New Therapeutics for Heart Failure Worsening: Focus on Vericiguat
Abstract
:1. Introduction to Heart Failure (HF) and to Worsening HF (WHF)
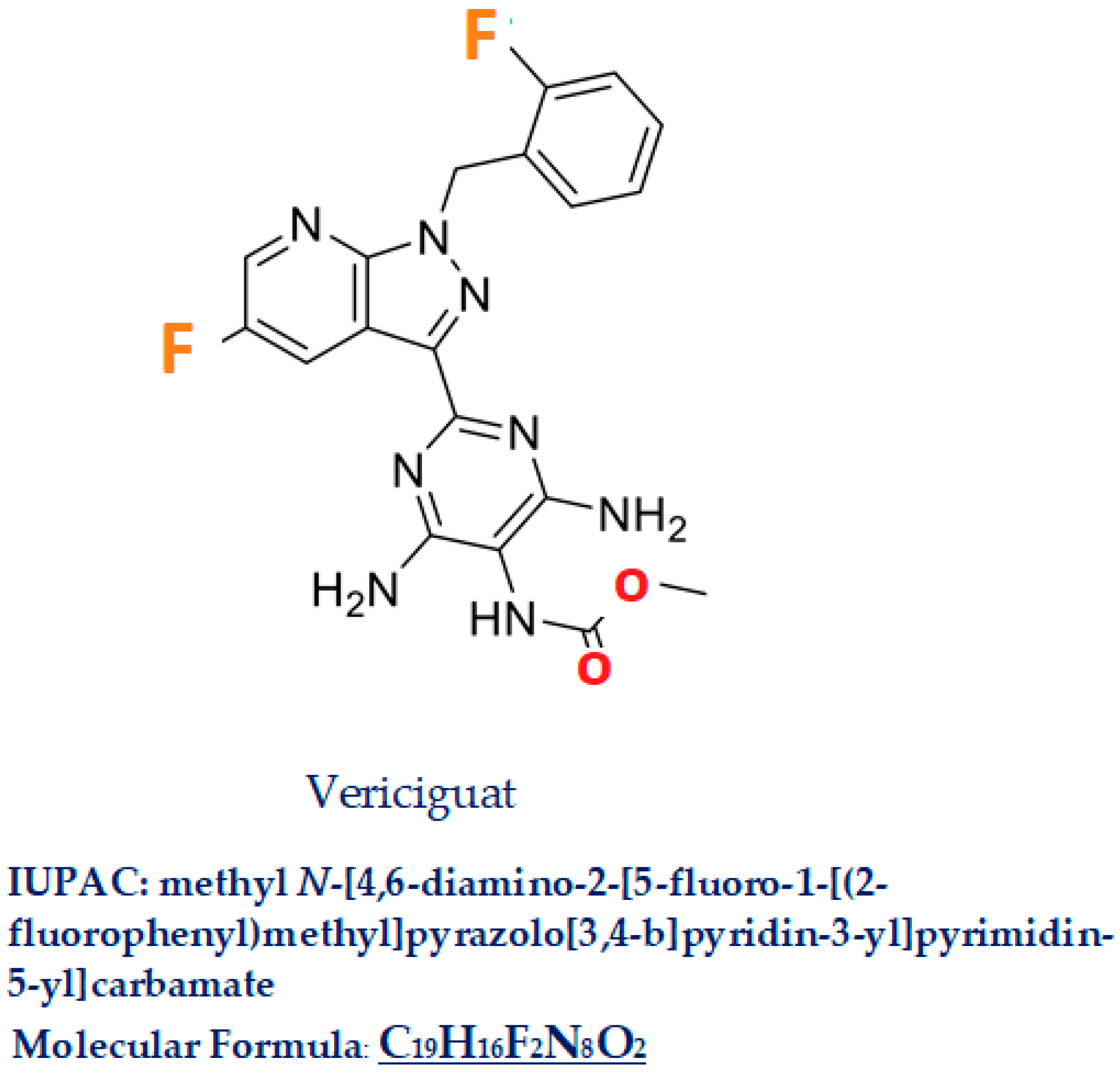
2. Vericiguat
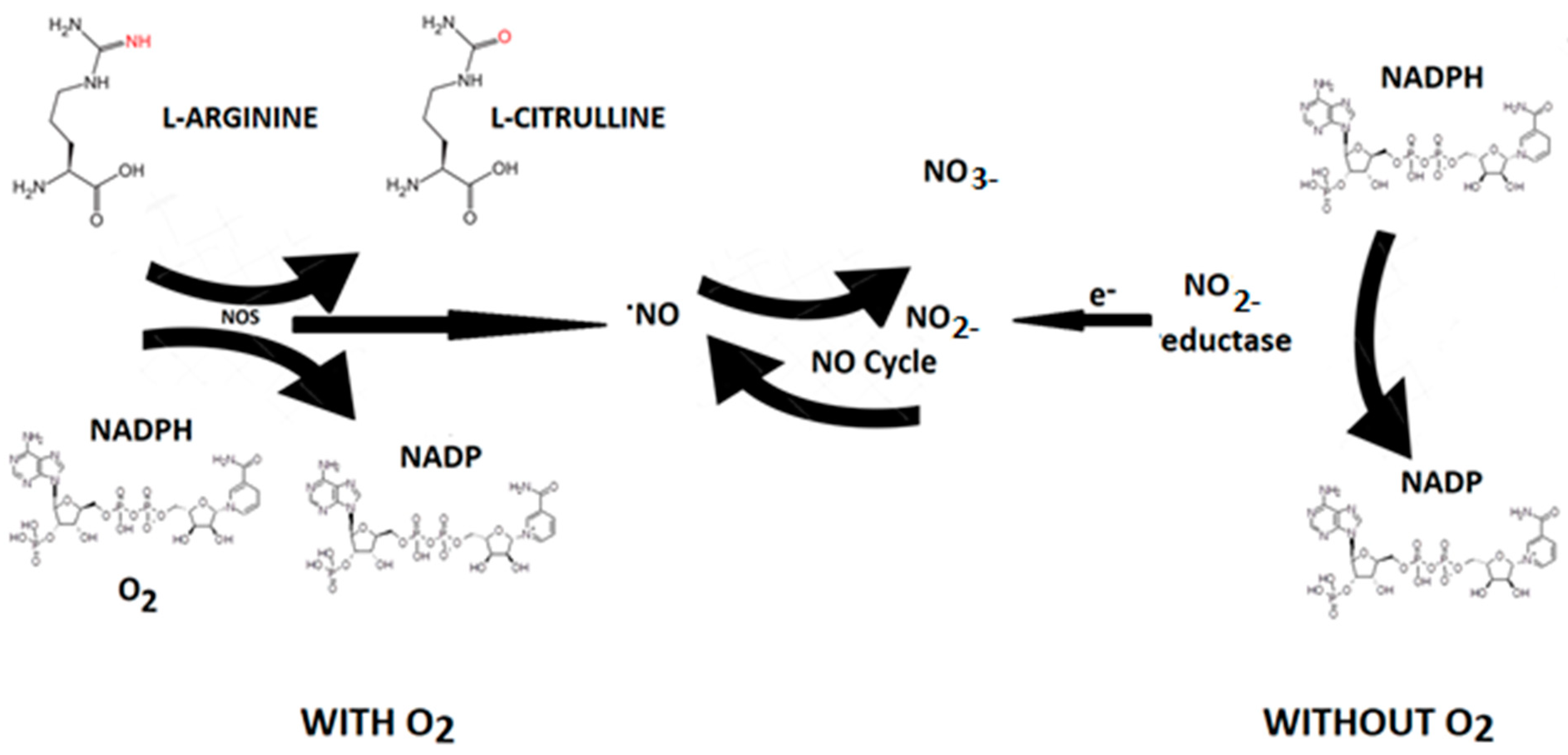
2.1. Nitric Oxide (NO)
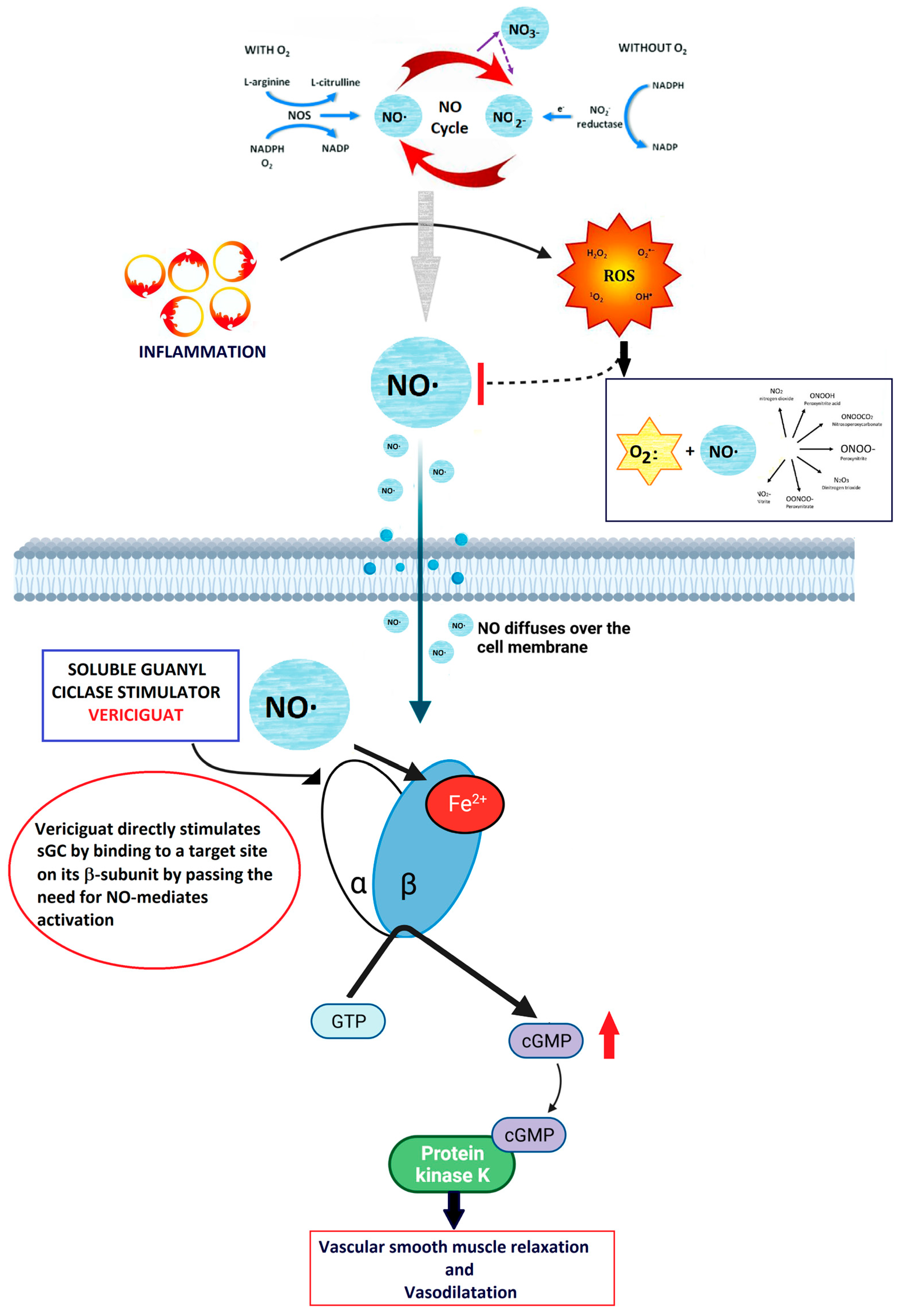
2.2. NO-sGC Pathway
2.3. Vericiguat Molecular Mechanism
2.4. Drug–Drug Interactions
2.5. Implementation of Personalized Therapy
2.6. Cost–Utility of the Addition of Vericiguat
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abovich, A.; Matasic, D.S.; Cardoso, R.; Ndumele, C.E.; Blumenthal, R.S.; Blankstein, R.; Gulati, M. The AHA/ACC/HFSA 2022 Heart Failure Guidelines: Changing the Focus to Heart Failure Prevention. Am. J. Prev. Cardiol. 2023, 15, 100527. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, M.; Sammartino, A.M.; Piepoli, M.; Adamo, M.; Pagnesi, M.; Rosano, G.; Metra, M.; Von Haehling, S.; Tomasoni, D. Heart Failure: An Update from the Last Years and a Look at the near Future. ESC Heart Fail. 2022, 9, 3667–3693. [Google Scholar] [CrossRef] [PubMed]
- Members, A.F.; McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B. Contemporary Pharmacological Treatment and Management of Heart Failure. Nat. Rev. Cardiol. