Abstract
With a common aim of restoring physiological function of defective cells, optogenetics and targeted gene therapies have shown great clinical potential and novelty in the branch of personalized medicine and inherited retinal diseases (IRDs). The basis of optogenetics aims to bypass defective photoreceptors by introducing opsins with light-sensing capabilities. In contrast, targeted gene therapies, such as methods based on CRISPR-Cas9 and RNA interference with noncoding RNAs (i.e., microRNA, small interfering RNA, short hairpin RNA), consists of inducing normal gene or protein expression into affected cells. Having partially leveraged the challenges limiting their prompt introduction into the clinical practice (i.e., engineering, cell or tissue delivery capabilities), it is crucial to deepen the fields of knowledge applied to optogenetics and targeted gene therapy. The aim of this in-depth and novel literature review is to explain the fundamentals and applications of optogenetics and targeted gene therapies, while providing decision-making arguments for ophthalmologists. First, we review the biomolecular principles and engineering steps involved in optogenetics and the targeted gene therapies mentioned above by bringing a focus on the specific vectors and molecules for cell signalization. The importance of vector choice and engineering methods are discussed. Second, we summarize the ongoing clinical trials and most recent discoveries for optogenetics and targeted gene therapies for IRDs. Finally, we then discuss the limits and current challenges of each novel therapy. We aim to provide for the first time scientific-based explanations for clinicians to justify the specificity of each therapy for one disease, which can help improve clinical decision-making tasks.
1. Introduction
Inherited retinal diseases (IRDs) encompass a group of genetically complex diseases that lead to gradual vision loss and blindness, with a prevalence of 1 case in 3000 individuals worldwide [1]. A recent study using computational analysis estimated that approximately 2.2 billion individuals worldwide are carriers of an IRD, from which 5.5 million individuals are expected to have clinical manifestations [2]. Furthermore, the financial burden associated with the diagnosis and treatment of IRDs is substantial in North America; costs attributable to IRDs in the United States was shown to range from nearly USD 13,500 million to USD 32,000 million [3]. Loss of wellbeing, followed by loss of productivity, were the major contributors to these expenses [3]. Consequently, there is a growing urge to develop novel treatments, such as gene therapies, to limit the progression of vision loss and the decrease in vision-related quality of life.
Numerous classification systems can be employed to categorize IRDs, such as the inheritance pattern, the clinical course of the course (i.e., stationary vs. progressive), based on the anatomical and functional disease features, or the phenotypic and genotypic characteristics [4,5]. A recent comparative cross-sectional study proposed a novel classification system based on the latter method, dividing the retinal dystrophies into six distinct categories, consisting of diffuse photoreceptor dystrophies, macular dystrophies, chorioretinal dystrophies, inner retinal and vitreoretinal dystrophies, systemic diseases associated with photoreceptor dystrophies, and congenital and stationary retinal diseases [5].
The genetic mutations involved in the pathogenesis of IRD have been shown to lead to the loss or degradation of the photoreceptor layer or retinal pigment epithelium (RPE) [6]. These mutations are inherited through the conventional inheritance patterns (i.e., autosomal dominant (AD), autosomal recessive (AR), X-linked, and mitochondrial inheritance patterns) [6]. Multiple studies have underscored the genetic epidemiology behind IRDs [7,8,9,10,11,12]; a recent study from Karali and colleagues identified by next-generation sequencing more than 1300 sequence variations in 132 genes, which included 866 potential genotypes for therapeutic avenues [7]. Gene therapy and optogenetics have generated tremendous interest over the past years to tackle the treatment of retinal dystrophies [13,14,15,16,17]. Herein, we review the advantages of these novel systems for the management of IRDs, the fundamental concepts involved in their engineering, and the most recent preclinical and clinical studies.
2. Optogenetics and Targeted Gene Therapies: Novel Advances in the Treatment of IRDs
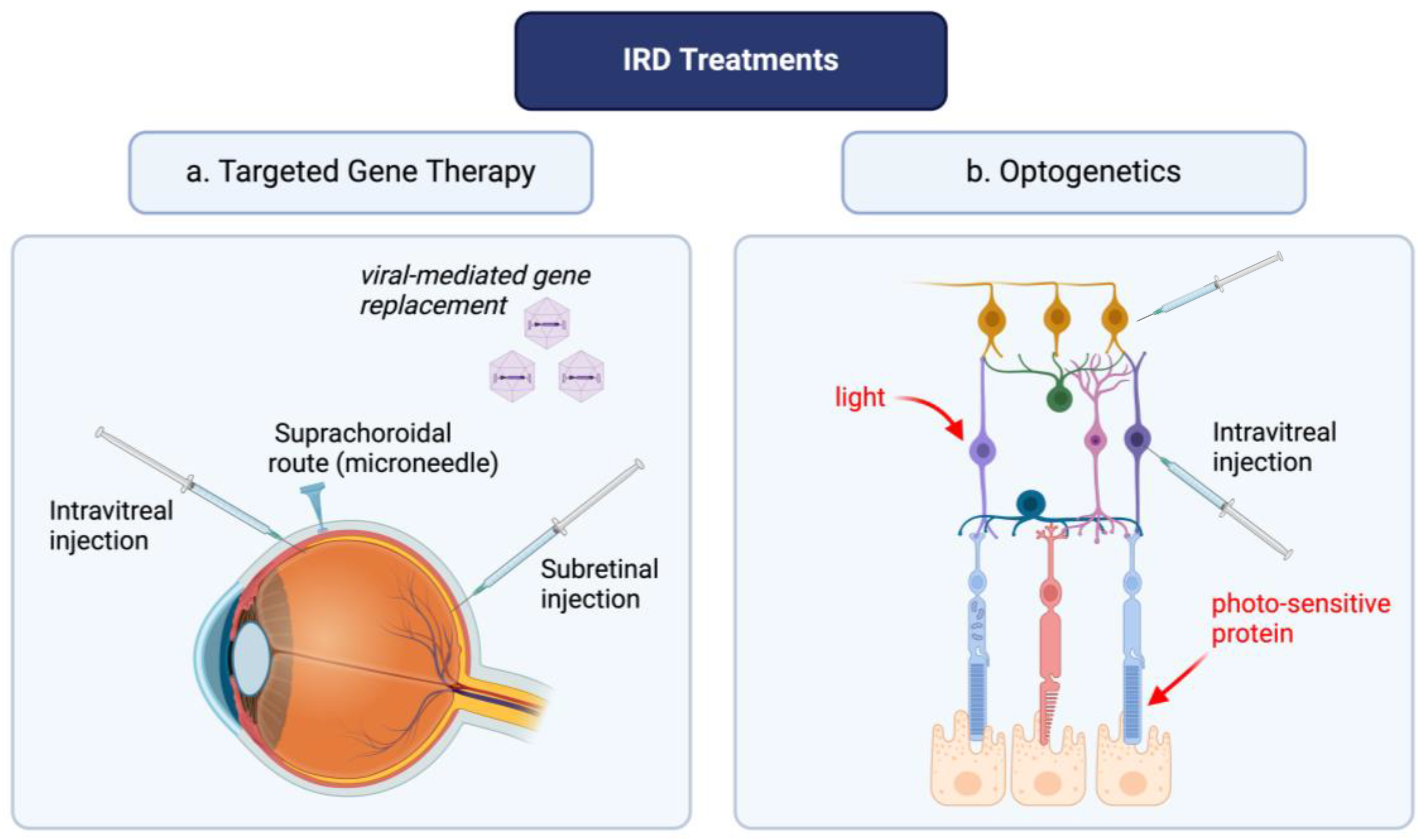
Current treatment of retinal dystrophies involves a multidisciplinary approach, given the limited clinical tools to halt disease progression in many disease subtypes [18]. The role of reactive oxygen species (ROS) in the pathogenesis of retinal diseases has been thoroughly described in previous studies and comprehensive literature reviews [19,20]. Briefly, the pro-oxidative environment ensuing gene mutations leads to photoreceptor degeneration, retinal ganglion cell death and retinal remodeling characterized by modulations in gene expression, migration of retinal cells, and neovascular modifications [21]. To delay disease progression in IRD, such as RP, multiple solutions have been proposed such as the use of antioxidants; in vivo studies have shown great potential of these agents in delaying disease progression [22,23]. However, challenges remain in the treatment of patients with gene mutations; the progression of the disease is inevitable and ultimately leads to blindness. Gene therapy and optogenetics are modern medicine tools whose primary aim is to treat genetic disorders at the level of the dysfunctional retinal cells (Figure 1). However, the engineering of these latter tools requires a multi-step approach, which is discussed in the ensuing sections.
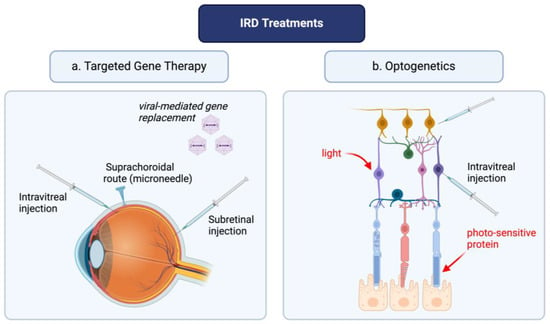
Figure 1.
Optogenetics and targeted gene therapy: a novel approach for inherited retinal disease management. The figure was created with BioRender.com.
3. Optogenetics
The concept of optogenetics emerged in the early 1980s, with the premise that light modulation could target and control specific neurons [24]. Throughout the years, scientific breakthroughs shaped the current modern concept of optogenetics, which aims to restore visual function by integrating opsins (i.e., light-sensitive proteins) into the retinal neurons. This process aims to control the activity of opsin-transfected cells by modulating light exposure/excitation. Prior to delving into the molecular principles involved in optogenetics, it is crucial to understand physiologic phototransduction.
3.1. Phototransduction
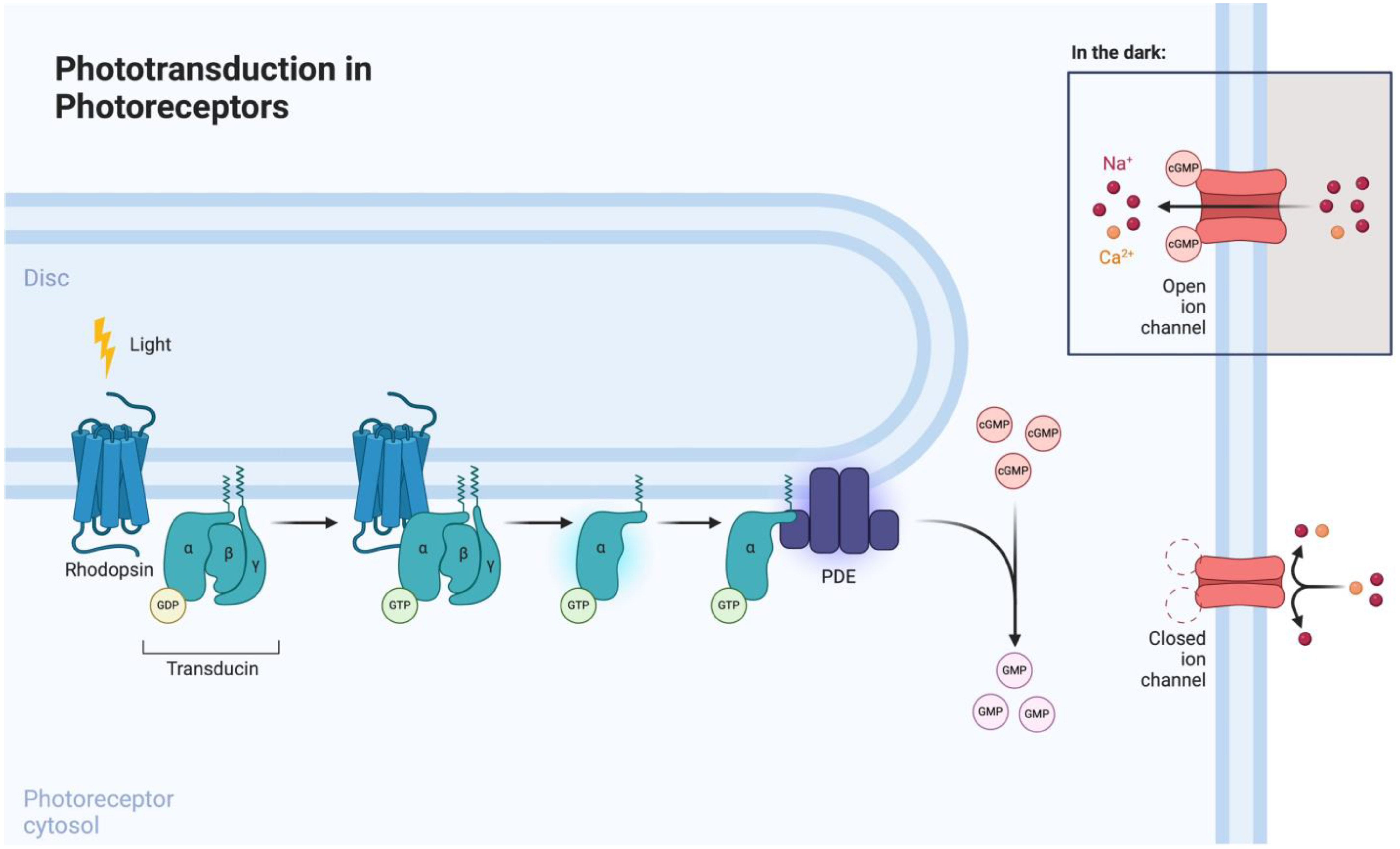
Phototransduction is mediated by photoreceptors (i.e., rods and cones), which are highly specialized neurons involved in visual function (Figure 2). Rods are involved in scotopic vision (i.e., vision at low levels of ambient light), whereas cones mediate photopic vision (i.e., vision at higher levels of ambient light) [25]. Both cell types consist of a highly organized anatomical structure, which encompasses an elongated outer segment, cilium, inner segment, cell body, and terminal synapse [26]. Rhodopsin, a rod-specific opsin and G-protein-coupled receptor (GPCR), is the key mediator of phototransduction, which takes place in the outer segment. Upon light exposure, rhodopsin undergoes photoisomerization, leading to the formation of its active form, metarhodopsin. Metarhodopsin activation subsequently induces transducin recruitment and intracellular GTP-mediated phosphodiesterase (PDE) signaling [27]. Therefore, by inducing the expression of opsins in degenerated photoreceptors, it is possible to modulate the phototransduction signaling pathway.
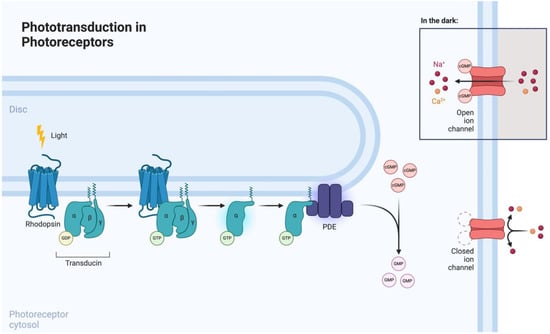
Figure 2.
Phototransduction signaling pathway in photoreceptors. Reprinted from “Phototransdution in Photoreceptors” by Biorender.com (2024). Retrieved from https://app.biorender.com/biorender-templates (accessed on 1 June 2024).
3.2. Optogenetic Engineering
To achieve an efficient optogenetic system, three main components are required: an opsin, a vector for transfection, and a safe surgical approach for drug delivery. Opsins form the backbone of optogenetics. In the ensuing section, we review the main categories of opsins and their advantages and disadvantages in optogenetics. The vector of transfection and surgical approaches are discussed in further sections.
