3772–3800. [Google Scholar] [CrossRef]
- Hilabi, B.S.; Alghamdi, S.A.; Almanaa, M. Impact of magnetic resonance imaging on healthcare in low- and middle-income countries. Cureus 2023, 15, e37698. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, Y.; Chen, J.; Gan, Y.; Su, C.; Zhang, H.; Long, E.; Yan, F.; Yang, Y. Measuring direct non-medical burden among patients with advanced non-small cell lung cancer in China: Is there a difference in health status? Front. Public Health 2023, 11, 1090623. [Google Scholar] [CrossRef] [PubMed]
- Azar, F.E.; Azami-Aghdash, S.; Pournaghi-Azar, F.; Mazdaki, A.; Rezapour, A.; Ebrahimi, P.; Yousefzadeh, N. Cost-effectiveness of lung cancer screening and treatment methods: A systematic review of systematic reviews. BMC Health Serv. Res. 2017, 17, 413. [Google Scholar] [CrossRef] [PubMed]
- Varkey, B. Principles of clinical ethics and their application to practice. Med. Princ. Pract. 2021, 30, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Farhud, D.D.; Zokaei, S. Ethical issues of artificial intelligence in medicine and healthcare. Iran. J. Public Health 2021, 50, i–v. [Google Scholar] [CrossRef] [PubMed]
- La’ah, A.S.; Chiou, S.-H. Cutting-edge therapies for lung cancer. Cells 2024, 13, 436. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.F.; Zhang, J.T.; Zhu, Y.G.; Cui, X.R.; Lu, Z.-M.; Yu, B.-T.; Wu, N. Chimeric antigen receptor T-cell therapy in lung cancer: Potential and challenges. Front. Immunol. 2021, 12, 782775. [Google Scholar] [CrossRef] [PubMed]
- Jhawar, S.R.; Thandoni, A.; Bommareddy, P.K.; Hassan, S.; Kohlhapp, F.J.; Goyal, S.; Schenkel, J.M.; Silk, A.W.; Zloza, A. Oncolytic viruses-natural and genetically engineered cancer immunotherapies. Front. Oncol. 2017, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xia, T.; Li, Z.; Gao, X.; Fang, X. Current status of clinical trial research and application of immune checkpoint inhibitors for non-small cell lung cancer. Front. Oncol. 2023, 13, 1213297. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, L.L.; Chen, J.H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef]
- Garcia-Robledo, J.E.; Rosell, R.; Ruíz-Patiño, A.; Sotelo, C.; Arrieta, O.; Zatarain-Barron, L.; Ordonez, C.; Jaller, E.; Rojas, L.; Russo, A.; et al. KRAS and MET in non-small-cell lung cancer: Two of the new kids on the ‘drivers’ block. Ther. Adv. Respir. Dis. 2022, 16, 17534666211066064. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.A.; Aisner, D.L.; Oxnard, G.R. Precision medicine in non-small cell lung cancer: Current standards in pathology and biomarker interpretation. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Kanan, M.; Alharbi, H.; Alotaibi, N.; Almasuood, L.; Aljoaid, S.; Alharbi, T.; Albraik, L.; Alothman, W.; Aljohani, H.; Alzahrani, A.; et al. AI-driven models for diagnosing and predicting outcomes in lung cancer: A systematic review and meta-analysis. Cancers 2024, 16, 674. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Mohanty, A.; Ramisetty, S.; Kulkarni, P.; Horne, D.; Pisick, E.; Salgia, R.; Singhal, S.S. Artificial intelligence and allied subsets in early detection and preclusion of gynecological cancers. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 189026. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, W.; Zhang, J.; Huang, A.; Yin, S.; Zhang, H.; Luo, Z.; Li, X.; Chen, Y.; Ma, L.; et al. Non-small cell lung cancer and metabolism research from 2013 to 2023: A visual analysis and bibliometric study. Front. Oncol. 2024, 14, 1322090. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Huang, G.; Qian, W.; Wang, X.; Zhang, H.; Li, Z.; Liu, Y.; Wang, Y.; Yu, H. Integrated analysis reveals the microenvironment of non-small cell lung cancer and a macrophage-related prognostic model. Transl. Lung Cancer Res. 2023, 12, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Hassanabad, M.F.; Hassanabad, A.F. Non-small cell lung cancer: Emerging molecular targeted and immunotherapeutic agents. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2021, 1876, 188636. [Google Scholar] [CrossRef] [PubMed]