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Metra, M.; Tomasoni, D.; Adamo, M.; Bayes-Genis, A.; Filippatos, G.; Abdelhamid, M.; Adamopoulos, S.; Anker, S.D.; Antohi, L.; Böhm, M.; et al. Worsening of Chronic Heart Failure: Definition, Epidemiology, Management and Prevention. A Clinical Consensus Statement by the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2023, 25, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global Burden of Heart Failure: A Comprehensive and Updated Review of Epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287. [Google Scholar] [CrossRef]
- Solomon Scott, D.; McMurray John, J.V.; Brian, C.; de Boer Rudolf, A.; DeMets, D.; Hernandez Adrian, F.; Inzucchi Silvio, E.; Kosiborod Mikhail, N.; Lam Carolyn, S.P.; Felipe, M.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Usman, M.S.; Anstrom, K.J.; Blaustein, R.O.; Bonaca, M.P.; Ezekowitz, J.A.; Freitas, C.; Lam, C.S.P.; Lewis, E.F.; Lindenfeld, J.; et al. Soluble Guanylate Cyclase Stimulators in Patients with Heart Failure with Reduced Ejection Fraction across the Risk Spectrum. Eur. J. Heart Fail. 2022, 24, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Armstrong Paul, W.; Burkert, P.; Anstrom Kevin, J.; Justin, E.; Hernandez Adrian, F.; Javed, B.; Lam Carolyn, S.P.; Piotr, P.; Voors Adriaan, A.; Gang, J.