Opsins
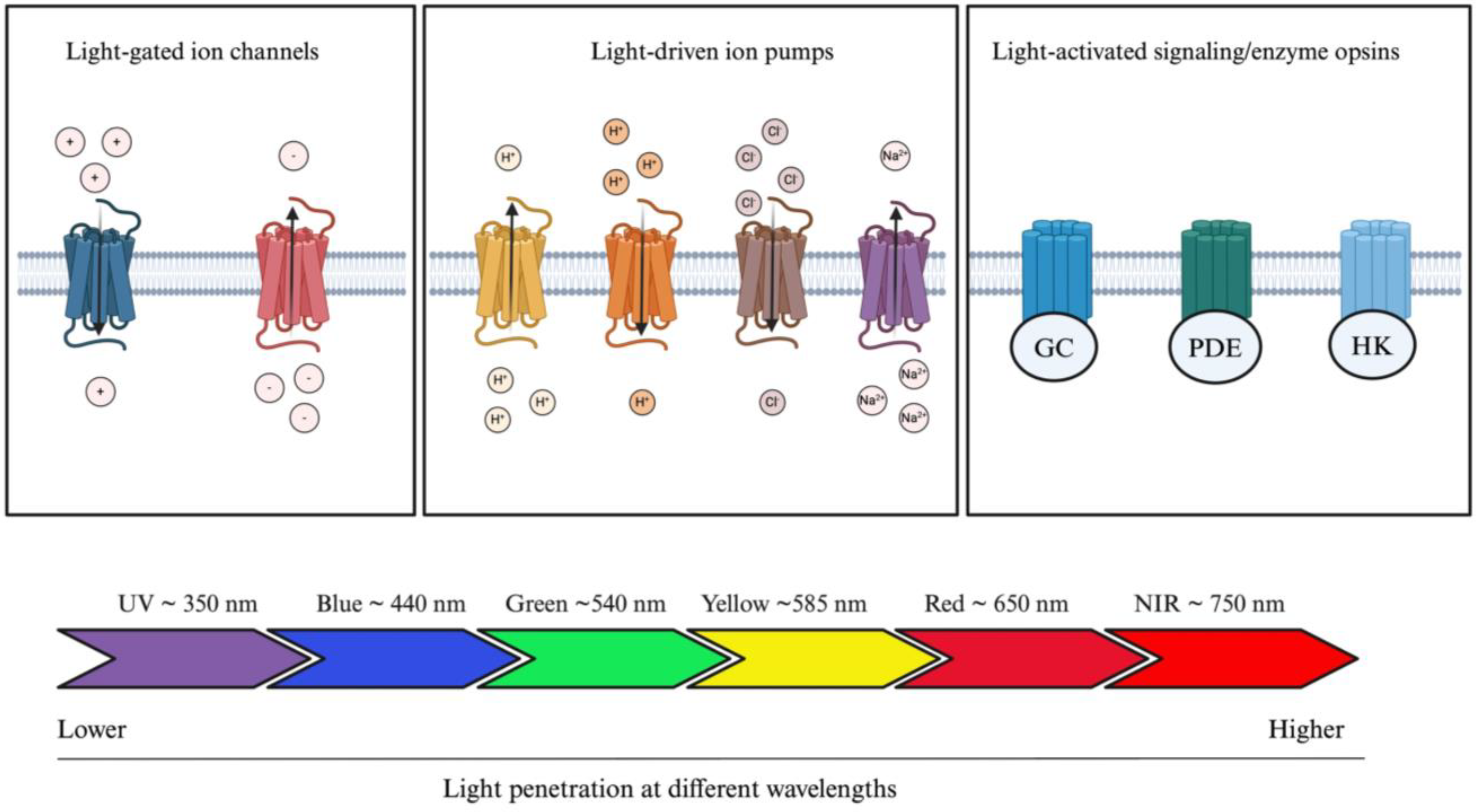
Opsins are G-protein-coupled receptors (GPCRs) and can be divided into two categories: microbial (also known as type 1 opsins, consisting of prokaryotes, algae, and fungi) or animal opsins (also known as type II opsins) [28]. Microbial opsins have been mostly studied for the treatment of IRD and can further be divided in three distinct subcategories, which consist of light-gated channels, light-driven ion pumps, and light-activated signaling/enzyme opsins (Figure 3) [29]. Microbial opsins use light as a substrate. Photostimulation induces opsin-mediated activation, silencing, or signaling pathway modulation of photoreceptors [30]. They function by absorbing light at a specific wavelength. Greater wavelengths are known to be associated with greater penetration rates (i.e., penetrating deeper into the skin) [31]. Blue light is the least penetrating at a wavelength of approximately 440 nm (peak sensitivity), whereas red light travels further given its approximate 650 nm wavelength (peak sensitivity) [32]. This phenomenon is partly explained by the different light scattering properties and absorption coefficients in human tissues [32,33]. Therefore, by modulating the wavelength properties of opsins, it is possible to modulate tissue penetration and opsin delivery within the human body. Given the greater penetrating ability of wavelengths greater than 650 nm, red or NIR wavelengths are preferred in optogenetic engineering, which is further discussed.
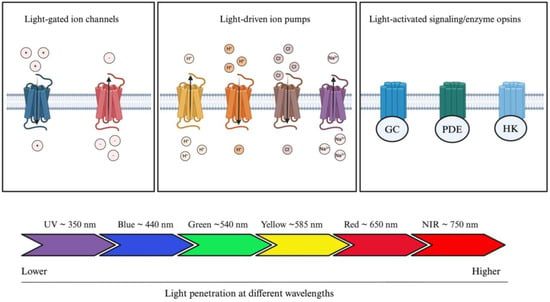
Figure 3.
Optogenetic actuators and their tissue-penetrating properties. Optogenetic actuators can be divided into three distinct groups: light-gated ion channels, light-driven ion pumps, and light-activated signaling/enzyme opsins. Light source can be modulated according to clinical application; blue light is the least penetrating, whereas red light penetrates the most human tissue. Abbreviations: GC, guanylyl cyclase; PDE, phosphodiesterase; HK, histidine kinase; UV, ultraviolet; NIR, near infrared. The figure was created with BioRender.com.
Light-gated channels encompass cation or anion channel rhodopsins (ChRs) [29]. These ChRs can be subdivided into further categories based on their maximal wavelength sensitivity: blue-opsins (i.e., opsins stimulated by blue light) or red-opsins (i.e., opsins stimulated by red-light). ChR2, an activating cation light-gated channel, is the most widely studied microbial rhodopsin throughout the literature. ChR2, derived from Chlamydomonas reinardtii, mediates the transport of Na+, K+, H+, and Ca2+ ions across the photoreceptor cell membrane following blue light stimulation and subsequent depolarization [34]. It is capable of further depolarizing the retinal ganglion cells (GCs), initiating electrical signals that replicate the role of photoreceptor cells. The objective of this method is to reinstate light sensitivity and facilitate the transmission of visual information to the brain. However, given the low absorption rate of blue light opsins, ChR2 is not a great candidate for deep tissue penetration, such as the RPE [29]. Furthermore, it was shown that blue light opsins require greater energy levels to be stimulated, which can further damage the retina or further aggravate the IRD [35,36]. To leverage these challenges, red opsins have shown greater potential in optogenetics (Table 1). However, an additional challenge with red opsins is their non-negligeable blue light sensitivity, which can lead to cross-talking in dual color systems. Chimeric opsins, as detailed in Table 1, can be used to minimize the cross-talk induced by red opsins. The signaling pathway of chimeric opsins can be divided into a two-step process [37]. First, the red opsins are inactivated by utilizing a long red stimulus. In the second step, immediately following the inactivation of red opsins, blue opsins are stimulated. This process allows for the exclusive stimulation of blue opsins, while red opsins are fully inactivated.
Light-driven ion pumps allow the unidirectional transport of single cations or anions across the cytoplasmic membrane. They can be subdivided into four distinct categories, consisting of outward or inward proton pumps, chloride pumps, and sodium pumps. Bacteriorhodopsin (BR) was the first ever characterized light-driven outward proton pump [38]. Halorhodopsin (HR) and archaerhodopsin (Archs) are also light-driven ion pumps [39]. The most recent discoveries involves the HR family opsins, with studies developing variants of NpHR [40,41]. Finally, the last category of opsins encompasses light-activated signaling/enzyme opsins, where intracellular signaling pathways can be modulated with light. Sensory rhodopsins (SR) were the first opsins from their category to be characterized. However, with recent advances, three novel opsins have been characterized, consisting of histidine kinase rhodopsin (HKR), guanylate cyclase rhodopsin (Rh-GC), and rhodopsin-phosphodiesterase (Rh-PDE) [42,43,44,45], which are all transmembrane proteins with eight domains. Overall, the evolving library of mutant microbial opsins is significantly contributing to the extending range of application of optogenetics and renders its suitability for various pathogenic mechanisms.

Table 1.
Summary of current and potential microbial opsins for optogenetics.
Table 1.
Summary of current and potential microbial opsins for optogenetics.
| Category | Opsin Examples a | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Light-gated ion channels | ||||
| Blue light opsins | ChR2 GtACRe | Rapid cell depolarization following stimulation (<50 μs) | Limited tissue penetration Requires high stimulus intensity in comparison to physiological rhodopsin and cones | [16,35,36,46] |
| Red-shift opsins | VChR1 ReaChR Chrimson ChrimsonR ChrimsonSA ChRmine frChRmine | Greater tissue penetration | Non-negligeable blue-light sensitivity that may lead to cross-activation | [47,48,49,50,51] |
| Chimeric opsins | Chronos/ChrimsonR CheRiff/ChrimsonR ChR2/ReaChR ChR2/ChrimsonR | Highly specific modulation of red or blue opsins | Low population of blue opsins can limit the excitatory potential | [52,53,54,55,56,57,58,59,60,61] |
| Light-driven ion pumps | ||||
| Hydrogen pumps | BR Arch Mac | Production of higher photocurrent rates and less interference with neurotransmission | [62,63] | |
| Sodium pumps | KR2 (DeNaR) | Efficient for neuron silencing | [64,65] | |
| Chloride pumps | HR NpHR eNpHR 2.0 eNpHR 3.0 | Rapid activation and inactivation kinetics Efficient for neuron silencing | Low levels of generated photocurrent with NpHR Requires high stimulus intensity in comparison to physiological rhodopsin and cones, as well as ChR2 | [66,67,68,69] |
| Light-activated signaling/enzyme opsins | ||||
| Sensory rhodopsins (SR) | SRI SRII | |||
| HKR Rh-GC Rh-PDE | Selective modulation of intracellular signaling pathways | [42,43,44,45] | ||
a Abbreviations: ChR, channelrhodopsin; BR, bacteriorhodopsin; Arch, archaerhodopsin-3; Mac, Leptosphaeria maculans; KR; Krokinobactereikastus rhodopsin; HR, halorhodopsin; NpHR, Natromonas pharaonis halorhodopsin; SR, sensory rhodopsin; HKR, histidine kinase rhodopsin; Rh-GC, guanylyl cyclase rhodopsin; Rh-PDE, phosphodiesterase rhodopsin.
4. Genome Editing with CRISPR-Cas9
Genome editing is a promising therapeutic strategy to correct underlying mutations that underly IRDs. First-generation genome editors, which include zinc-finger nucleases (ZNFs) and transcription-activator like effector nucleases (TALENs), have been studied extensively but with limited success [70,71]. Both are endonucleases with modifiable domains that can introduce double-strand DNA breaks (DSBs) at specific loci. However, they have been limited by their complexity [72]. More recently, genome editing has focused on the clustered regularly interspaced short palindromic repeats (CRISPR)—the CRISPR-associated protein 9 (Cas9) system. CRISPR-Cas9 system is composed of nucleases discovered in bacteria, in which they function as an adaptative immune system protecting prokaryotes against bacteriophage and plasmid infection [73]. CRISPR-Cas9 is more promising than first-generation genome editors due to its relative simplicity, adaptability, and specificity [72,74,75].
In bacteria, CRISPR systems rely on two small RNAs that detect foreign pathogenic nucleic acids and guide their endogenous Cas protein to the infecting nucleic material and cleave it, thus protecting the host [73]. Three types (I-III) of CRISPR systems have been discovered and studied. In 2012, a protocol for describing CRISPR-Cas9 as a genome editing tool was first published, and since then, it has been investigated as a therapeutic tool for IRDs [73,76,77]. For genome editing, the RNAs are merged into a single, chimeric guide RNA (gRNA), combining the functions of both prokaryote RNAs. The gRNA must contain a protospacer adjacent motif (PAM) sequence and an accompanying 20 nucleotide target sequence, which will guide the Cas9 protein to the target site where Cas9 will introduce a double-strand break (DSB) [77]. This DSB will be repaired by cellular mechanisms such as non-homologous end-joining (NHEJ) or homology-directed repair (HDR). Thus, unlike TALENs and ZFNs, which require modification of the nuclease, CRISPR-Cas9 is much simpler as its selectivity is achieved by adjusting the gRNA and not the Cas9 endonuclease.
System Engineering
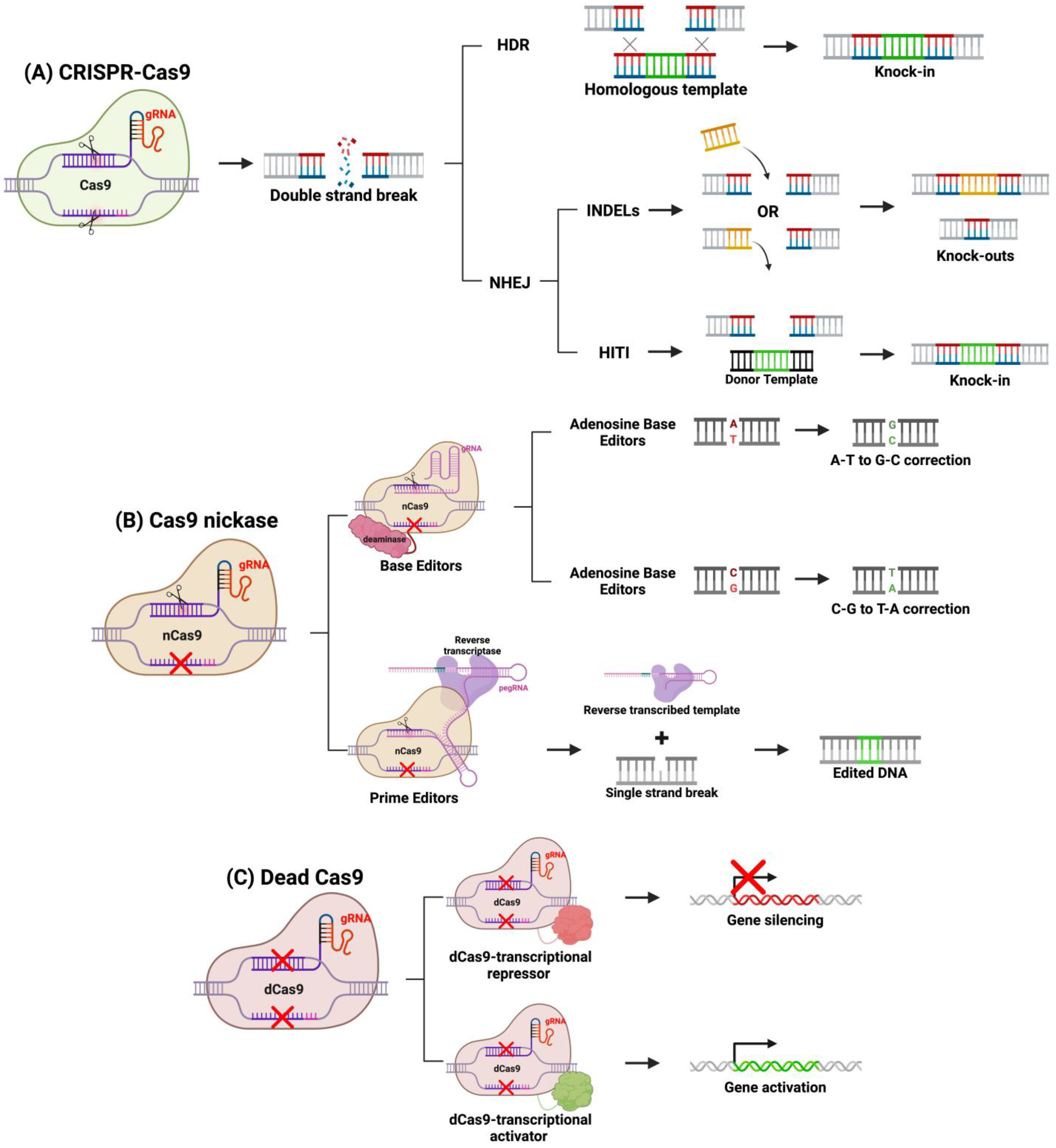
Over the past decade, genome editing applications of CRISPR-Cas9 have been expanded, and various methods have been developed to modify the system to edit genes in diverse ways. For example, DSBs using a traditional Cas9 protein favor NHEJ or HDR repair, and through protocol design, a specific DNA repair pathway can be activated over the other (Figure 4). NHEJ introduces small insertions and deletions (indels) disrupting protein function. This repair pathway is active throughout the cell cycle in all dividing and non-dividing cells, making it ideal in the quiescent retina [77,78,79]. NHEJ is useful to knock-out gain-of-function or dominant-negative mutations such as Rhodopsin (Rho) gene mutations in autosomal dominant retinitis pigmentosa (ADRP) [78,79]. NHEJ can be leveraged to restore normal expression through a “reduction and replacement” strategy. This strategy involves reducing the expression of the mutated gene product, then transfecting a wild-type gene, thus restoring phototransduction signaling pathway [80]. This method is ideal in restoring normal protein expression in the presence of a toxic mutant. Another group expanded upon the “reduction and replacement” strategy by developing a method called homology-independent targeted integration (HITI), where the “replacement” transgene is integrated at the site of the Cas9 DSB [72,79,81]. By designing the DNA template to contain a homologous cleavage site as the gene-of-interest, the researchers demonstrated that integration is achieved through NHEJ [81]. This is achieved by Cas9 cleaving both the template and target gene, and NHEJ will repair both breaks by integrating the template into the genome. HITI can be a preferred strategy compared to “reduction and replacement” strategy because the complementary DNA (cDNA) is integrated into the host genome at its endogenous locus, ensuring long-term expression in dividing cells and endogenous gene regulation. Homology-directed repair (HDR) is another cellular DNA repair mechanism that is activated in response to DSB introduced by Cas9 and requires a homologous template like HITI. In the presence of a homologous sequence, HDR will integrate the sequence into the Cas9 cleavage site. Therapeutically, it can be designed to correct single-nucleotide or monoallelic mutations involved in IRDs. HDR-based CRISPR genome editing was shown to correct small 5 bp deletions or a point mutation in mouse models of IRDs [82,83]. However, HDR-based CRISPR-therapeutics may not be the most effective option for IRDs, as HDR is active primarily in mitotically active cells [72,79]. Since most cells of the retina are post-mitotic, HDR will be downregulated in the retina, and thus NHEJ-based CRISPR therapies are more suited for gene editing for IRDs [72,79].