- Ghose, S.; Radhakrishnan, V.; Bhattacharya, S. Ethics of cancer care: Beyond biology and medicine. Ecancermedicalscience 2019, 13, 911. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision medicine, AI, and the future of personalized health care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Ringborg, U.; von Braun, J.; Celis, J.; Baumann, M.; Berns, A.; Eggermont, A.; Heard, E.; Heitor, M.; Chandy, M.; Chen, C.-J.; et al. Strategies to decrease inequalities in cancer therapeutics, care and prevention: Proceedings on a conference organized by the Pontifical Academy of Sciences and the European Academy of Cancer Sciences, Vatican City, 2023. Mol. Oncol. 2024, 18, 245–279. [Google Scholar] [CrossRef]
- Camerini, A.; Conte, A.D.; Pezzuto, A.; Scotti, V.; Facchinetti, F.; Ciccone, L.P.; Perna, M.; Sartori, G.; Puccetti, C.; Ricci, A.; et al. Selection criteria and treatment outcome for advanced non-small cell lung cancer (NSCLC) patients unfit for platinum-based first-line therapy: Results of the MOON-OSS observational trial. Cancers 2022, 14, 6074. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Nam, A.; Srivastava, S.; Jones, J.; Lomenick, B.; Singhal, S.S.; Guo, L.; Warden, C.; Cho, H.; Li, A.; et al. Acquired resistance to KRAS G12C small molecule inhibition via genetic/non-genetic mechanisms in lung cancer. Sci. Adv. 2023, 9, eade3816. [Google Scholar] [CrossRef] [PubMed]
- Ramisetty, S.R.; Subbalakshmi, A.R.; Pareek, S.; Achuthan, S.; Bhattacharya, S.; Mohanty, A.; Singhal, S.S.; Salgia, R.; Kulkarni, P. Leveraging cancer phenotypic plasticity for novel treatment strategies. J. Clin. Med. 2024, 13, 3337. [Google Scholar] [CrossRef] [PubMed]

| Surgical Approach | Description | Benefits | Complications |
|---|---|---|---|
| Minimally Invasive Techniques | Utilizes VATS or robotic-assisted surgery, reducing trauma and enhancing precision. | Reduced postoperative pain, shorter hospital stays, faster recovery, improved cosmetic outcomes. | Risk of air leaks, prolonged chest tube duration, potential for incomplete tumor removal in complex cases. |
| Traditional Open Thoracotomy | Involves a larger chest incision for direct access, suitable for extensive or complex cases. | Allows for thorough tumor removal, suitable for larger tumors or complex anatomical situations. | Higher risk of postoperative pain, longer hospital stays, increased infection rates, longer recovery periods. |
| Specific Considerations | Selection based on tumor size, location, and patient factors. | Provides tailored treatment options, balances tumor removal with preservation of lung function. | Risk of complications varies based on the extent of resection (e.g., pneumonectomy vs. lobectomy vs. segmentectomy). |
| Advancement | Overview | Advantages | Examples |
|---|---|---|---|
| Molecular targeted therapies | Target specific genetic alterations (e.g., EGFR, ALK, and KRAS) using tyrosine kinase inhibitors (TKIs). | Improved survival, quality of life, and disease management. | Erlotinib, Crizotinib, Sotorasib |
| Immunotherapy | Use of immune checkpoint inhibitors (e.g., pembrolizumab and nivolumab) to enhance immune response. | Durable responses, potential for long-term remission. | Pembrolizumab, Nivolumab |
| Advanced imaging techniques | 4D-CT and MRI integration for precise diagnosis and treatment planning. | Better tumor delineation, accurate targeting, improved monitoring. | 4D-CT, MRI |
| Minimally invasive surgery | VATS and robotic-assisted surgery for lung resections. | Reduced pain, shorter hospital stays, faster recovery. | VATS, Da Vinci Surgical System |
| Radiotherapy advances | SBRT and IMRT for precise, high-dose radiation therapy. | High precision, minimized side effects, improved outcomes. | SBRT, IMRT |
| Aspect | Details | Current Limitations | Prospective Developments |