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Anstrom, K.J.; Armstrong, P.W.; For the VICTORIA Study Group. Comparing the Benefit of Novel Therapies Across Clinical Trials: Insights From the VICTORIA Trial. Circulation 2020, 142, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.N.; Diez, J.; Gustafsson, F.; Mentz, R.J.; Senni, M.; Jankowska, E.A.; Bauersachs, J. Practical Patient Care Considerations with Use of Vericiguat After Worsening Heart Failure Events. J. Card. Fail. 2023, 29, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, D.; Adamo, M.; Bozkurt, B.; Heidenreich, P.; McDonagh, T.; Rosano, G.M.C.; Virani, S.A.; Zieroth, S.; Metra, M. Aiming at Harmony. Comparing and Contrasting International HFrEF Guidelines. Eur. Heart J. Suppl. 2022, 24 (Suppl. L), L20–L28. [Google Scholar] [CrossRef] [PubMed]
- Spadafora, L.; Bernardi, M.; Sarto, G.; Simeone, B.; Forte, M.; D’Ambrosio, L.; Betti, M.; D’Amico, A.; Cammisotto, V.; Carnevale, R.; et al. Towards the Fifth Pillar for the Treatment of Heart Failure with Reduced Ejection Fraction: Vericiguat in Older and Complex Patients. Am. J. Cardiovasc. Drugs 2024, 24, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Inoue, T.; Terui, Y.; Takeuchi, K.; Susukita, K.; Arai, M.; Sato, H.; Satoh, T.; Yamamoto, S.; Yaoita, N.; et