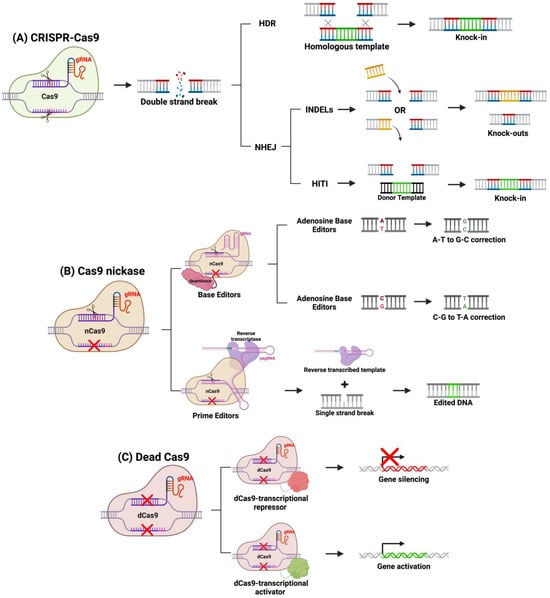
Figure 4.
CRISPR-based gene editing methods. (A) Using a fully functional system, CRISPR-Cas9 will target a specific sequence by the gRNA and will cause a DSB. Knock-ins can be achieved through HDR or HITI mechanisms, whereas knockouts are achieved through NHEJ indels. (B) Cas9 nickases can be linked to deaminases (base editors) or reverse transcriptase (prime editors) to precisely correct mutations. (C) Dead Cas9 can be linked to transcription factors to alter gene expression. Figure created with BioRender.com.
Newer developments in CRISPR therapeutics have modified the Cas9 protein by altering its cleaving function. Several of these strategies now exist, one of which is fusing catalytically inactivated or “dead” Cas9 (dCas9) to transcriptional regulators. Silencing the expression of this toxic products from mutated genes in IRD can be achieved by fusing a dCas9 to a transcriptional repressor and targeting this construct to the mutant allele with a specific gRNA [84]. As a result, only the dominantly expressed toxic allele is repressed, restoring normal rhodopsin signaling. Alternatively, dCas9 can be fused to transcriptional activators to upregulate silent genes to replace loss-of-function mutants [85]. Many genes have counterparts with homologous function, but in certain cell types, only one of such genes is expressed. For example, in rhodopsin-deficient mouse models of RP, there is a lack of rhodopsin-dependent signaling in rods [85,86]. In cones, M-opsin provides a homologous function to rhodopsin, but it is silenced in rods. Thus, by transfecting cells with a transcriptional activator of M-opsin fused to dCas9, M-opsin can be transactivated in rods, restoring their photon-sensitive signaling, which will improve retinal function and attenuate retinal degeneration [85]. dCas9 allows the targeting of transcriptional regulators to specific genomic sites, opening the possibility of therapeutically altering gene expression instead of modifying DNA sequence.
dCas9 are generated by inactivating Cas9 catalytic sites. Alternatively, Cas9 can be mutated to inactivate only one catalytic site generating Cas 9 nickases (Cas9n) that only cleave one DNA strand [87,88]. New editing tools, called DNA base editors (BEs) and prime editors (PEs), are composed of Cas9n fused to a deaminase or reverse transcriptase, respectively. The enzyme and Cas9n fusion protein can precisely correct single-point mutations at the site of a single strand break [87,88]. BEs are further categorized into cytidine deaminase (cytosine BE or CBE) or deoxyadenosine deaminase (adenosine BE or ABE) [87,88]. The CBE or ABE will be guided by a gRNA to a target sequence, where CBEs deaminate a C-G base pair to a U-G base pair, or ABEs deaminate a T-A base pair to an I-T base pairs. Cas9n will then cleave the non-deaminated strand, creating a single-strand break that will be repaired by host mechanisms and resolve the point mutation [88]. The overall nucleotide edit is a C to T transition by CBEs and an A to G transition by ABEs, and a more recent protocol has expanded this system to generate C to G transitions using C to G BEs (CGBEs) [87,88,89]. This strategy can be extremely promising for resolving mutations that underly several IRDs [90].
Unlike BEs, PEs possess the potential to repair both point mutations and transcribe short sequences that can be integrated through host DNA repair mechanisms [91]. PEs have a unique gRNA termed a prime editing guide RNA (pegRNA), which directs the PE to a specific locus and provides the template for reverse transcriptase. Once the nickase makes a single-strand break, the template transcribed by the reverse transcriptase is integrated into the host genome at the target locus through host repair mechanisms [91,92]. The utilization of reverse transcription allows for the correction of any transition or transverse mutations, enabling precise correction of IRD-causing point mutations with minimal off-target edits [79,93]. Furthermore, unlike BEs, PEs can also correct small indels. A limitation to PEs is their large size, which hampers its delivery via adeno-associated virus (AAV) vectors and therefore may limit the effectiveness of this strategy [91]. These CRISPR-Cas9 strategies demonstrate the promise of the Cas9 in treating IRDs. Long-term success of these strategies would rely on their safety and efficacy within the retina. Importantly, one study in Cas9 knock-in mice evaluated the long-term effects of Cas9 within the eye [94]. This study demonstrated the maintenance of the structural and functional components of both the retina and RPE, which is key pre-clinical evidence demonstrating the promise of Cas9-based strategies in long-term treatment of IRDs [94].
5. RNA Interference for IRDs
Our genome is constantly being transcribed into mRNA that is then translated to protein. mRNA is processed and spliced prior to translation, which serves as a second wave of regulation to ensure proper protein expression. The time between transcription and translation where mRNA is processed, modified, and spliced is a window of opportunity for RNA interference (RNAi) therapies to alter gene expression without having to act at the gene level.
While mRNA is being transcribed, our genome is simultaneously transcribing non-coding RNA (ncRNA), which are not translated into protein but serve as mRNA regulators. They play an important functional role in the retina, regulating factors in several cellular pathways that when dysregulated can lead to disease. As such, ncRNAs and their role in regulating RNAs can be central to disease pathophysiology and can be leveraged therapeutically. RNAi is a growing therapeutic field based on ncRNA, which include micro-RNA (miRNAs), small interfering RNA (siRNA), short hairpin RNA (shRNA), and antisense oligonucleotides (ASOs) (Table 2).

Table 2.
Summary of RNAi-based methods.
System Engineering
miRNAs are short nucleic acids, typically 20–25 base pairs, that are endogenously expressed in human genomes. They are transcribed in the nucleus as single primary miRNA and later cleaved by nuclear and cytosolic RNAses II, Drosha and Dicer [95]. The final product of this enzymatic activity is a double-stranded miRNA that binds argonaute (AGO) proteins and forms RNA-induced silencing complexes (RISC) [95]. Within RISCs, miRNAs serve as guides, homing this complex to an mRNA target complimentary to the miRNA. Once the miRNA anneals to its target, the RISC complex interferes with translation or activates mRNA degradation. This strategy can be potentiated, as one miRNA can target hundreds of endogenous mRNA genes, highlighting its therapeutic potential [95,99]. Therapeutic miRNA can be delivered to the retina in AAV vectors through subretinal injections to slow down retinal degeneration in IRD mouse models [97,98].
siRNAs are 21–23 bp, double-stranded RNAs that were first discovered in plants and later in mammalian cells, where they protect host cells from viral pathogens [96]. Therapeutically, siRNAs are activated intracellularly by cytoplasmic Dicer that cleaves long double-stranded pre-siRNA molecules [95]. Similar to miRNA, siRNAs are incorporated into RISC complexes and serve as a guide to target host mRNA for degradation [95]. Furthermore, a single siRNA can target multiple mRNAs, as they are recycled for multiple rounds of mRNA cleavage, similar to miRNA [95]. Early studies in IRDs had limited success due to a lack of stability and efficiency of siRNAs [100,101]. However, a recent study demonstrated that intravitreal injections of chemically modified siRNA termed tetra-valent siRNA (tetra-siRNA) in mouse and pig retinas could effectively and safely silence their target [101]. Despite the promise of such modifications, siRNAs studied to treat age-related macular degeneration have been shown to trigger immune responses, as non-internalized siRNA trigger extracellular receptors that initiate immune cascades causing retinal degeneration [102]. Both these studies demonstrate the promise and current limitations of siRNA-therapies in silencing toxic mutant expression in IRDs [101,102].
shRNAs are 19–22 base pair, double-stranded RNA connected by a 4–11 base pair hairpin loop that integrates into RISC complexes and silences or degrades specific mRNA, similar to miRNA and siRNA. However, they are unique as they are delivered in an exogenous DNA expression vector, typically an AAV vector, and not as an RNA effector molecule. This strategy allows for sustainable long-term expression by the host cells. This strategy is favorable, especially in the quiescent retina, as it eliminates the requirement of continuous administration, which is the case for miRNA and siRNA [95]. In ADRP, shRNA has been shown to be functionalized as a “knockdown and replace” strategy similar to CRISPR methods previously outlined [95,103].
ASOs are a class of single-stranded DNA or RNA molecules, typically 15–30 base pairs in length. Functionally, they interfere with mRNA translation by silencing, degrading, or altering splicing of their target. Silencing is achieved by designing an ASO that will interfere with ribosomal binding to the target mRNA. Degradation occurs when ASOs are developed to recruit RNases to degrade mRNA. Finally, splicing can be manipulated by designing ASOs that target defective splice sites to restore normal splicing. In clinical use, ASOs have been limited by nuclease degradation within cells, but modification of chemical modification of ASOs, forming phosphonodiamidite morpholino oligonucleotides (PMOs), can improve delivery by reducing degradation [104].
6. Vectors for Optogenetics and Targeted Gene Therapy
Gene therapies and optogenetic systems are most efficiently introduced to target cells through vectors, which maximize transduction efficiency, while ensuring that therapies with multiple constructs such as CRISPR, composed of Cas9 and gRNAs, transduce cells concurrently (Table 3). These vectors can be injected by different routes (i.e., subretinal, intravitreal, and suprachoroidal), allowing efficient transduction into photoreceptors and the RPE.

Table 3.
Pros and cons of vector-based gene therapy delivery methods.
The first vectors studied for gene therapy were adenoviruses (AdVs). AdVs, despite being very efficient transducers, caused significant inflammation, which has limited their use as therapeutic vectors [105]. AAVs have since replaced AdVs. They belong to the family of parvoviruses and are reliant on co-infection, primarily of AdVs, for replication. Their genome is quite simple, containing a single strand of DNA of about 4.8 kb, which is surrounded by a protein shell that will interact with carbohydrates on the surface of target cells to transfect cells [107].
AAVs have been the preferred vector for genetic editing and have become popular for several reasons. The first is that AAVs have a low integration rate into the host genome, eliminating the possibility of integration-related mutations that can cause loss-of-function mutations or activate oncogenes. Secondly, AAVs are very efficient at delivering constructs due to their high diffusion and transduction capacity, especially in quiescent retinal cells [108]. Furthermore, AAVs are non-pathogenic helper-dependent viruses that require co-infection to replicate, reducing the possibility of activation. Finally, various serotypes exist (AAV1-13), and combining these serotypes to create pseudotypes can further refine delivery [105]. For example, a common pseudotype is AAV2/8, which contains AAV2 genome in an AVV8 capsid [105]. Although they are the preferred vector in gene-editing studies in IRDs, there are some key limitations that have led to the investigation in other vectors. Of note, AAVs are immunogenic. However, most importantly in the context of IRD gene therapy, AAVs are limited by their small packaging size. With a genome of 4.8 kb, they can hardly accommodate Cas9. For CRISPR-based therapeutics, dual AAV systems are frequently used, which limits transduction efficiency, as both AAVs need to transduce the same cell for the desired effect [108]. This limited size is also insufficient to treat certain IRDs. Specifically, the most frequently mutated gene in Usher syndrome, MYO7A (7 kb) [110], and in Stargardt disease, ABCA4 (128 kb) [111], exceed the carrying capacity of AAVs. However, for most other IRDs, such as RP, LCA, and Cone-rod dystrophy, AAV or dual-AAV systems have been shown to effectively transduce with CRISPR and RNAi construct in preclinical models and clinical trials [81,112]. Conversely, AAVs have also shown great efficacy and safety for optogenetic system delivery [113,114].
Due to the limited packaging size of AAVs, lentiviruses (LVs) have been studied for gene therapy for diseases such as Stargardt disease and Usher syndrome that require larger packaging size. LVs can transduce large genes, as their carrying capacity is almost double that of AAVs, with a genome of around 8 kb [109]. LVs were first derived from HIV-1, but these had poor transduction of photoreceptors, and improved transduction has been demonstrated with equine infectious-anemia-virus-derived (EIAV) LVs [109]. LVs are limited by several factors. First, they are more likely to cause an immune response compared to AAVs [105]. More importantly, however, LVs naturally integrate into the host genome, which yields long-lasting expression, but also poses a safety concern due to insertional mutagenesis that can activate oncogenes [105]. Importantly, modifications can be made to LVs to reduce their integration capacity [105]. LVs are used in IRD studies, specifically for gene therapies for Stargardt disease and Usher syndrome and may be preferred to delivering large CRISPR constructs such as PEs [108,109]. Conversely, in optogenetics, lentiviral platforms, such as the OPTO-BLUE and Light-On systems, have been successfully used to induce light-controlled expression of reporter proteins [115,116].
