Whole Body Vibration Training Has No Effect on Vascular Endothelial and Inflammatory Markers in Young Healthy Women
Abstract
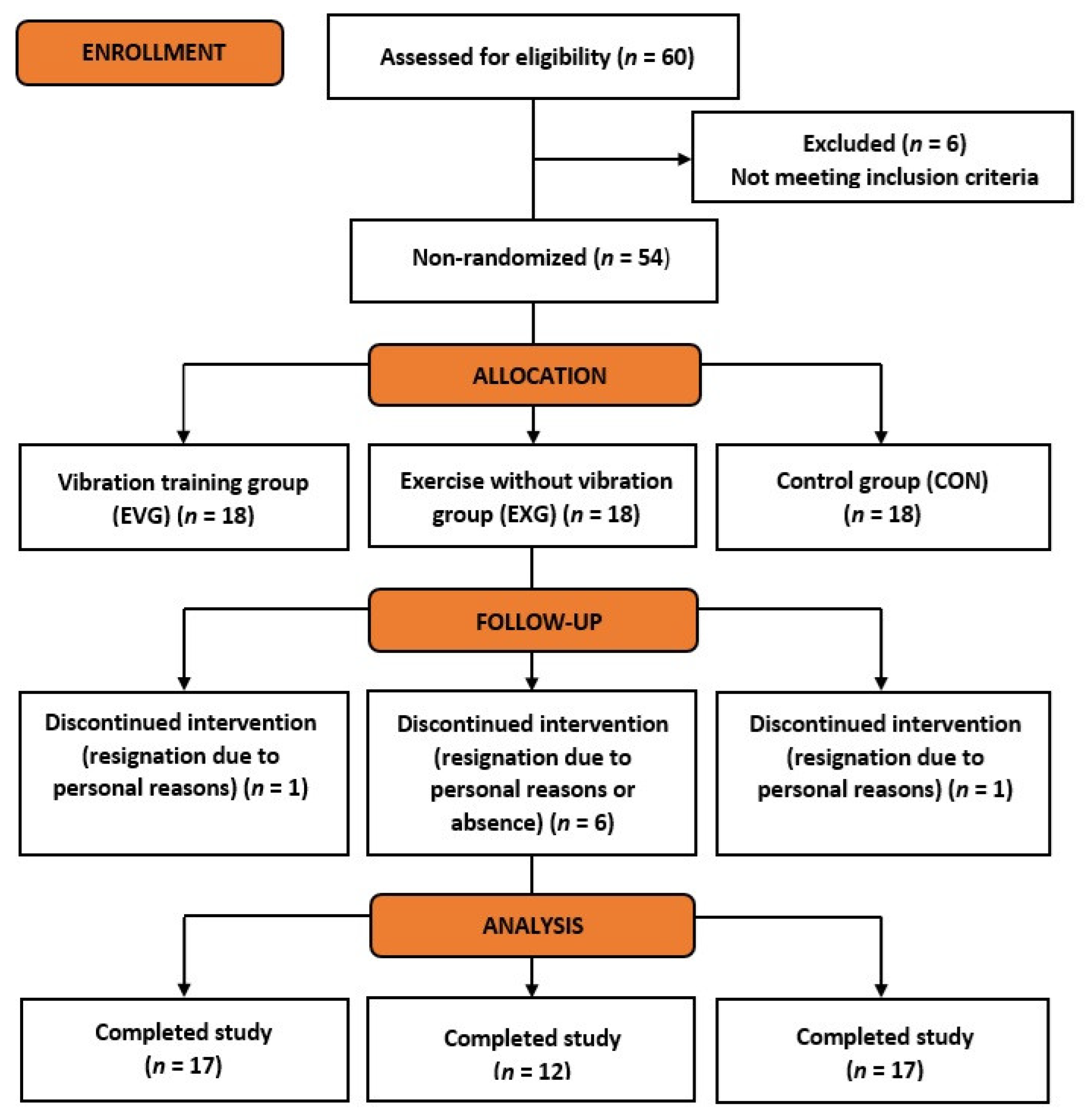
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Protocol
2.3. Anthropometric Measurements
2.4. Nutritional Analysis and Physical Activity Level
2.5. Venous Blood Collection and Biochemical Assays
2.6. Exercise Program
- maintaining a standing position on the platform (Figure 2);
- dynamic half-squats;
- maintaining a standing position on the platform with the back to the platform while holding taut bands attached to the base (Figure 3);
- dynamic side squats on the platform in a lunge position with the right lower limb while simultaneously raising the opposite upper limb;
- dynamic side squats on the platform in a lunge position with the left lower limb while simultaneously raising the opposite upper limb (Figure 4);
- maintaining a half-squat position.