al. Evaluating Haemodynamic Changes: Vericiguat in Patients with Heart Failure with Reduced Ejection Fraction. ESC Heart Fail. 2024, ehf2.14802. [Google Scholar] [CrossRef]
- Nelson, M.B.; Gilbert, O.N.; Duncan, P.W.; Kitzman, D.W.; Reeves, G.R.; Whellan, D.J.; Mentz, R.J.; Chen, H.; Hewston, L.A.; Taylor, K.M.; et al. Intervention Adherence in REHAB-HF: Predictors and Relationship with Physical Function, Quality of Life, and Clinical Events. J. Am. Heart Assoc. 2022, 11, e024246. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Kitzman, D.W.; Nelson, M.B.; Pastva, A.M.; Duncan, P.; Whellan, D.J.; Mentz, R.J.; Chen, H.; Upadhya, B.; Reeves, G.R. Frailty and Effects of a Multidomain Physical Rehabilitation Intervention Among Older Patients Hospitalized for Acute Heart Failure: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Molloy, C.; Long, L.; Mordi, I.R.; Bridges, C.; Sagar, V.A.; Davies, E.J.; Coats, A.J.; Dalal, H.; Rees, K.; Singh, S.J.; et al. Exercise-Based Cardiac Rehabilitation for Adults with Heart Failure. Cochrane Database Syst. Rev. 2024, 2024. [Google Scholar] [CrossRef]
- PubChem. Vericiguat. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/54674461 (accessed on 11 June 2024).