Nanoparticles are the newest vectors that have shown immense promise in gene therapy delivery in other organs. Most notably, lipid-based cationic nanoparticles were used in COVID-19 vaccines [108]. Compared to viral vectors, they are easy to produce at scale in liquid form, and they can be chemically modified or incorporate different ligands to alter transduction efficiency based on cell target [109]. Nanoparticles also have a better safety profile as they are less immunogenic and possess less insertional mutagenesis risk [108]. Furthermore, given their lower invasive nature, they induce lesser levels of inflammation and limit tissue damage [117]. Finally, there are diverse subtypes of nanoparticles of varying sizes that can incorporate large constructs, a key limitation to AAVs [108,109]. Despite current limited use of these vectors in IRD clinical trials, they are expected to be the future vectors of gene therapy for IRDs. Some early studies have demonstrated some promising results. One study demonstrated that intravitreal injections of PEGylated-ECO nanoparticle carrying plasmid ABCA4-DNA was shown to be safe and effective in mouse models of Stargardt disease [118]. Furthermore, using upconversion nanoparticles, researchers were able to use near-infrared (NIR) light sources to activate optogenetic proteins [119,120,121]. Overall, the possibility to deliver nanoparticles through an intravitreal injection would be a clear advantage over viral vectors that require subretinal injections, a much more complicated procedure. However, it is to note that other non-viral delivery methods have been explored for the delivery of optogenetics constructs, such as the use of electroporation [122] and biopolymers (e.g., hydrogels) [123,124]. Given the clinical significance and importance of AAVs in drug delivery, we mainly focus on these delivery systems in the ensuing section.
7. Recent Advances in Optogenetics
Among the range of innovative approaches for vision restoration, optogenetic therapy stands out for its ability to confer light sensitivity onto remaining retinal neurons through the introduction of ectopic light-responsive proteins [125]. This technique can prove therapeutic to a wider range of patients as it can treat the disease independently to the underlying gene defect. A wide array of optogenetic actuators have been utilized paired to various promoters to explore their potential in restoring vision. This section aims to elucidate the extent to which meaningful improvement in vision can be achieved by modulating retinal ganglion cells (RGCs), bipolar cells (BCs), or photoreceptors with optogenetic actuators. Herein, we provide a summary of the recent periclinal studies published within the last five years (Table 4).

Table 4.
Summary of preclinical phase study advances for optogenetics in the treatment of inherited retinal diseases a.
7.1. Delivery of Optogenetic Actuators to Retinal Ganglion Cells
Despite the loss of photoreceptor cells in many cases of IRD, the remaining retinal layers, including RGCs, often remain intact and maintain communication with the brain through the optic nerve, providing an avenue for stimulation to potentially restore vision. Targeting RGCs could potentially treat patients, regardless of disease stage, with loss of all photoreceptors.
Recent advancements are aimed at developing a ChR with improved channel kinetics that is more photosensitive with the goal of restoring daylight vision [133]. This resulted in the creation of a modified ChR by replacing the amino acid sequences related to ion-conduction in mVChR1 with ChR2 counterparts. This chimeric opsin, ex3mV1CO, has shown greater sensitivity compared to mVChR1 [133]. Additionally, VEPs were recorded 17 months after transfection. Apart from ChR2, the application of other light-sensitive proteins with absorption spectra shifted towards the red end of the spectrum for optogenetic treatments of IRD has been explored. ReaCh or CrimsonR respond to longer wavelengths of light, such as red or NIR. The use of these red-shifted opsins holds promise in enhancing light sensitivity in patients by reaching deeper into the retina. With the goal of more specific opsin targeting to the membrane, Gauvain et al. optimized an AAV2.7m8-ChR-tDT vector [128]. Upon single IV injection of the ChrimsonR construct, primates were found to have an estimated restored visual acuity of 20/249 based on MEA recordings. In contrast to mice and other non-human mammals, primates possess a fovea similar to that of humans, rendering them optimal subjects for in vivo studies of vision restoration. McGregor et al. used IV delivery of ChrimsonR and the calcium sensor GCaMP6s, using a dual AAV2 vector and in vivo imaging to demonstrate optogenetic responses of RGCs in non-human primates [131]. They found that the ChrimsonR mediated optogenetic responses of inner retinal neurons, which persisted 14 months after IV injection. Furthermore, additional variants for ChR2, such as CoChR, were optimized in terms of light sensitivity and kinetics by an increase in the deactivation time [132].
A recent preclinical study utilized Chronos, a blue channel rhodopsin [129]. Chronos is reported to be tenfold more light-sensitive than ChR2, threefold more than ChrimsonR, with a longer excitation wavelength (with peak excitation at 500 nm) with fast on/off kinetics resulting in a substantial decrease in the risk of potential phototoxic effects [48]. By estimating the spatial resolution of the retina, a recent study on nonhuman primates concluded that targeting ganglion has the potential to yield visual acuity surpassing the threshold for legal blindness [130]. Ferrari et al. injected macaque retinas with an AAV2 encoding CatCh (human codon optimized ChR variant bearing L132C mutation [143]) under a RCG-specific promoter. They used a classical linear-non-linear (LN) model for the CatCh reactivated macaque retina to simulate the spiking response of the reactivated retina to an acuity test (the random E test) and performed Bayesian decoding at different time points following stimulation to predict quantitatively the best visual acuity one can expect in a patient. Based on the spatial resolution of the retinas, their model predicted that a patient should be able to discriminate letters corresponding to a visual acuity of 20/72. In contrast to epiretinal implants, which have been shown to activate distant ganglion cells and thus limit vision restoration, the reactivated GCs in the transduced primate retina were only sensitive to the stimulation of their dendritic field and soma.
Microbial opsins are limited as to their low light sensitivity or slow kinetics due to the lack of signal amplification. Additionally, these opsins lack adaptation to changes in natural light [134]. Conversely, type 2 opsins frequently attach covalently to 11-cis-retinal, initiating metabotropic signaling that indirectly affect ion channels upon light exposure. Furthermore, in the mammalian retina, it is expected that animal opsins would elicit a diminished immunogenic response [144]. A novel approach to confer light sensitivity involves the utilization of cone opsins, specifically the vertebrate middle wave opsin (MW-opsin) [134]. However, it is limited in its latency as its off-response time is >10 s [135]. In two sets of behavioral tests, rd1 mice expressing the MW-opsin displayed a pronounced inclination toward the dark compartment during a light avoidance task. Additionally, these mice demonstrated exploratory behavior, specifically an ability to differentiate between constant and pulsating light, discern moving lines of varying spatial frequencies, and investigate unfamiliar objects across diverse natural light environments. Electrophysiological assessments conducted in the V1 region revealed enhanced stimulus detection capabilities, with responses adapting to fluctuations in brightness. Operationally, this therapy can prove suitable in both indoor and outdoor light levels, circumventing the need for intensifying goggles. Visual restoration was similarly achieved with the introduction of a Gleobacter/human chimeric rhodopsin (coGHCR) in a murine model [139]. To study the functional outcome of different optogenetic targets, a direct comparison of AAV-mediated expression of CoChR in ON-BP cells versus RGCs was performed using a TKO mouse model (Opn4−/− Gnat1−/− Cnga3−/−) [138]. MEA recordings from the retina showed that the threshold light intensity to elicit a spike potential in RGCs was 1 log unit lower (2.0 × 1013 photons/cm2/s) compared to bipolar cells (2.4 × 1014 photons/cm2/s) [138]. Additionally, when comparing RGC targeting to BC targeting at equivalent light intensity, the RGCs targeted transfections demonstrated higher visual acuity [138]. Furthermore, significant pupil constriction was observed in TKO mice with RGC expression, not in those where BCs were targeted [138]. Thus, the authors concluded there is a higher efficacy of restored vision when targeting RGC compared to ON BC. However, in recent investigations, both animal-derived opsins (hOPN4) and microbial opsins (ReaChR) were targeted to bipolar cells and retinal ganglion cells (RGCs) to assess and contrast their response times and sensitivity. They concluded that bipolar targeted optogenetic tools exhibited higher light sensitivity and faster kinetics when compared to RGC targets [145]. Thus, there is no unanimous agreement regarding the favored cell type for targeting.
7.2. Delivery of Optogenetic Actuators to Bipolar Cells
As cell loss is mainly restricted to the outer retina in IRDs, BCs also remain mainly intact even in later stage disease [137]. Specifically targeting BCs is thought to better mimic the intrinsic processing features of the retinal circuitry, providing ON and OFF responses at the downstream RGCs. Unlike RGCs, BCs lack lateral extensions, resulting in a more focal activation pattern [135]. Additionally, when directly imparting light upon RGCs, one bypasses the parallel presynaptic processing of visual information such as luminance, directed movement, and contrast done by bipolar and amacrine networks. A BC-targeted approached would thus be an ideal method to preserve inner retinal processing [136]. However, downstream retinal neurons are affected by significant remodeling at end-stage RD compared to RGCs.
Studies have shown the potential of optogenetic actuator delivery to BCs. Injection of MCO1, a multi-characteristic, highly photosensitive opsin targeted onto ON BC of rd10 mice, resulted in stable expression up to 4 months after delivery [135]. Additionally, MCO1 has a broad spectral response, allowing for vision restoration in multiple color environments [146]. Significant improved visually guided behavioral outcomes showing light sensitivity were quantified through water maze and optomotor assays. Notably, this engineered opsin showed improved optomotor response at ambient light levels <10 mW*/mm2 [135]. Furthermore, Gaub et al. targeted retinal ON-bipolar cells of rd1 mice with a rhodopsin construct under control of the 4xgrm6 promoter. MEA recordings showed robust responses with an amplitude akin to WT across a broad spectrum of light strengths of treated retinas [147]. Firing rates were similar to wild-type retinas. Additionally, the results of behavioral tests showed restoration of innate light avoidance and temporal pattern recognition. The treated mice were able to distinguish between light and dark as well as between static and moving spatial patterns. Similarly, Kralik et al. transducted a modified GPCR construct comprising of the transmembrane region of melanopsin paired with the intracellular segment specific to ON-bipolar cells found in mGluR6 into the retina of rd1 mice. This engineered opsin restored cortical light responses. By activating the mGluR6 signaling cascade, these chimeric opsins were demonstrated to be 3–4 log units more sensitive than microbial alternatives [148].
7.3. Delivery of Optogenetic Actuators to Photoreceptors
Few studies have further demonstrated the clinical significance of optogenetic actuators in photoreceptor function modulation [69,141]. However, even following optogenetic therapy, cones are likely to continue on a degenerative path. Thus, this method is not optimal for patients with advanced disease where there is only a narrow window to target cones.
7.4. Clinical Trials
The first clinical trials of optogenetic treatment for IRDs are currently underway (Table 5). Various companies and research institutions are making notable progress in clinical trials.

Table 5.
Clinical trials of optogenetic therapies for vision restoration.
For instance, RetroSense Therapeutics, now under Abbvie, is investigating the use of ChR2 to target retinal ganglion cells (RGCs) through intravitreal delivery (NCT02556736). The CAG-vector-driven intravitreal delivery of ChR2 achieved its primary endpoint with no serious adverse events reported. A total of 9/14 of patients reported adverse events (64.29%), with the most common being increased intraocular pressure (3/14). GenSight Biologics is pursuing similar goals by targeting RGC using ChrimsonR, with encouraging results, whereby the introduction of rAAV2.7m8-CAG-ChrimsonR-tdTomato intravitreally paired with stimulating medical goggles showed partial visual function recovery in a patient with non-syndromic retinitis pigmentosa (NCT03326336) [153]. Bionic Sight LLC has reported success in restoring light perception and motion detection in all 12 of its patients with RP using Chronos to target RGCs and a neural impulse producing device (NCT04278131). Their findings were dose dependent, where the highest dose group has the most vision restored [129]. Zhongmou Therapeutics similarly targeted RGCs in the same patient population with intravitreal injection of the CatCh, a variant of channelrhodopsin ChR2-L132C (NCT06292650). There are early reports of improvement in functional visual abilities, minimum light sensitivity, and overall visual performance across various simulated lighting conditions [151]. Nanoscope Therapeutics Inc. is exploring different optogenetic tools targeting ON Bipolar Cells (ON BCs), NCT04919473, NCT05417126, and NCT04945772. The Phase IIa trial STARLIGHT assessed the impact of their innovative treatment, MCO-010 (a ChR2 mutant and Chrimson), on individuals diagnosed with Stargardt disease. Patients demonstrated clinical meaningful improvements in best-corrected visual acuity. No serious adverse events were observed [149]. The same vector was used in the dose-escalated open-label safety study on 11 patients with RP, all showing vision improvement [152]. Additionally, a Phase IIb trial characterized the optogenetic therapy in patients with advanced RP, achieving its primary and key secondary endpoints with statistical significance and no serious adverse events. Nanoscope intends to submit a Biologics License Application to the FDA in the second half of 2024 [150]. In a different vein, with prospects of targeting remaining cone photoreceptor cells, the retrospective EyeConic trial NCT05294978 at University Hospital, Basel, Switzerland, is attempting to estimate proportion of IRD patients with remaining cone photoreceptors using an OCT diagnostic test. The progress in clinical trials underscores the potential of optogenetic therapy to provide improved treatment those affected by inherited retinal degenerative diseases.
8. Recent Advances in Targeted Gene Therapy
In recent years, significant advancements have occurred in the field gene therapy, particularly in treating inherited retinal diseases [154]. Addressing IRDs is influenced by the disease’s inheritance pattern. For autosomal recessive IRDs, such as some RP, LCA, achromatopsia, Stargardt disease, cone-rod dystrophies, and syndromic IRDs, which are characterized by a loss of function in the relevant protein, the focus lies on gene augmentation. Meanwhile, in traditionally dominantly inherited conditions, such as 20% of RP, gene therapy strategies revolve around gene suppression, sometimes combined with gene augmentation. Herein, we summarize the primary gene therapy techniques presently being investigated for the advancement of therapeutic strategies aimed at managing IRDs through preclinical studies (Table 6).

Table 6.
Summary of preclinical phase study advances for targeted gene therapy in the treatment of inherited retinal diseases a.
8.1. Retinitis Pigmentosa
Several in vivo and in vitro studies are testing the capabilities of gene editor systems in the treatment of IRD. Su and colleagues studied base editing in rd10 mice, a model of autosomal recessive RP (Pde6b mutation identified by Chang et al., 2002 [176]). Base editing is limited to single-nucleotide conversions and can correct pathogenic substitutions without generation of DNA double-strand breaks (DSBs). This strategy can target most of the identified disease causing single-nucleotide variants. However, its use is limited as it can cause a significant amount of off-target bystander edits. Additionally, the dual AAV-approach can bypass the AAV cargo limitation. These dual vector strategies have been utilized for delivering large gene supplements effectively in preclinical trials. Subretinal introduction of a split dual AAV8-ABE vector restored PDE6B expression, preserved photoreceptor cells, and restored partial retinal function (−50% rescued photopic ERG amplitude) [155]. Retinal layers were still visible 6 months after injection. However, 8.84% of bystander editing was detected near the target locus [155], outlining potential limits for its translation in clinical trials. Alternative non-viral strategies of delivery of editing constructs also include combining CRISPR/Cas9 with electroporation to enable the delivery of naked DNA to the retina [157]. This technique potentially reduces off-target effects due to the removal of bacterial elements of the plasmid and the temporary expression of Cas9. Nonetheless it also presents certain limitations, such as safety concerns, transfection efficiency (greater cell death during electroporation), and achieving adequate retinal coverage.
In the treatment of autosomal dominant RP, Liu and colleagues harnessed allele-specific sgRNAs for T17M to target the mutant allele RHO-Ti7M in both 293 T and patient-derived iPSCs [158].