|---|---|---|---|
| Personalized medicine | Genomic profiling and liquid biopsy technologies for tailored treatment plans. | Limited availability and high costs. | Broader implementation and cost reduction through technological advances. |
| Integration of artificial intelligence | AI and machine learning for optimizing diagnosis, staging, and treatment plans. | Requires extensive data and validation. | Enhanced data collection, validation, and integration into clinical workflows. |
| Development of novel therapies | Emerging therapies like CAR-T cells, oncolytic viruses, and next-generation inhibitors. | Early stages of research, high costs, and potential side effects. | Extensive clinical trials, cost-effective production, and refinement of therapies. |
| Combination therapies | Combining modalities such as targeted therapies with immunotherapies or radiation with systemic treatments. | Potential for increased toxicity and complex treatment regimens. | Optimizing combinations and dosing to minimize toxicity while maximizing efficacy. |
| Global collaboration and equity | Efforts to improve access to advanced diagnostics and therapies worldwide, addressing healthcare disparities. | Variability in healthcare infrastructure and funding across regions. | International partnerships and funding initiatives to standardize access globally. |
| Patient-centered care | Emphasizing quality of life, supportive care measures, and incorporating patient preferences in treatment. | Requires comprehensive care models and integration of multidisciplinary teams. | Development of integrated care models that prioritize patient experience and outcomes. |
| Challenges | Description | Current Limitations | Future Directions |
|---|---|---|---|
| Healthcare disparities | Limited access to advanced diagnostic tools and therapies in lower socioeconomic and rural areas. | Inconsistent healthcare policies and lack of infrastructure. | Policy reforms and investment in healthcare infrastructure to ensure equitable access. |
| Economic and ethical issues | High cost of treatments posing economic burdens and ethical dilemmas in equitable access. | Need for policy reforms and financial support mechanisms. | Implementation of cost-effective strategies and ethical guidelines for treatment access. |
| Resistance mechanisms | Development of resistance to targeted therapies and immunotherapies limiting long-term effectiveness. | Ongoing research required to understand and overcome resistance. | Research into new targets and combination strategies to overcome resistance. |
| Complex tumor biology | Heterogeneity of NSCLC complicating treatment due to diverse molecular alterations. | Personalized approaches needed for effective treatment of diverse tumor profiles. | Advancements in molecular profiling and personalized treatment plans. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, P.; Singhal, S.; Kulkarni, P.; Horne, D.; Malhotra, J.; Salgia, R.; Singhal, S.S. Advances in Non-Small Cell Lung Cancer: Current Insights and Future Directions. J. Clin. Med. 2024, 13, 4189. https://doi.org/10.3390/jcm13144189
Garg P, Singhal S, Kulkarni P, Horne D, Malhotra J, Salgia R, Singhal SS. Advances in Non-Small Cell Lung Cancer: Current Insights and Future Directions. Journal of Clinical Medicine. 2024; 13(14):4189. https://doi.org/10.3390/jcm13144189
Chicago/Turabian StyleGarg, Pankaj, Sulabh Singhal, Prakash Kulkarni, David Horne, Jyoti Malhotra, Ravi Salgia, and Sharad S. Singhal. 2024. "Advances in Non-Small Cell Lung Cancer: Current Insights and Future Directions" Journal of Clinical Medicine 13, no. 14: 4189. https://doi.org/10.3390/jcm13144189
APA StyleGarg, P., Singhal, S., Kulkarni, P., Horne, D., Malhotra, J., Salgia, R., & Singhal, S. S. (2024). Advances in Non-Small Cell Lung Cancer: Current Insights and Future Directions. Journal of Clinical Medicine, 13(14), 4189. https://doi.org/10.3390/jcm13144189