- Sheikhi, N.; Bahraminejad, M.; Saeedi, M.; Mirfazli, S.S. A Review: FDA-Approved Fluorine-Containing Small Molecules from 2015 to 2022. Eur. J. Med. Chem. 2023, 260, 115758. [Google Scholar] [CrossRef] [PubMed]
- Markham, A.; Duggan, S. Vericiguat: First Approval. Drugs 2021, 81, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Shiba, M.; Kato, T.; Morimoto, T.; Yaku, H.; Inuzuka, Y.; Tamaki, Y.; Ozasa, N.; Seko, Y.; Yamamoto, E.; Yoshikawa, Y.; et al. Heterogeneity in Characteristics and Outcomes of Patients Who Met the Indications for Vericiguat Approved by the Japanese Agency: From the KCHF Registry. J. Card. Fail. 2023, 29, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, M.; Gerisch, M.; Lobmeyer, M.; Besche, N.; Thomas, D.; Gerrits, M.; Lemmen, J.; Mueck, W.; Radtke, M.; Becker, C. Metabolism and Pharmacokinetic Drug–Drug Interaction Profile of Vericiguat, A Soluble Guanylate Cyclase Stimulator: Results from Preclinical and Phase I Healthy Volunteer Studies. Clin. Pharmacokinet. 2020, 59, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Ruehs, H.; Klein, D.; Frei, M.; Grevel, J.; Austin, R.; Becker, C.; Roessig, L.; Pieske, B.; Garmann, D.; Meyer, M. Population Pharmacokinetics and Pharmacodynamics of Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. Clin. Pharmacokinet. 2021, 60, 1407–1421. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Arrington, L.; Patel, Y.; Passarell, J.; Wenning, L.; Blaustein, R.O.; Armstrong, P.W.; Meyer, M.; Becker, C.; Gheyas, F. Population Pharmacokinetics of Vericiguat in Patients with Heart Failure with Reduced Ejection Fraction: An Integrated Analysis. Clin. Pharma Ther. 2022, 112, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214377s000lbl.pdf (accessed on 11 June 2024).
- Australian Prescriber. New Drug: Vericiguat for Chronic Heart Failure. Aust. Prescr. 2023, 46, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Sharina, I.; Martin, E. Cellular Factors That Shape the Activity or Function of Nitric Oxide-Stimulated Soluble Guanylyl Cyclase. Cells 2023, 12, 471. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; You, B.; Chen, S.-P.; Liao, J.K.; Sun, J. Tumor Necrosis Factor-α Downregulates Endothelial Nitric Oxide Synthase mRNA Stability via Translation Elongation Factor 1-α 1. Circ. Res. 2008, 103, 591–597. [Google Scholar] [CrossRef]
- Breitenstein, S.; Roessig, L.; Sandner, P.; Lewis, K.S. Novel sGC Stimulators and sGC Activators for the Treatment of Heart Failure. In Heart Failure; Bauersachs, J., Butler, J., Sandner, P., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2016; Volume 243, pp. 225–247. [Google Scholar] [CrossRef]
- Kemp-Harper, B.; Schmidt, H.H.H.W. cGMP in the Vasculature. In cGMP: Generators, Effectors and Therapeutic Implications; Schmidt, H.H.H.W., Hofmann, F., Stasch, J.-P., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 191, pp. 447–467. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Ayalasomayajula, S.; Blaustein, R.O.; Gheyas, F. Vericiguat, a Novel sGC Stimulator: Mechanism of Action, Clinical, and Translational Science. Clin. Transl. Sci. 2023, 16, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, C.Y. Nitric Oxide: An Old Drug but with New Horizons in Ophthalmology—A Narrative Review. Ann. Transl. Med. 2023, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S. Nitric Oxide: Discovery and Impact on Clinical Medicine. J. R. Soc. Med. 1999, 92, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Michel, L.Y.M.; Balligand, J.-L. Nitric Oxide Signalling in Cardiovascular Health and Disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef] [PubMed]