Examination of treated retinas revealed a lasting therapeutic impact (up to 11 months post-injection), including enhanced retinal function and preservation of photoreceptors in treated mice [158]. WGS analysis confirmed no bystander editing. Both in vitro and in vivo assessments indicated that SaCas9/17-Sg2 did not disrupt the WT RHO allele. A different strategy, a mutation-independent gene ablation and replacement system, has been used in the RHO-autosomal dominant (ad) RP humanized mouse model hRHOC110R/hRHOWT. Guided by two single-guide RNAs (sgRNAs) in a dual vector approach, approximately 60% of the target DNA underwent editing in the transduced area. Ablation and replacement methodology significantly improves photoreceptor survival and function in the humanized adRP mouse model for 12 months. In contrast, gene replacement therapy exhibited modest results in the same model [159].
In the same vein, “reduction and replacement” systems in the Rho-P23H knock-in mouse model of adRP have favorable results. Moreover, in ADRP human retinal explants and mutant pig models, researchers fused Cas9 to the transcriptional repressor domain, Krüppel-associated box (KRAB), and targeted this fusion protein to mutant Rho with a specific gRNA. This RhopCRISPRi transfection resulted in 74–84% decreased promoter activity, resulting in preservation of photoreceptor cell layer thickness [84]. Both the transcriptional activators NRL and NR2E3 are involved in rod photoreceptor cell differentiation and cell homeostasis. Their modulation is shown to be a viable therapeutic strategy for RP. In three different mouse models of retinal degeneration, AAV-mediated CRISPR-Cas9 targeting Nrl in post-mitotic photoreceptors improves rod survival and preserves cone function [163].
In another approach, Nolan and colleagues proposed using CRISPR therapeutic editing to enhance aerobic glycolysis in photoreceptors over mitochondrial oxidation, making them more resilient to stress [165]. PHD2 ablation by subretinal injections of AAV8:U6-gRNAs_PHD2 (i.e., prolyl hydroxylase domain 2), targeting rod specific aerobic glycolysis via PHD-HIF (i.e., hypoxia-inducible factor) reprogramming, rescued degeneration in both recessive and dominant RP mouse models without inducing toxicity. This glycolytic reprogramming strategy confers two main advantages over mutation-specific CRISPR-based homologous repair. It could offer cost-effective treatment for diseases caused by multiple mutations and treat both dividing and non-dividing cells.
8.2. Leber Congenital Amaurosis
Another group demonstrated the therapeutic potential of ABE conversion of a nonsense mutation in rd12 mice, which is a model for Rpe65-LCA [170]. Subretinal delivery via a dual-AAV serotype 9 vector appropriately induced an A to G transition in the RPE, and it was sustained 3 months after injection. In order to offset the high off-target editing rates resulting from exogenous vector administration, the group delivered a base editor and joint sgRNA ribonucleoprotein (RNP) complex for the correction of the same pathogenic variant in an identical in vivo model [166]. Both studies restored the RPE65 protein, and ERG determined the restoration resulted in significant functional recovery. However, the RNP-mediated complex approach demonstrated markedly greater editing efficiency with reduced indels compared to the classical ABE approach. Similar findings in other studies also suggest that base editing stands out as a more effective and applicable approach for disease treatment.
This time undertaking a prime editing approach, the same group recently employed a trans-RNA-splicing dual AAV strategy, intravitreally injecting of two AAV8 vectors encoding N-PE and C-PE, co-injected with an additional AAV delivering pegRNA and mCherry into the rd12 mice [93]. Subretinal administration achieved 23% delivery efficiency across the RPE, with an editing efficacy of 6.4% (range: 4.1–7.4%) [93]. Editing efficiency in solely the exposed regions was estimated to be 28%, without bystander editing, indels, or off-target effects in the rd12 mouse RPE. Moreover, ERG results suggest some rescue of the disease phenotype with improved visual function [93]. LV-mediated and AAV-mediated base editing yielded higher editing efficiencies (16 ± 3% and 11 ± 5%) than AAV- delivered PE2 (6.4 ± 3%). However, no observable off-targets or undesired indels were found in rd12 mice, whereas the ABE-mediated approach showed relevant rates of bystander edits (7.7 ± 5%). Another separate investigation conducted in rd12 mice explored the use of AAV-mediated delivery of a different prime editor to provide a method for precise correction of the Rpe65 mutation in eyes. She and colleagues delivered a dual AAV8-split PE3 (PE2 with an additional sgRNA) construct subretinally into the same mouse model [167]. Their approach yielded an editing rate of 11.4 ± 2.3% in RPE cells, with a maximal rate of 15.9%, partially restoring RPE65 expression. This intervention also enhanced photoreceptor function and viability, rescued rod and cone function, and slowed cone degeneration.
As a proof-of-concept study, Suh et al. previously reported restauration of visual function in a rd12 mouse model using a lentiviral vector-delivered base editing strategy [171]. In seeking to enhance the on-target correction rate and reduce off-target editing, they selected an evolved ABE variant more compatible with the A6 PAM sequence to deliver to the RPE mouse [169]. NG-ABE and sgRNA-A6 were packaged into a single lentivirus vector and injected subretinally into rd12 mice. The average frequency of functionally restored alleles was (27 ± 12%). Alternative packaging into dual AAV vectors, followed by ERG testing, showed slower rescue (7 weeks in AAV vs. 3 weeks in LV delivery). To evaluate the long-term survival of cone photoreceptors, rd12Gnat1−/−-cone-function-dominant mice were injected with LV-NG-ABE-A6. ERG assessment revealed partial restoration of M-cone (36%) and S-cone (30%) function in the treated mice when compared to Gnat−/− mice. Significant protection against cone loss was conferred as cones were still detected at 6 months. In another non-viral approach, empty virus-like particles (eVLPs) were used to deliver ABE RNPs to the RPE cells of rd12 mice, targeting the Rpe65 gene by subretinal injection (ABE7.10-NG-eVLPs), achieving 12% correction efficiency, with no significant bystander editing (Banskota et al., 2022) [168].
CRISPR–Cas9 nuclease and antisense oligonucleotides have been used to bypass one of the most common deep-intronic IRD variants splicing defect-inducing mutation in CEP290, another representative LCA-causing gene, in mice, primates, and human patients [177]. A different genome-editing method, termed “EDIT-101”, in which a pair of highly active and CEP290-specific saCas9 gRNAs were delivered on a human CEP290 IVS26 knock-in mouse model and in somatic primate cells, achieved a clinically efficacious rate of productive editing [172].
8.3. Stargardt Disease
With regards to correcting mutations in Stargardt disease, Wimmer and colleagues introduced split PE2 plasmids into HEK293 cells containing an ABCA4 mutation to evaluate editing efficiency in vitro. Their study utilized a bioluminescence resonance energy transfer (BRAT)-based editing sensor as a measurement tool and observed corrections of up to 92% in the ABCA4 gene [178]. In search of an optimization strategy to deliver large transgenes through AAV-based gene therapy, a similar dual AAV vector construct coined “REVeRT” by Riedmayr and colleagues was tested on a small cohort of mice, successfully reconstituting the ABCA4 gene [173]. Similarly, McClements and colleagues administered an ABCA4 overlapping dual vector system into Abca4−/− mice [174]. Analysis via Western blotting revealed low levels of ABCA4 expression levels throughout the neural retina of Abca4−/− mice. However, treatment resulted in a decrease in bisretinoid accumulation and fundus autofluorescence levels, suggesting therapeutic potential.
8.4. Clinical Trials
As previously mentioned, AAV vectors face a limitation in their payload capacity, which restricts their application in gene therapies involving larger genes. However, despite this constraint, the notable success of Luxturna studies in treating Leber congenital amaurosis, followed by clinical trials addressing conditions like RP, Leber hereditary optic neuropathy, choroideremia, Stargardt disease, and achromatopsia, all employing various serotypes with strong tropism for retinal pigment epithelium, photoreceptors, and retinal ganglion cells, has firmly established AAV vectors as the preferred choice for ocular gene therapy.
A cumulative total of 2081 patients were enrolled across all the studies, as outlined in Table 7. The majority of these trials (52 out of 63) were classified as early phase (I/II) clinical trials, with 43 prioritizing safety as the primary outcome, while 20 emphasized treatment efficacy. Among the registered clinical trials, the targeted gene or protein varied across the studies: RPE65 was the focus in 10 trials and post-market surveillance studies, RPGR in 10 studies, ND4 in 8 studies, CHM/REP1 in 8 studies, CEP450 in 5 studies, RHO in 4 studies, USH2A in 3 studies, CNGA3 in 2 studies, CYP4V2 in 2 studies, and there was one trial each for LCA5 and RLBP1.

Table 7.
Clinical trials of targeted gene therapy for vision restoration a.
9. Future Perspectives
Personalized medicine is centered on the notion that every disease, although overlapping in pathology, will be experienced uniquely by a patient based on genetic and molecular presentation. To a further extent, the treatment a patient receives can be tailored to their unique genetic and cellular background. Such an approach requires precise diagnosis, which in the care for IRDs entails identifying disease causing mutations through sequencing. In the context of gene editing or RNAi, the identification of a specific mutation will decide the optimal treatment approach. For example, a point mutation would be best addressed with a BE or PE, whereas a dominant negative mutation causing toxic protein expression would best by a “knockdown and replace” strategy, based on RNAi or CRISPR methods. Furthermore, genome size greatly influences vector choice; AAV vectors are limited to genomes lesser than 5 kb, therefore limiting its use in many IRDs. Conversely, in cases where spatial genome delivery is unachievable, optogenetics is a great alternative. Illumination provides an activation of opsins within a specific tissue area, therefore leveraging the need for tissue-specific deliveries.
Future perspectives regarding IRD treatment encompass protocols to produce retinal organoids, as they can more accurately replicate human tissue composition [179,180]. Retinal organoids do not only serve as a model for drug development but have promising applications in clinical practice. Personalized, patient-derived retinal organoids could be grown in a laboratory setting, and these patient-specific models could then be screened with multiple therapeutics. This would determine a patient’s response to a specific treatment to facilitate the optimal treatment approach [108]. The possibility to test a model that more closely resembles or is identical to human tissue will have great use in preclinical gene therapy development and future clinical practice.
Furthermore, with the increasing landscape of artificial intelligence (AI) and machine-learning technologies, promising avenues are being explored in terms of IRD screening, diagnosis, and management [181]. Ideally, AI-based technologies will be a clinical tool that allows for the earlier detection of IRDs by accelerating diagnosis through the identification of specific disease markers that can be missed with current clinical practices. As AI continues to be studied in ophthalmology, it will gradually be integrated into clinical practice, serving as a useful tool in the diagnosis and care of IRDs.
Finally, the landscape of optogenetics and gene therapy applications is greater; these tools have shown promising results in the treatment of non-retinal diseases as well such as glaucoma [182,183,184].
10. Conclusions
IRDs compromise a spectrum of clinical and genetic disorders that manifest at multiple ages, exhibiting varying severity levels and involving mutations in several genes [108]. Genetic testing for IRD-associated mutations has become standard in today’s clinical workup when IRDs are suspected, with panels capable of testing for approximately 271 known IRD-associated genes [185,186]. This is a feat that has largely been attributed to developments in next-generation sequencing but is still limited by the cost and availability of these diagnostic tests [185]. Despite access to extensive diagnostic tests, the lack of approved gene therapies can be attributed to the difficulty of translating preclinical studies into clinical therapies. This has proven to be a significant hurdle, with the most promising CRISPR and RNAi preclinical studies in model organisms failing to translate into clinical treatments. The limitation of preclinical models has led to the development of human-derived organoids, which unlike traditional cell culture, are three-dimensional multicellular constructs generated from induced-pluripotent stem cells that attempt to recapitulate human organs at a much smaller scale. Overall, numerous factors are to be considered when choosing the optimal treatment plan, based on disease characteristics, patient needs, technical resources within the clinical facility, and clinician expertise. Furthermore, with the scarce long-term safety data, use of targeted therapies should be limited to specific patient pools, based on current clinical trials.