- Blanton, R.M. cGMP Signaling and Modulation in Heart Failure. J. Cardiovasc. Pharmacol. 2020, 75, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Gheorghiade, M.; Borlaug, B.A.; Pieske, B.; Vaduganathan, M.; Burnett, J.C.; Roessig, L.; Stasch, J.; Solomon, S.D.; Paulus, W.J.; et al. The cGMP Signaling Pathway as a Therapeutic Target in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2013, 2, e000536. [Google Scholar] [CrossRef]
- Busse, W.W.; Melén, E.; Menzies-Gow, A.N. Holy Grail: The Journey towards Disease Modification in Asthma. Eur. Respir. Rev. 2022, 31, 210183. [Google Scholar] [CrossRef] [PubMed]
- Stasch, J.-P.; Evgenov, O.V. Soluble Guanylate Cyclase Stimulators in Pulmonary Hypertension. In Pharmacotherapy of Pulmonary Hypertension; Humbert, M., Evgenov, O.V., Stasch, J.-P., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 218, pp. 279–313. [Google Scholar] [CrossRef]
- Böger, R.H. Asymmetric Dimethylarginine, an Endogenous Inhibitor of Nitric Oxide Synthase, Explains the “L-Arginine Paradox” and Acts as a Novel Cardiovascular Risk Factor. J. Nutr. 2004, 134, 2842S–2847S. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Dempsey, S.; Daneva, Z.; Azam, M.; Li, N.; Li, P.-L.; Ritter, J. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef]
- Mangmool, S.; Duangrat, R.; Parichatikanond, W.; Kurose, H. New Therapeutics for Heart Failure: Focusing on cGMP Signaling. Int. J. Mol. Sci. 2023, 24, 12866. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, F.; Campora, A.; Barilli, M.; Palazzuoli, A. Vericiguat in Heart Failure: Characteristics, Scientific Evidence and Potential Clinical Applications. Biomedicines 2022, 10, 2471. [Google Scholar] [CrossRef]
- Numata, G.; Takimoto, E. Cyclic GMP and PKG Signaling in Heart Failure. Front. Pharmacol. 2022, 13, 792798. [Google Scholar] [CrossRef]
- Dies, R.M.; Jackson, C.N.; Flanagan, C.J.; Sinnathamby, E.S.; Spillers, N.J.; Potharaju, P.; Singh, N.; Varrassi, G.; Ahmadzadeh, S.; Shekoohi, S.; et al. The Evolving Role of Vericiguat in Patients with Chronic Heart Failure. Cureus 2023, 15, e49782. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lindberg, F.; Christodorescu, R.M.; Ferrini, M.; Kumler, T.; Toutoutzas, K.; Dattilo, G.; Bayes-Genis, A.; Moura, B.; Amir, O.; et al. Physician Perceptions, Attitudes, and Strategies towards Implementing Guideline-directed Medical Therapy in Heart Failure with Reduced Ejection Fraction. A Survey of the Heart Failure Association of the ESC and the ESC Council for Cardiology Practice. Eur. J. Heart Fail. 2024, 26, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Kjeldsen, K.P.; Delpón, E.; Semb, A.G.; Cerbai, E.; Dobrev, D.; Savarese, G.; Sulzgruber, P.; Rosano, G.; Borghi, C.; et al. Facing the Challenge of Polypharmacy When Prescribing for Older People with Cardiovascular Disease. A Review by the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. Eur. Heart J.—Cardiovasc. Pharmacother. 2022, 8, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Kerwagen, F.; Ohlmeier, C.; Evers, T.; Herrmann, S.; Bayh, I.; Michel, A.; Kruppert, S.; Wilfer, J.; Wachter, R.; Böhm, M.; et al. Real-World Characteristics and Use Patterns of Patients Treated with Vericiguat: A Nationwide Longitudinal Cohort Study in Germany. Eur. J. Clin. Pharmacol. 2024, 80, 931–940. [Google Scholar] [CrossRef]
- Merck Sharp & Dohme LLC. A Pivotal Phase 3 Randomized, Placebo-Controlled Clinical Study to Evaluate the Efficacy and Safety of the sGC Stimulator Vericiguat/MK-1242 in Adults with Chronic Heart Failure with Reduced Ejection Fraction; Clinical trial registration NCT05093933; clinicaltrials.gov. 2024. Available online: https://clinicaltrials.gov/study/NCT05093933 (accessed on 1 January 2024).