Author Contributions
Conceptualization, M.K., S.K.A. and C.X.Q.; writing—original draft preparation, M.K., N.T. and A.A.; writing—review and editing, M.K., N.T. and C.X.Q.; supervision, S.K.A. and C.X.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sahel, J.-A.; Marazova, K.; Audo, I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb. Perspect. Med. 2014, 5, a017111. [Google Scholar] [CrossRef]
- Hanany, M.; Rivolta, C.; Sharon, D. Worldwide carrier frequency and genetic prevalence of autosomal recessive inherited retinal diseases. Proc. Natl. Acad. Sci. USA 2020, 117, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Cheung, S.; Fasso-Opie, A.; Galvin, O.; Moniz, L.S.; Earle, D.; Durham, T.; Menzo, J.; Li, N.; Duffy, S.; et al. The Impact of Inherited Retinal Diseases in the United States of America (US) and Canada from a Cost-of-Illness Perspective. Clin. Ophthalmol. 2021, 15, 2855–2866. [Google Scholar] [CrossRef] [PubMed]
- Cremers, F.P.M.; Boon, C.J.F.; Bujakowska, K.; Zeitz, C. Special Issue Introduction: Inherited Retinal Disease: Novel Candidate Genes, Genotype-Phenotype Correlations, and Inheritance Models. Genes. 2018, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Sabbaghi, H.; Madani, S.; Ahmadieh, H.; Daftarian, N.; Suri, F.; Khorrami, F.; Saviz, P.; Shahriari, M.H.; Motevasseli, T.; Fekri, S.; et al. A health terminological system for inherited retinal diseases: Content coverage evaluation and a proposed classification. PLoS ONE 2023, 18, e0281858. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yosef, T. Inherited Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 13467. [Google Scholar] [CrossRef] [PubMed]
- Karali, M.; Testa, F.; Di Iorio, V.; Torella, A.; Zeuli, R.; Scarpato, M.; Romano, F.; Onore, M.E.; Pizzo, M.; Melillo, P.; et al. Genetic epidemiology of inherited retinal diseases in a large patient cohort followed at a single center in Italy. Sci. Rep. 2022, 12, 20815. [Google Scholar] [CrossRef]
- Chen, T.-C.; Huang, D.-S.; Lin, C.-W.; Yang, C.-H.; Yang, C.-M.; Wang, V.Y.; Lin, J.-W.; Luo, A.C.; Hu, F.-R.; Chen, P.-L. Genetic characteristics and epidemiology of inherited retinal degeneration in Taiwan. NPJ Genom. Med. 2021, 6, 16. [Google Scholar] [CrossRef]
- El Shamieh, S.; Maltese, P.E. Editorial: The genetics of inherited retinal diseases in understudied ethnic groups: Novel associations, challenges, and perspectives. Front. Genet. 2022, 13, 990782. [Google Scholar]
- Lin, S.; Vermeirsch, S.; Pontikos, N.; Martin-Gutierrez, M.P.; Daich Varela, M.; Malka, S.; Schiff, E.; Knight, H.; Wright, G.; Jurkute, N.; et al. Spectrum of Genetic Variants in the Most Common Genes Causing Inherited Retinal Disease in a Large Molecularly Characterized United Kingdom Cohort. Ophthalmol. Retin. 2024, 8, 699–709. [Google Scholar] [CrossRef]
- Schlottmann, P.G.; Luna, J.D.; Labat, N.; Yadarola, M.B.; Bainttein, S.; Esposito, E.; Ibañez, A.; Barbaro, E.I.; Álvarez Mendiara, A.; Picotti, C.P.; et al. Nationwide genetic analysis of more than 600 families with inherited eye diseases in Argentina. NPJ Genom. Med. 2023, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Pontikos, N.; Arno, G.; Jurkute, N.; Schiff, E.; Ba-Abbad, R.; Malka, S.; Gimenez, A.; Georgiou, M.; Wright, G.; Armengol, M.; et al. Genetic Basis of Inherited Retinal Disease in a Molecularly Characterized Cohort of More than 3000 Families from the United Kingdom. Ophthalmology 2020, 127, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Nuzbrokh, Y.; Ragi, S.D.; Tsang, S.H. Gene therapy for inherited retinal diseases. Ann. Transl. Med. 2021, 9, 1278. [Google Scholar] [CrossRef] [PubMed]
- Leroy, B.P.; Fischer, M.D.; Flannery, J.G.; MacLaren, R.E.; Dalkara, D.; Scholl, H.P.N.; Chung, D.C.; Spera, C.; Viriato, D.; Banhazi, J. Gene Therapy for Inherited Retinal Disease: Long-Term Durability of Effect. Ophthalmic Res. 2022, 66, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Arrigo, A.; Aragona, E.; Manitto, M.P.; Saladino, A.; Bandello, F.; Battaglia Parodi, M. Gene Therapy in Inherited Retinal Diseases: An Update on Current State of the Art. Front. Med. 2021, 8, 750586. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.R.; Moore, A.T. Optogenetic approaches to therapy for inherited retinal degenerations. J. Physiol. 2022, 600, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- McClements, M.E.; Staurenghi, F.; MacLaren, R.E.; Cehajic-Kapetanovic, J. Optogenetic Gene Therapy for the Degenerate Retina: Recent Advances. Front. Neurosci. 2020, 14, 570909. [Google Scholar] [CrossRef] [PubMed]
- Murro, V.; Banfi, S.; Testa, F.; Iarossi, G.; Falsini, B.; Sodi, A.; Signorini, S.; Iolascon, A.; Russo, R.; Mucciolo, D.P.; et al. A multidisciplinary approach to inherited retinal dystrophies from diagnosis to initial care: A narrative review with inputs from clinical practice. Orphanet J. Rare Dis. 2023, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Geng, Z.; Khattak, S.; Ji, X.; Wu, D.; Dang, Y. Role of Oxidative Stress in Retinal Disease and the Early Intervention Strategies: A Review. Oxid. Med. Cell Longev. 2022, 2022, 7836828. [Google Scholar] [CrossRef]
- Ren, X.; Léveillard, T. Modulating antioxidant systems as a therapeutic approach to retinal degeneration. Redox Biol. 2022, 57, 102510. [Google Scholar] [CrossRef]
- García-Ayuso, D.; Di Pierdomenico, J.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Retinal Ganglion Cell Death as a Late Remodeling Effect of Photoreceptor Degeneration. Int. J. Mol. Sci. 2019, 20, 4649. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Rogers, B.S.; Campochiaro, P.A. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell Physiol. 2007, 213, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Rogers, B.S.; Lu, L.; Campochiaro, P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2006, 103, 11300–11305. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Rubart, M.; Zhu, W. Optogenetics: Background, Methodological Advances and Potential Applications for Cardiovascular Research and Medicine. Front. Bioeng. Biotechnol. 2019, 7, 466. [Google Scholar] [CrossRef] [PubMed]
- Grassmeyer, J.J.; Munakomi, S. Photopic Vision. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Perkins, B.D.; Fadool, J.M. Photoreceptor structure and development analyses using GFP transgenes. Methods Cell Biol. 2010, 100, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Pepe, I.M. Recent Advances in Our Understanding of Rhodopsin and Phototransduction. Prog. Retin. Eye Res. 2001, 20, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Terakita, A. The opsins. Genome Biol. 2005, 6, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fang, H.; Liu, D.; Zhang, Y.; Adu-Amankwaah, J.; Yuan, J.; Tan, R.; Zhu, J. Applications and challenges of rhodopsin-based optogenetics in biomedicine. Front. Neurosci. 2022, 16, 966772. [Google Scholar] [CrossRef] [PubMed]
- Josselyn, S.A. The past, present and future of light-gated ion channels and optogenetics. elife 2018, 7, e42367. [Google Scholar] [CrossRef]
- Zhao, Z.; Fairchild, P.W. Dependence of Light Transmission through Human Skin on Incident Beam Diameter at Different Wavelengths; SPIE: Bellingham, WA, USA, 1998; Volume 3254, pp. 354–360. [Google Scholar]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Chernov, K.G.; Redchuk, T.A.; Omelina, E.S.; Verkhusha, V.V. Near-infrared fluorescent proteins, biosensors, and optogenetic tools engineered from phytochromes. Chem. Rev. 2017, 117, 6423–6446. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K.; Hegemann, P. The form and function of channelrhodopsin. Science 2017, 357, eaan5544. [Google Scholar] [CrossRef]
- Lagali, P.S.; Balya, D.; Awatramani, G.B.; Münch, T.A.; Kim, D.S.; Busskamp, V.; Cepko, C.L.; Roska, B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat. Neurosci. 2008, 11, 667–675. [Google Scholar] [CrossRef]
- Bi, A.; Cui, J.; Ma, Y.-P.; Olshevskaya, E.; Pu, M.; Dizhoor, A.M.; Pan, Z.-H. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 2006, 50, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Rindner, D.J.; Lur, G. Practical considerations in an era of multicolor optogenetics. Front. Cell Neurosci. 2023, 17, 1160245. [Google Scholar] [CrossRef]
- Oesterhelt, D.; Stoeckenius, W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat. New Biol. 1971, 233, 149–152. [Google Scholar] [CrossRef]
- Duebel, J.; Marazova, K.; Sahel, J.-A. Optogenetics. Curr. Opin. Ophthalmol. 2015, 26, 226–232. [Google Scholar] [CrossRef]
- Gradinaru, V.; Thompson, K.R.; Deisseroth, K. eNpHR: A Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008, 36, 129–139. [Google Scholar] [CrossRef]
- Gradinaru, V.; Mogri, M.; Thompson, K.R.; Henderson, J.M.; Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science 2009, 324, 354–359. [Google Scholar] [CrossRef]
- Yoshida, K.; Tsunoda, S.P.; Brown, L.S.; Kandori, H. A unique choanoflagellate enzyme rhodopsin exhibits light-dependent cyclic nucleotide phosphodiesterase activity. J. Biol. Chem. 2017, 292, 7531–7541. [Google Scholar] [CrossRef]
- Avelar, G.M.; Schumacher, R.I.; Zaini, P.A.; Leonard, G.; Richards, T.A.; Gomes, S.L. A rhodopsin-guanylyl cyclase gene fusion functions in visual perception in a fungus. Curr. Biol. 2014, 24, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Luck, M.; Mathes, T.; Bruun, S.; Fudim, R.; Hagedorn, R.; Nguyen, T.M.T.; Kateriya, S.; Kennis, J.T.; Hildebrandt, P.; Hegemann, P. A photochromic histidine kinase rhodopsin (HKR1) that is bimodally switched by ultraviolet and blue light. J. Biol. Chem. 2012, 287, 40083–40090. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Tsunoda, S.P.; Hibi, M.; Kandori, H. Molecular Properties of New Enzyme Rhodopsins with Phosphodiesterase Activity. ACS Omega 2020, 5, 10602–10609. [Google Scholar] [CrossRef] [PubMed]
- Vierock, J.; Rodriguez-Rozada, S.; Dieter, A.; Pieper, F.; Sims, R.; Tenedini, F.; Bergs, A.C.; Bendifallah, I.; Zhou, F.; Zeitzschel, N. BiPOLES is an optogenetic tool developed for bidirectional dual-color control of neurons. Nat. Commun. 2021, 12, 4527. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Knutsen, P.M.; Muller, A.; Kleinfeld, D.; Tsien, R.Y. ReaChR: A red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 2013, 16, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.; et al. Independent optical excitation of distinct neural populations. Nat. Methods 2014, 11, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Vierock, J.; Oishi, S.; Rodriguez-Rozada, S.; Taniguchi, R.; Yamashita, K.; Wiegert, J.S.; Nishizawa, T.; Hegemann, P.; Nureki, O. Crystal structure of the red light-activated channelrhodopsin Chrimson. Nat. Commun. 2018, 9, 3949. [Google Scholar] [CrossRef] [PubMed]
- Marshel, J.H.; Kim, Y.S.; Machado, T.A.; Quirin, S.; Benson, B.; Kadmon, J.; Raja, C.; Chibukhchyan, A.; Ramakrishnan, C.; Inoue, M. Cortical layer–specific critical dynamics triggering perception. Science 2019, 365, eaaw5202. [Google Scholar] [CrossRef] [PubMed]
- Kishi, K.E.; Kim, Y.S.; Fukuda, M.; Inoue, M.; Kusakizako, T.; Wang, P.Y.; Ramakrishnan, C.; Byrne, E.F.; Thadhani, E.; Paggi, J.M. Structural basis for channel conduction in the pump-like channelrhodopsin ChRmine. Cell 2022, 185, 672–689. [Google Scholar] [CrossRef]
- Christoffel, D.J.; Walsh, J.J.; Heifets, B.D.; Hoerbelt, P.; Neuner, S.; Sun, G.; Ravikumar, V.K.; Wu, H.; Halpern, C.H.; Malenka, R.C. Input-specific modulation of murine nucleus accumbens differentially regulates hedonic feeding. Nat. Commun. 2021, 12, 2135. [Google Scholar] [CrossRef]
- Bauer, J.; Weiler, S.; Fernholz, M.H.; Laubender, D.; Scheuss, V.; Hübener, M.; Bonhoeffer, T.; Rose, T. Limited functional convergence of eye-specific inputs in the retinogeniculate pathway of the mouse. Neuron 2021, 109, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, M.; van Bommel, B.; Wang, R.; Mikhaylova, M.; Wiegert, J.S.; Oertner, T.G.; Gee, C.E. Spike-timing-dependent plasticity rewards synchrony rather than causality. Cereb. Cortex 2023, 33, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Hooks, B.M.; Lin, J.Y.; Guo, C.; Svoboda, K. Dual-channel circuit mapping reveals sensorimotor convergence in the primary motor cortex. J. Neurosci. 2015, 35, 4418–4426. [Google Scholar] [CrossRef] [PubMed]
- Rindner, D.J.; Proddutur, A.; Lur, G. Cell-type-specific integration of feedforward and feedback synaptic inputs in the posterior parietal cortex. Neuron 2022, 110, 3760–3773. [Google Scholar] [CrossRef] [PubMed]
- Joffe, M.E.; Maksymetz, J.; Luschinger, J.R.; Dogra, S.; Ferranti, A.S.; Luessen, D.J.; Gallinger, I.M.; Xiang, Z.; Branthwaite, H.; Melugin, P.R. Acute restraint stress redirects prefrontal cortex circuit function through mGlu5 receptor plasticity on somatostatin-expressing interneurons. Neuron 2022, 110, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Yu, J.; Huang, X.; Sesack, S.R.; Huang, Y.H.; Schlüter, O.M.; Cao, J.-L.; Dong, Y. Cortical and thalamic interaction with amygdala-to-accumbens synapses. J. Neurosci. 2020, 40, 7119–7132. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.A.; Xie, C.; Chaichim, C.; Nguyen, J.H.; McClusky, H.E.; Killcross, S.; Power, J.M.; McNally, G.P. Complementary roles for ventral pallidum cell types and their projections in relapse. J. Neurosci. 2020, 40, 880–893. [Google Scholar] [CrossRef]
- Birdsong, W.T.; Jongbloets, B.C.; Engeln, K.A.; Wang, D.; Scherrer, G.; Mao, T. Synapse-specific opioid modulation of thalamo-cortico-striatal circuits. elife 2019, 8, e45146. [Google Scholar] [CrossRef]
- Chiu, C.Q.; Martenson, J.S.; Yamazaki, M.; Natsume, R.; Sakimura, K.; Tomita, S.; Tavalin, S.J.; Higley, M.J. Input-specific NMDAR-dependent potentiation of dendritic GABAergic inhibition. Neuron 2018, 97, 368–377. [Google Scholar] [CrossRef]
- Husson, S.J.; Liewald, J.F.; Schultheis, C.; Stirman, J.N.; Lu, H.; Gottschalk, A. Microbial light-activatable proton pumps as neuronal inhibitors to functionally dissect neuronal networks in C. elegans. PLoS ONE 2012, 7, e40937. [Google Scholar] [CrossRef]
- Chow, B.Y.; Han, X.; Dobry, A.S.; Qian, X.; Chuong, A.S.; Li, M.; Henninger, M.A.; Belfort, G.M.; Lin, Y.; Monahan, P.E. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 2010, 463, 98–102. [Google Scholar] [CrossRef]
- Inoue, K.; Ono, H.; Abe-Yoshizumi, R.; Yoshizawa, S.; Ito, H.; Kogure, K.; Kandori, H. A light-driven sodium ion pump in marine bacteria. Nat. Commun. 2013, 4, 1678. [Google Scholar] [CrossRef]
- Hososhima, S.; Kandori, H.; Tsunoda, S.P. Ion transport activity and optogenetics capability of light-driven Na+-pump KR2. PLoS ONE 2021, 16, e0256728. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.-P.; Brauner, M.; Liewald, J.F.; Kay, K.; Watzke, N.; Wood, P.G.; Bamberg, E.; Nagel, G.; Gottschalk, A. Multimodal fast optical interrogation of neural circuitry. Nature 2007, 446, 633–639. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wu, J.-L.; Hu, N.-Y.; Zhuang, J.-P.; Li, W.-P.; Zhang, S.-R.; Li, X.-W.; Yang, J.-M.; Gao, T.-M. Distinct projections from the infralimbic cortex exert opposing effects in modulating anxiety and fear. J. Clin. Investig. 2021, 131, e145692. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Boyden, E.S. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE 2007, 2, e299. [Google Scholar] [CrossRef] [PubMed]
- Busskamp, V.; Duebel, J.; Balya, D.; Fradot, M.; Viney, T.J.; Siegert, S.; Groner, A.C.; Cabuy, E.; Forster, V.; Seeliger, M.; et al. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 2010, 329, 413–417. [Google Scholar] [CrossRef]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013, 14, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-T.; Leng, Q.; Mixson, A.J. Zinc Finger Nucleases: Tailor-made for Gene Therapy. Drugs Future 2012, 37, 183–196. [Google Scholar] [CrossRef]
- Pulman, J.; Sahel, J.-A.; Dalkara, D. New Editing Tools for Gene Therapy in Inherited Retinal Dystrophies. CRISPR J. 2022, 5, 377–388. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR–Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. CRISPR/Cas gene therapy. J. Cell. Physiol. 2021, 236, 2459–2481. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.B.T.; Platt, R.J.; Zhang, F. Therapeutic genome editing: Prospects and challenges. Nat. Med. 2015, 21, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.L.; Du, S.W.; Palczewski, K. Genome editing, a superior therapy for inherited retinal diseases. Vis. Res. 2023, 206, 108192. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Li, J.; Tang, X.; Yu, W.; Zhao, M. CRISPR/SaCas9-based gene editing rescues photoreceptor degeneration throughout a rhodopsin-associated autosomal dominant retinitis pigmentosa mouse model. Exp. Biol. Med. 2023, 248, 1818–1828. [Google Scholar] [CrossRef]
- Suzuki, K.; Tsunekawa, Y.; Hernandez-Benitez, R.; Wu, J.; Zhu, J.; Kim, E.J.; Hatanaka, F.; Yamamoto, M.; Araoka, T.; Li, Z.; et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 2016, 540, 144–149. [Google Scholar] [CrossRef]
- Hu, S.; Du, J.; Chen, N.; Jia, R.; Zhang, J.; Liu, X.; Yang, L. In Vivo CRISPR/Cas9-Mediated Genome Editing Mitigates Photoreceptor Degeneration in a Mouse Model of X-Linked Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2020, 61, 31. [Google Scholar] [CrossRef]
- Da Costa, B.L.; Li, Y.; Levi, S.R.; Tsang, S.H.; Quinn, P.M.J. Generation of CRB1 RP Patient-Derived iPSCs and a CRISPR/Cas9-Mediated Homology-Directed Repair Strategy for the CRB1 c.2480G>T Mutation. In Retinal Degenerative Diseases XIX; Ash, J.D., Pierce, E., Anderson, R.E., Bowes Rickman, C., Hollyfield, J.G., Grimm, C., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2023; Volume 1415, pp. 571–576. Available online: https://link.springer.com/10.1007/978-3-031-27681-1_83 (accessed on 15 May 2024).
- Burnight, E.R.; Wiley, L.A.; Mullin, N.K.; Adur, M.K.; Lang, M.J.; Cranston, C.M.; Jiao, C.; Russell, S.R.; Sohn, E.H.; Han, I.C.; et al. CRISPRi-Mediated Treatment of Dominant Rhodopsin-Associated Retinitis Pigmentosa. CRISPR J. 2023, 6, 502–513. [Google Scholar] [CrossRef]
- Böhm, S.; Splith, V.; Riedmayr, L.M.; Rötzer, R.D.; Gasparoni, G.; Nordström, K.J.V.; Wagner, J.E.; Hinrichsmeyer, K.S.; Walter, J.; Wahl-Schott, C.; et al. A gene therapy for inherited blindness using dCas9-VPR–mediated transcriptional activation. Sci. Adv. 2020, 6, eaba5614. [Google Scholar] [CrossRef]
- Riedmayr, L.M.; Hinrichsmeyer, K.S.; Karguth, N.; Böhm, S.; Splith, V.; Michalakis, S.; Becirovic, E. dCas9-VPR-mediated transcriptional activation of functionally equivalent genes for gene therapy. Nat. Protoc. 2022, 17, 781–818. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Kurt, I.C.; Zhou, R.; Iyer, S.; Garcia, S.P.; Miller, B.R.; Langner, L.M.; Grünewald, J.; Joung, J.K. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat. Biotechnol. 2021, 39, 41–46. [Google Scholar] [CrossRef]
- Kabra, M.; Shahi, P.K.; Wang, Y.; Sinha, D.; Spillane, A.; Newby, G.A.; Saxena, S.; Tong, Y.; Chang, Y.; Abdeen, A.A.; et al. Nonviral base editing of KCNJ13 mutation preserves vision in a model of inherited retinal channelopathy. J. Clin. Investig. 2023, 133, e171356. [Google Scholar] [CrossRef]
- Costa, B.L.D.; Levi, S.R.; Eulau, E.; Tsai, Y.-T.; Quinn, P.M.J. Prime Editing for Inherited Retinal Diseases. Front. Genome Ed. 2021, 3, 775330. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Jo, D.H.; Cho, C.S.; Shin, J.H.; Seo, J.H.; Yu, G.; Gopalappa, R.; Kim, D.; Cho, S.-R.; Kim, J.H.; et al. Application of prime editing to the correction of mutations and phenotypes in adult mice with liver and eye diseases. Nat. Biomed. Eng. 2021, 6, 181–194. [Google Scholar] [CrossRef]
- Mohan, K.; Dubey, S.K.; Jung, K.; Dubey, R.; Wang, Q.J.; Prajapati, S.; Roney, J.; Abney, J.; Kleinman, M.E. Long-Term Evaluation of Retinal Morphology and Function in Rosa26-Cas9 Knock-In Mice. Int. J. Mol. Sci. 2023, 24, 5186. [Google Scholar] [CrossRef]
- Gemayel, M.C.; Bhatwadekar, A.D.; Ciulla, T. RNA therapeutics for retinal diseases. Expert Opin. Biol. Ther. 2021, 21, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. 2017, 13, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Carrella, S.; Di Guida, M.; Brillante, S.; Piccolo, D.; Ciampi, L.; Guadagnino, I.; Garcia Piqueras, J.; Pizzo, M.; Marrocco, E.; Molinari, M.; et al. miR-181a/b downregulation: A mutation-independent therapeutic approach for inherited retinal diseases. EMBO Mol. Med. 2022, 14, e15941. [Google Scholar] [CrossRef] [PubMed]
- Anasagasti, A.; Lara-López, A.; Milla-Navarro, S.; Escudero-Arrarás, L.; Rodríguez-Hidalgo, M.; Zabaleta, N.; González Aseguinolaza, G.; De La Villa, P.; Ruiz-Ederra, J. Inhibition of MicroRNA 6937 Delays Photoreceptor and Vision Loss in a Mouse Model of Retinitis Pigmentosa. Pharmaceutics 2020, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Caiazzi, J.; Biscans, A.; Alterman, J.F.; Echeverria, D.; McHugh, N.; Hassler, M.; Jolly, S.; Giguere, D.; Cipi, J.; et al. Single intravitreal administration of a tetravalent siRNA exhibits robust and efficient gene silencing in mouse and pig photoreceptors. Mol. Ther.-Nucleic Acids 2024, 35, 102088. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, M.E.; Kaneko, H.; Cho, W.G.; Dridi, S.; Fowler, B.J.; Blandford, A.D.; Albuquerque, R.J.; Hirano, Y.; Terasaki, H.; Kondo, M.; et al. Short-interfering RNAs Induce Retinal Degeneration via TLR3 and IRF3. Mol. Ther. 2012, 20, 101–108. [Google Scholar] [CrossRef]
- Cideciyan, A.V.; Sudharsan, R.; Dufour, V.L.; Massengill, M.T.; Iwabe, S.; Swider, M.; Lisi, B.; Sumaroka, A.; Marinho, L.F.; Appelbaum, T.; et al. Mutation-independent rhodopsin gene therapy by knockdown and replacement with a single AAV vector. Proc. Natl. Acad. Sci. USA 2018, 115, E8547–E8556. [Google Scholar] [CrossRef]
- Fenner, B.J.; Tan, T.-E.; Barathi, A.V.; Tun, S.B.B.; Yeo, S.W.; Tsai, A.S.H.; Lee, S.Y.; Cheung, C.M.G.; Chan, C.M.; Mehta, J.S.; et al. Gene-Based Therapeutics for Inherited Retinal Diseases. Front. Genet. 2022, 12, 794805. [Google Scholar] [CrossRef]
- Kovacs, K.D.; Ciulla, T.A.; Kiss, S. Advancements in ocular gene therapy delivery: Vectors and subretinal, intravitreal, and suprachoroidal techniques. Expert. Opin. Biol. Ther. 2022, 22, 1193–1208. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, H.; Colosi, P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010, 18, 80–86. [Google Scholar] [CrossRef]
- Ebner, L.J.A.; Grimm, C. AAV Serotypes and Their Suitability for Retinal Gene Therapy. In Retinal Degenerative Diseases XIX; Ash, J.D., Pierce, E., Anderson, R.E., Bowes Rickman, C., Hollyfield, J.G., Grimm, C., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2023; Volume 1415, pp. 131–134. Available online: https://link.springer.com/10.1007/978-3-031-27681-1_20 (accessed on 4 May 2024).
- Chien, Y.; Hsiao, Y.-J.; Chou, S.-J.; Lin, T.-Y.; Yarmishyn, A.A.; Lai, W.-Y.; Lee, M.-S.; Lin, Y.-Y.; Lin, T.-W.; Hwang, D.-K.; et al. Nanoparticles-mediated CRISPR-Cas9 gene therapy in inherited retinal diseases: Applications, challenges, and emerging opportunities. J. Nanobiotechnol. 2022, 20, 511. [Google Scholar] [CrossRef]
- Bordet, T.; Behar-Cohen, F. Ocular gene therapies in clinical practice: Viral vectors and nonviral alternatives. Drug Discov. Today 2019, 24, 1685–1693. [Google Scholar] [CrossRef]
- Lopes, V.S.; Williams, D.S. Gene Therapy for the Retinal Degeneration of Usher Syndrome Caused by Mutations in MYO7A. Cold Spring Harb. Perspect. Med. 2015, 5, a017319. [Google Scholar] [CrossRef]
- Al-Khuzaei, S.; Broadgate, S.; Foster, C.R.; Shah, M.; Yu, J.; Downes, S.M.; Halford, S. An Overview of the Genetics of ABCA4 Retinopathies, an Evolving Story. Genes 2021, 12, 1241. [Google Scholar] [CrossRef]
- Mellen, R.W.; Calabro, K.R.; McCullough, K.T.; Crosson, S.M.; Cova, A.D.L.; Fajardo, D.; Xu, E.; Boye, S.L.; Boye, S.E. Development of an AAV-CRISPR-Cas9-based treatment for dominant cone-rod dystrophy 6. Mol. Ther.-Methods Clin. Dev. 2023, 30, 48–64. [Google Scholar] [CrossRef]
- Bali, B.; Gruber-Dujardin, E.; Kusch, K.; Rankovic, V.; Moser, T. Analyzing efficacy, stability, and safety of AAV-mediated optogenetic hearing restoration in mice. Life Sci. Alliance 2022, 5, e202101338. [Google Scholar] [CrossRef]
- Mendoza, S.D.; El-Shamayleh, Y.; Horwitz, G.D. AAV-mediated delivery of optogenetic constructs to the macaque brain triggers humoral immune responses. J. Neurophysiol. 2017, 117, 2004–2013. [Google Scholar] [CrossRef]
- Zárate, R.V.; Arancibia, D.; Fernández, A.; Signorelli, J.R.; Larrondo, L.F.; Andrés, M.E.; Zamorano, P. Optimization of the Light-On system in a lentiviral platform to a light-controlled expression of genes in neurons. Electron. J. Biotechnol. 2021, 51, 50–57. [Google Scholar] [CrossRef]
- Arancibia, D.; Pol, I.; Vargas-Fernández, M.; Zárate, R.V.; Signorelli, J.R.; Zamorano, P. OPTO-BLUE: An Integrated Bidirectional Optogenetic Lentiviral Platform for Controlled Light-Induced Gene Expression. Int. J. Mol. Sci. 2023, 24, 9537. [Google Scholar] [CrossRef]
- Yi, Z.; All, A.H.; Liu, X. Upconversion Nanoparticle-Mediated Optogenetics. Adv. Exp. Med. Biol. 2021, 1293, 641–657. [Google Scholar] [CrossRef]
- Sun, D.; Sun, W.; Gao, S.-Q.; Lehrer, J.; Wang, H.; Hall, R.; Lu, Z.-R. Intravitreal Delivery of PEGylated-ECO Plasmid DNA Nanoparticles for Gene Therapy of Stargardt Disease. Pharm. Res. 2024, 41, 807–817. [Google Scholar] [CrossRef]
- Lin, Y.; Yao, Y.; Zhang, W.; Fang, Q.; Zhang, L.; Zhang, Y.; Xu, Y. Applications of upconversion nanoparticles in cellular optogenetics. Acta Biomater. 2021, 135, 1–12. [Google Scholar] [CrossRef]
- Chen, S.; Weitemier, A.Z.; Zeng, X.; He, L.; Wang, X.; Tao, Y.; Huang, A.J.Y.; Hashimotodani, Y.; Kano, M.; Iwasaki, H.; et al. Near-infrared deep brain stimulation via upconversion nanoparticle–mediated optogenetics. Science 2018, 359, 679–684. [Google Scholar] [CrossRef]
- Wu, C.; Su, B.; Xin, N.; Tang, J.; Xiao, J.; Luo, H.; Wei, D.; Luo, F.; Sun, J.; Fan, H. An upconversion nanoparticle-integrated fibrillar scaffold combined with a NIR-optogenetic strategy to regulate neural cell performance. J. Mater. Chem. B 2023, 11, 430–440. [Google Scholar] [CrossRef]
- Gu, L.; Shivalingaiah, S.; Ficinski, M.; Wong, E.; Mohanty, S. Non-viral delivery and optimized optogenetic stimulation of retinal ganglion cells led to behavioral restoration of vision. Nat. Preced. 2012. [Google Scholar] [CrossRef]
- Hsieh, F.-Y.; Han, H.-W.; Chen, X.-R.; Yang, C.-S.; Wei, Y.; Hsu, S. Non-viral delivery of an optogenetic tool into cells with self-healing hydrogel. Biomaterials 2018, 174, 31–40. [Google Scholar] [CrossRef]
- Johannsmeier, S.; Torres, M.; Ripken, T.; Heinemann, D.; Heisterkamp, A. Hydrogels for Efficient Light Delivery in Optogenetic Applications; SPIE: Bellingham, WA, USA, 2018; Volume 10482, pp. 27–35. [Google Scholar]
- Emiliani, V.; Entcheva, E.; Hedrich, R.; Hegemann, P.; Konrad, K.R.; Lüscher, C.; Mahn, M.; Pan, Z.-H.; Sims, R.R.; Vierock, J.; et al. Optogenetics for light control of biological systems. Nat. Rev. Methods Primers 2022, 2, 55. [Google Scholar] [CrossRef]
- Kwan, W.C.; Brunton, E.K.; Begeng, J.M.; Richardson, R.T.; Ibbotson, M.R.; Tong, W. Timing is Everything: Stochastic Optogenetic Stimulation Reduces Adaptation in Retinal Ganglion Cells. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; IEEE: Sydney, Australia, 2023; pp. 1–4. Available online: https://ieeexplore.ieee.org/document/10340849/ (accessed on 30 May 2024).