- Wang, L.; Huo, X.; Sun, H.; Liu, F.; Huang, R.; Zhao, Q. Cost-Utility Analysis of Add-on Vericiguat for the Treatment of Chronic Heart Failure with Reduced Ejection Fraction in China. BMC Public Health 2024, 24, 1275. [Google Scholar] [CrossRef] [PubMed]
- Chew, D.S.; Li, Y.; Bigelow, R.; Cowper, P.A.; Anstrom, K.J.; Daniels, M.R.; Davidson-Ray, L.; Hernandez, A.F.; O’Connor, C.M.; Armstrong, P.W.; et al. Cost-Effectiveness of Vericiguat in Patients with Heart Failure with Reduced Ejection Fraction: The VICTORIA Randomized Clinical Trial. Circulation 2023, 148, 1087–1098. [Google Scholar] [CrossRef] [PubMed]



| VICTORIA [9] (Patients: 5050) | VICTOR [48] (Patients: 6000) | |
|---|---|---|
| Vericiguat 2526 female 1921 male Placebo 2524 female 1921 male | Ongoing | |
| Study drug | Vericiguat vs. placebo | Vericiguat vs. placebo |
| Key inclusion criteria | Chronic HF with NYHA functional class II–IV EF < 45% Receiving HF GDMT Prior HF hospitalization within 6 months or outpatient intravenous diuretic for HF within 3 months Elevated NT-proBNP levels | Chronic HF with NYHA functional class II–IV EF ≤ 40% Elevated NT-proBNP levels |
| Primary endpoint | Time to First Occurrence of Composite Endpoint of Cardiovascular (CV) Death or Heart Failure (HF) Hospitalization | Time to First Occurrence of Composite Endpoint of Cardiovascular (CV) Death or Heart Failure (HF) Hospitalization The first event of CV death or HF hospitalization as confirmed by a clinical event committee (CEC) |
| Select secondary endpoints | Time to CV death Time to first HFH Time to total (first and recurrent) HFHs Time to total first occurrence of composite of all-cause mortality or HFH Time to all-cause mortality Safety and tolerability | Time to first occurrence of CV First event of CV death as confirmed by CEC Time to first occurrence of HF hospitalization The first event of HF hospitalization as confirmed by CEC Time to total HF hospitalizations, all events of HF hospitalization as confirmed by CEC Safety and tolerability |
| Randomized Double-blind 1:1 | 2.5 mg od/5 mg od/10 mg od (up-titration at 2-week intervals) Placebo | 2.5, 5.0, or 10.0 mg orally once daily Placebo |
| Median follow-up | 10.8 months | 40.0 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, P.; Vitiello, L.; Milani, F.; Volterrani, M.; Rosano, G.M.C.; Tomino, C.; Bonassi, S. New Therapeutics for Heart Failure Worsening: Focus on Vericiguat. J. Clin. Med. 2024, 13, 4209. https://doi.org/10.3390/jcm13144209
Russo P, Vitiello L, Milani F, Volterrani M, Rosano GMC, Tomino C, Bonassi S. New Therapeutics for Heart Failure Worsening: Focus on Vericiguat. Journal of Clinical Medicine. 2024; 13(14):4209. https://doi.org/10.3390/jcm13144209
Chicago/Turabian StyleRusso, Patrizia, Laura Vitiello, Francesca Milani, Maurizio Volterrani, Giuseppe M. C. Rosano, Carlo Tomino, and Stefano Bonassi. 2024. "New Therapeutics for Heart Failure Worsening: Focus on Vericiguat" Journal of Clinical Medicine 13, no. 14: 4209. https://doi.org/10.3390/jcm13144209