- Hososhima, S.; Ueno, S.; Okado, S.; Inoue, K.; Konno, M.; Yamauchi, Y.; Inoue, K.; Terasaki, H.; Kandori, H.; Tsunoda, S.P. A light-gated cation channel with high reactivity to weak light. Sci. Rep. 2023, 13, 7625. [Google Scholar] [CrossRef]
- Gauvain, G.; Akolkar, H.; Chaffiol, A.; Arcizet, F.; Khoei, M.A.; Desrosiers, M.; Jaillard, C.; Caplette, R.; Marre, O.; Bertin, S.; et al. Optogenetic therapy: High spatiotemporal resolution and pattern discrimination compatible with vision restoration in non-human primates. Commun. Biol. 2021, 4, 125. [Google Scholar] [CrossRef]
- Yan, B.; Viswanathan, S.; Brodie, S.E.; Deng, W.-T.; Coleman, K.E.; Hauswirth, W.W.; Nirenberg, S. A clinically viable approach to restoring visual function using optogenetic gene therapy. Mol. Ther.-Methods Clin. Dev. 2023, 29, 406–417. [Google Scholar] [CrossRef]
- Ferrari, U.; Deny, S.; Sengupta, A.; Caplette, R.; Trapani, F.; Sahel, J.-A.; Dalkara, D.; Picaud, S.; Duebel, J.; Marre, O. Towards optogenetic vision restoration with high resolution. PLoS Comput. Biol. 2020, 16, e1007857. [Google Scholar] [CrossRef] [PubMed]
- McGregor, J.E.; Godat, T.; Dhakal, K.R.; Parkins, K.; Strazzeri, J.M.; Bateman, B.A.; Fischer, W.S.; Williams, D.R.; Merigan, W.H. Optogenetic restoration of retinal ganglion cell activity in the living primate. Nat. Commun. 2020, 11, 1703. [Google Scholar] [CrossRef] [PubMed]
- Ganjawala, T.H.; Lu, Q.; Fenner, M.D.; Abrams, G.W.; Pan, Z.-H. Improved CoChR Variants Restore Visual Acuity and Contrast Sensitivity in a Mouse Model of Blindness under Ambient Light Conditions. Mol. Ther. 2019, 27, 1195–1205. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sugano, E.; Tabata, K.; Hatakeyama, A.; Sakajiri, T.; Fukuda, T.; Ozaki, T.; Suzuki, T.; Sayama, T.; Tomita, H. Development of an optogenetic gene sensitive to daylight and its implications in vision restoration. NPJ Regen. Med. 2021, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.H.; Holt, A.; Salari, A.; Veit, J.; Visel, M.; Levitz, J.; Aghi, K.; Gaub, B.M.; Sivyer, B.; Flannery, J.G.; et al. Restoration of high-sensitivity and adapting vision with a cone opsin. Nat. Commun. 2019, 10, 1221. [Google Scholar] [CrossRef]
- Batabyal, S.; Gajjeraman, S.; Pradhan, S.; Bhattacharya, S.; Wright, W.; Mohanty, S. Sensitization of ON-bipolar cells with ambient light activatable multi-characteristic opsin rescues vision in mice. Gene Ther. 2021, 28, 162–176. [Google Scholar] [CrossRef]
- Kralik, J.; Van Wyk, M.; Stocker, N.; Kleinlogel, S. Bipolar cell targeted optogenetic gene therapy restores parallel retinal signaling and high-level vision in the degenerated retina. Commun. Biol. 2022, 5, 1116. [Google Scholar] [CrossRef]
- Gaub, B.M.; Berry, M.H.; Holt, A.E.; Isacoff, E.Y.; Flannery, J.G. Optogenetic Vision Restoration Using Rhodopsin for Enhanced Sensitivity. Mol. Ther. 2015, 23, 1562–1571. [Google Scholar] [CrossRef]
- Lu, Q.; Ganjawala, T.H.; Krstevski, A.; Abrams, G.W.; Pan, Z.-H. Comparison of AAV-Mediated Optogenetic Vision Restoration between Retinal Ganglion Cell Expression and ON Bipolar Cell Targeting. Mol. Ther.-Methods Clin. Dev. 2020, 18, 15–23. [Google Scholar] [CrossRef]
- Katada, Y.; Yoshida, K.; Serizawa, N.; Lee, D.; Kobayashi, K.; Negishi, K.; Okano, H.; Kandori, H.; Tsubota, K.; Kurihara, T. Highly sensitive visual restoration and protection via ectopic expression of chimeric rhodopsin in mice. iScience 2023, 26, 107716. [Google Scholar] [CrossRef]
- Idzhilova, O.S.; Kolotova, D.E.; Smirnova, G.R.; Abonakour, A.; Dolgikh, D.A.; Petrovskaya, L.E.; Kirpichnikov, M.P.; Ostrovsky, M.A.; Malyshev, A.Y. Nonselective Expression of Short-Wavelength Cone Opsin Improves Learning in Mice with Retinal Degeneration in a Visually Guided Task. Dokl. Biol. Sci. 2023, 510, 167–171. [Google Scholar] [CrossRef]
- Nikonov, S.; Aravand, P.; Lyubarsky, A.; Nikonov, R.; Luo, A.J.; Wei, Z.; Maguire, A.M.; Phelps, N.T.; Shpylchak, I.; Willett, K.; et al. Restoration of Vision and Retinal Responses After Adeno-Associated Virus–Mediated Optogenetic Therapy in Blind Dogs. Trans. Vis. Sci. Tech. 2022, 11, 24. [Google Scholar] [CrossRef]
- Khabou, H.; Garita-Hernandez, M.; Chaffiol, A.; Reichman, S.; Jaillard, C.; Brazhnikova, E.; Bertin, S.; Forster, V.; Desrosiers, M.; Winckler, C.; et al. Noninvasive gene delivery to foveal cones for vision restoration. JCI Insight 2018, 3, e96029. [Google Scholar] [CrossRef]
- Chaffiol, A.; Caplette, R.; Jaillard, C.; Brazhnikova, E.; Desrosiers, M.; Dubus, E.; Duhamel, L.; Macé, E.; Marre, O.; Benoit, P.; et al. A New Promoter Allows Optogenetic Vision Restoration with Enhanced Sensitivity in Macaque Retina. Mol. Ther. 2017, 25, 2546–2560. [Google Scholar] [CrossRef]
- Simon, C.-J.; Sahel, J.-A.; Duebel, J.; Herlitze, S.; Dalkara, D. Opsins for vision restoration. Biochem. Biophys. Res. Commun. 2020, 527, 325–330. [Google Scholar] [CrossRef]
- Gilhooley, M.J.; Lindner, M.; Palumaa, T.; Hughes, S.; Peirson, S.N.; Hankins, M.W. A systematic comparison of optogenetic approaches to visual restoration. Mol. Ther.-Methods Clin. Dev. 2022, 25, 111–123. [Google Scholar] [CrossRef]
- Wright, W.W.; Gajjeraman, S.; Batabyal, S.; Pradhan, S.; Bhattacharya, S.; Mahapatra, V.; Tripathy, A.; Mohanty, S.K. Restoring vision in mice with retinal degeneration using multicharacteristic opsin. Neurophoton. 2017, 4, 041505. [Google Scholar] [CrossRef]
- Gaub, B.M.; Berry, M.H.; Visel, M.; Holt, A.; Isacoff, E.Y.; Flannery, J.G. Optogenetic Retinal Gene Therapy with the Light Gated GPCR Vertebrate Rhodopsin. In Retinal Gene Therapy; Boon, C.J.F., Wijnholds, J., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1715, pp. 177–189. Available online: http://link.springer.com/10.1007/978-1-4939-7522-8_12 (accessed on 12 May 2024).
- Cehajic-Kapetanovic, J.; Eleftheriou, C.; Allen, A.E.; Milosavljevic, N.; Pienaar, A.; Bedford, R.; Davis, K.E.; Bishop, P.N.; Lucas, R.J. Restoration of Vision with Ectopic Expression of Human Rod Opsin. Curr. Biol. 2015, 25, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, V.H.; Lam, B.L.; Zak, V.; Mohanty, S.; Bataybal, S.; Chang, J.; Ayyagari, A.; Chavala, S.H.; Piltz-Seymour, J.; Koester, J.; et al. MCO-010 intravitreal optogenetic therapy in Stargardt disease. 6-month outcomes from the Phase 2 STARLIGHT trial. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3546. [Google Scholar]
- Nanoscope Therapeutics Announces Positive Top-Line Results from Randomized Controlled Trial of MCO-010 for Retinitis Pigmentosa. 2024. Available online: https://nanostherapeutics.com/2024/03/26/nanoscope-therapeutics-announces-top-line-results-from-ph2-trial-of-mco-010-for-retinitis-pigmentosa/ (accessed on 5 May 2024).
- PackGene Biotech lnc. Zhongmou Therapeutics Unveils Promising Clinical Data for Innovative Retinitis Pigmentosa Gene Therapy. 2024. Available online: https://www.packgene.com/frontier/240401/ (accessed on 17 June 2024).
- Eramian, D. Nanoscope Therapeutics, Inc. Positive Data from Nanoscope’s Phase 1/2a Trial of Gene Therapy to Restore Vision in Patients Blinded by Retinitis Pigmentosa to Be Featured at 2021. 2021. Available online: https://nanostherapeutics.com/2021/10/08/positive-data-from-nanoscopes-phase-1-2a-trial-of-gene-therapy-to-restore-vision-in-patients-blinded-by-retinitis-pigmentosa-to-be-featured-at-2021-american-society-of-retina-specialists-meet/ (accessed on 17 June 2024).
- Sahel, J.-A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J.N.; Esposti, S.D.; Delaux, A.; De Saint Aubert, J.-B.; De Montleau, C.; et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 2021, 27, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, D.; Sahoo, N.; Mariappan, I.; Narayanan, R. Gene therapy in retinal diseases: A review. Indian J. Ophthalmol. 2021, 69, 2257. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; She, K.; Song, L.; Jin, X.; Li, R.; Zhao, Q.; Xiao, J.; Chen, D.; Cheng, H.; Lu, F.; et al. In vivo base editing rescues photoreceptors in a mouse model of retinitis pigmentosa. Mol. Ther.-Nucleic Acids 2023, 31, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, W.; Zhang, S.; Feng, Y.; Xu, W.; Qi, J.; Zhang, Q.; Xu, C.; Liu, S.; Zhang, J.; et al. Vision rescue via unconstrained in vivo prime editing in degenerating neural retinas. J. Exp. Med. 2023, 220, e20220776. [Google Scholar] [CrossRef] [PubMed]
- Vagni, P.; Perlini, L.E.; Chenais, N.A.L.; Marchetti, T.; Parrini, M.; Contestabile, A.; Cancedda, L.; Ghezzi, D. Gene Editing Preserves Visual Functions in a Mouse Model of Retinal Degeneration. Front. Neurosci. 2019, 13, 945. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiao, J.; Jia, R.; Zhang, F.; Meng, X.; Li, Y.; Yang, L. Allele-specific gene-editing approach for vision loss restoration in RHO-associated retinitis pigmentosa. eLife 2023, 12, e84065. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-H.; Tsai, Y.-T.; Huang, I.-W.; Cheng, C.-H.; Hsu, C.-W.; Cui, X.; Ryu, J.; Quinn, P.M.J.; Caruso, S.M.; Lin, C.-S.; et al. CRISPR genome surgery in a novel humanized model for autosomal dominant retinitis pigmentosa. Mol. Ther. 2022, 30, 1407–1420. [Google Scholar] [CrossRef]
- Orlans, H.O.; McClements, M.E.; Barnard, A.R.; Martinez-Fernandez De La Camara, C.; MacLaren, R.E. Mirtron-mediated RNA knockdown/replacement therapy for the treatment of dominant retinitis pigmentosa. Nat. Commun. 2021, 12, 4934. [Google Scholar] [CrossRef]
- Tornabene, P.; Ferla, R.; Llado-Santaeularia, M.; Centrulo, M.; Dell’Anno, M.; Esposito, F.; Marrocco, E.; Pone, E.; Minopoli, R.; Iodice, C.; et al. Therapeutic homology-independent targeted integration in retina and liver. Nat. Commun. 2022, 13, 1963. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Cai, B.; Tian, Y.; Liu, X.; Liang, C.; Gao, Q.; Li, B.; Ding, Y.; Li, R.; Zhou, Q.; et al. Therapeutic In Vivo Gene Editing Achieved by a Hypercompact CRISPR-Cas12f1 System Delivered with All-in-One Adeno-Associated Virus. Adv. Sci. 2024, 11, e2308095. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Mookherjee, S.; Chaitankar, V.; Hiriyanna, S.; Kim, J.-W.; Brooks, M.; Ataeijannati, Y.; Sun, X.; Dong, L.; Li, T.; et al. Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat. Commun. 2017, 8, 14716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ming, C.; Fu, X.; Duan, Y.; Hoang, D.A.; Rutgard, J.; Zhang, R.; Wang, W.; Hou, R.; Zhang, D.; et al. Gene and mutation independent therapy via CRISPR-Cas9 mediated cellular reprogramming in rod photoreceptors. Cell Res. 2017, 27, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Nolan, N.D.; Cui, X.; Robbings, B.M.; Demirkol, A.; Pandey, K.; Wu, W.H.; Hu, H.F.; Jenny, L.A.; Lin, C.-S.; Hass, D.T.; et al. CRISPR editing of anti-anemia drug target rescues independent preclinical models of retinitis pigmentosa. Cell Rep. Med. 2024, 5, 101459. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-K.; Jo, D.H.; Lee, S.-N.; Cho, C.S.; Jeong, Y.K.; Jung, Y.; Yu, J.; Kim, J.H.; Woo, J.-S.; Bae, S. High-purity production and precise editing of DNA base editing ribonucleoproteins. Sci. Adv. 2021, 7, eabg2661. [Google Scholar] [CrossRef] [PubMed]
- She, K.; Liu, Y.; Zhao, Q.; Jin, X.; Yang, Y.; Su, J.; Li, R.; Song, L.; Xiao, J.; Yao, S.; et al. Dual-AAV split prime editor corrects the mutation and phenotype in mice with inherited retinal degeneration. Signal Transduct. Target. Ther. 2023, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Banskota, S.; Raguram, A.; Suh, S.; Du, S.W.; Davis, J.R.; Choi, E.H.; Wang, X.; Nielsen, S.C.; Newby, G.A.; Randolph, P.B.; et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 2022, 185, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H.; Suh, S.; Foik, A.T.; Leinonen, H.; Newby, G.A.; Gao, X.D.; Banskota, S.; Hoang, T.; Du, S.W.; Dong, Z.; et al. In vivo base editing rescues cone photoreceptors in a mouse model of early-onset inherited retinal degeneration. Nat. Commun. 2022, 13, 1830. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Jang, H.-K.; Cho, C.S.; Han, J.H.; Ryu, G.; Jung, Y.; Bae, S.; Kim, J.H. Visual function restoration in a mouse model of Leber congenital amaurosis via therapeutic base editing. Mol. Ther.-Nucleic Acids 2023, 31, 16–27. [Google Scholar] [CrossRef]
- Suh, S.; Choi, E.H.; Leinonen, H.; Foik, A.T.; Newby, G.A.; Yeh, W.-H.; Dong, Z.; Kiser, P.D.; Lyon, D.C.; Liu, D.R.; et al. Restoration of visual function in adult mice with an inherited retinal disease via adenine base editing. Nat. Biomed. Eng. 2020, 5, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.L.; Stefanidakis, M.; Wilson, C.J.; Baral, R.; Barrera, L.A.; Bounoutas, G.S.; Bumcrot, D.; Chao, H.; Ciulla, D.M.; DaSilva, J.A.; et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 2019, 25, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Riedmayr, L.M.; Hinrichsmeyer, K.S.; Thalhammer, S.B.; Mittas, D.M.; Karguth, N.; Otify, D.Y.; Böhm, S.; Weber, V.J.; Bartoschek, M.D.; Splith, V.; et al. mRNA trans-splicing dual AAV vectors for (epi)genome editing and gene therapy. Nat. Commun. 2023, 14, 6578. [Google Scholar] [CrossRef] [PubMed]
- McClements, M.E.; Barnard, A.R.; Singh, M.S.; Charbel Issa, P.; Jiang, Z.; Radu, R.A.; MacLaren, R.E. An AAV Dual Vector Strategy Ameliorates the Stargardt Phenotype in Adult Abca4−/− Mice. Hum. Gene Ther. 2019, 30, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.; Yang, P.; Ban, Q.; Yang, Y.; Wang, M.; Chien, C.; Chen, S.; Sun, N.; Zhu, Y.; Liu, H.; et al. Dual Supramolecular Nanoparticle Vectors Enable CRISPR/Cas9-Mediated Knockin of Retinoschisin 1 Gene—A Potential Nonviral Therapeutic Solution for X-Linked Juvenile Retinoschisis. Adv. Sci. 2020, 7, 1903432. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. Retinal degeneration mutants in the mouse. Vis. Res. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Cideciyan, A.V. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog. Retin. Eye Res. 2010, 29, 398–427. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, T.; Sawinski, H.; Urban, A.M.; Motlik, J.; Stieger, K. Rapid and Reliable Quantification of Prime Editing Targeting within the Porcine ABCA4 Gene Using a BRET-Based Sensor. Nucleic Acid. Ther. 2023, 33, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Regent, F.; Chen, H.Y.; Kelley, R.A.; Qu, Z.; Swaroop, A.; Li, T. A simple and efficient method for generating human retinal organoids. Mol. Vis. 2020, 26, 97–105. [Google Scholar]
- Chichagova, V.; Hilgen, G.; Ghareeb, A.; Georgiou, M.; Carter, M.; Sernagor, E.; Lako, M.; Armstrong, L. Human iPSC differentiation to retinal organoids in response to IGF1 and BMP4 activation is line- and method-dependent. Stem Cells 2020, 38, 195–201. [Google Scholar] [CrossRef]
- Daich Varela, M.; Sen, S.; De Guimaraes, T.A.C.; Kabiri, N.; Pontikos, N.; Balaskas, K.; Michaelides, M. Artificial intelligence in retinal disease: Clinical application, challenges, and future directions. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 3283–3297. [Google Scholar] [CrossRef] [PubMed]
- Sulak, R.; Liu, X.; Smedowski, A. The concept of gene therapy for glaucoma: The dream that has not come true yet. Neural Regen. Res. 2024, 19, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Prosseda, P.P.; Alvarado, J.A.; Wang, B.; Kowal, T.J.; Ning, K.; Stamer, W.D.; Hu, Y.; Sun, Y. Optogenetic stimulation of phosphoinositides reveals a critical role of primary cilia in eye pressure regulation. Sci. Adv. 2020, 6, eaay8699. [Google Scholar] [CrossRef] [PubMed]
- Kowal, T.J.; Prosseda, P.P.; Ning, K.; Wang, B.; Alvarado, J.; Sendayen, B.E.; Jabbehdari, S.; Stamer, W.D.; Hu, Y.; Sun, Y. Optogenetic modulation of intraocular pressure in a glucocorticoid-induced ocular hypertension mouse model. Transl. Vis. Sci. Technol. 2021, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Ballios, B.G.; Pierce, E.A.; Huckfeldt, R.M. Gene editing technology: Towards precision medicine in inherited retinal diseases. Semin. Ophthalmol. 2021, 36, 176–184. [Google Scholar] [CrossRef]
- Koulisis, N.; Nagiel, A. Precision Therapy for Inherited Retinal Disease. Clin. Lab. Med. 2020, 40, 189–204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).