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Vascular Endothelial Growth Factor (VEGF)
3.3. Endothelial Nitric Oxide Synthase (eNOS)
3.4. High-Sensitivity C-Reactive Protein (hsCRP)
4. Discussion
Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, M.; Kargl, C.K.; Ferguson, R.; Gavin, T.P.; Hellsten, Y. Exercise-induced Skeletal Muscle Angiogenesis: Impact of Age, Sex, Angiocrines and Cellular Mediators. Eur. J. Appl. Physiol. 2023, 123, 1415–1432. [Google Scholar] [CrossRef] [PubMed]
- Königstein, K.; Dipla, K.; Zafeiridis, A. Training the Vessels: Molecular and Clinical Effects of Exercise on Vascular Health-A Narrative Review. Cells 2023, 12, 2544. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [PubMed]
- Mouliou, D.S. C-Reactive Protein: Pathophysiology, Diagnosis, False Test Results and a Novel Diagnostic Algorithm for Clinicians. Diseases 2023, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gan, L.; Deng, Y.; Zhu, S.; Li, G.; Nasser, M.I.; Liu, N.; Zhu, P. Cardiovascular Disease and Exercise: From Molecular Mechanisms to Clinical Applications. J. Clin. Med. 2022, 11, 7511. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Ruilope, L.M.; Santos-Lozano, A.; Wilhelm, M.; Kränkel, N.; Fiuza-Luces, C.; Lucia, A. Exercise Benefits in Cardiovascular Diseases: From Mechanisms to Clinical Implementation. Eur. Heart J. 2023, 44, 1874–1889. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, T.; Murakami, M. Regular Physical Exercise Improves Physical Motor Functions and Biochemical Barkers in Middle-Age and Elderly Women. J. Phys. Act. Health 2009, 6, 55–62. [Google Scholar] [CrossRef] [PubMed]
- LeCheminant, J.; Tucker, L.; Russell, K. Physical Activity and C-Reactive Protein Levels: The Confounding Role of Body Fat. J. Phys. Act. Health 2011, 8, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Hammonds, T.L.; Gathright, E.C.; Goldstein, C.M.; Penn, M.S.; Hughes, J.W. Effects of Exercise on C-Reactive Protein in Healthy Patients and in Patients with Heart Disease: A Meta-Analysis. Heart Lung 2016, 45, 273–282. [Google Scholar] [CrossRef]
- Maierean, S.; Webb, R.; Banach, M.; Mazidi, M. The Role of Inflammation and the Possibilities of Inflammation Reduction to Prevent Cardiovascular Events. Eur. Heart J. Open 2022, 2, oeac039. [Google Scholar] [CrossRef]
- Campbell, K.L.; Campbell, P.T.; Ulrich, C.M.; Wener, M.; Alfano, C.M.; Foster-Schubert, K.; Rudolph, R.E.; Potter, J.D.; McTiernan, A. No Reduction in C-Reactive Protein Following a 12-Month Randomized Controlled Trial of Exercise in Men and Women. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1714–1718. [Google Scholar] [CrossRef]
- Stewart, L.K.; Earnest, C.P.; Blair, S.N.; Church, T.S. Effects of Different Doses of Physical Activity on C-Reactive Protein Among Women. Med. Sci. Sports Exerc. 2010, 42, 701–707. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S. Effects of Aerobic Exercise on C-Reactive Protein, Body Composition, and Maximum Oxygen Consumption in Adults: A Meta-Analysis of Randomized Controlled Trials. Metabolism 2006, 55, 1500–1507. [Google Scholar] [CrossRef]
- Daray, L.A.; Henagan, T.M.; Zanovec, M.; Earnest, C.P.; Johnson, L.G.; Winchester, J.; Tuuri, G.; Stewart, L.K. Endurance and Resistance Training Lowers C-Reactive Protein in Young, Healthy Females. Appl. Physiol. Nutr. Metab. 2011, 36, 660–670. [Google Scholar] [CrossRef]
- Cunha, P.M.; Ribeiro, A.S.; Nunes, J.P.; Tomeleri, C.M.; Nascimento, M.A.; Moraes, G.K.; Sugihara, P., Jr.; Barbosa, D.S.; Venturini, D.; Cyrino, E.S. Resistance Training Performed with Single-Set is Sufficient to Reduce Cardiovascular Risk Factors in Untrained Older Women: The Randomized Clinical Trial. Active Aging Longitudinal Study. Arch. Gerontol. Geriatr. 2019, 81, 171–175. [Google Scholar] [CrossRef]
- Tomeleri, C.M.; Souza, M.F.; Burini, R.C.; Cavaglieri, C.R.; Ribeiro, A.S.; Antunes, M.; Nunes, J.P.; Venturini, D.; Barbosa, D.S.; Sardinha, L.B.; et al. Resistance Training Reduces Metabolic Syndrome and Inflammatory Markers in Older Women: A Randomized Controlled Trial. J. Diabetes 2018, 10, 328–337. [Google Scholar] [CrossRef]
- Martins, R.A.; Neves, A.P.; Coelho-Silva, M.J.; Veríssimo, M.T.; Teixeira, A.M. The Effect of Aerobic Versus Strength-Based Training on High-Sensitivity C-Reactive Protein in Older Adults. Eur. J. Appl. Physiol. 2010, 110, 161–169. [Google Scholar] [CrossRef]
- Tao, X.; Chen, Y.; Zhen, K.; Ren, S.; Lv, Y.; Yu, L. Effect of Continuous Aerobic Exercise on Endothelial Function: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Physiol. 2023, 14, 1043108. [Google Scholar] [CrossRef]
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Oggioni, C.; Jakovljevic, D.G.; Mathers, J.C. Exercise Modalities and Endothelial Function: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Sports Med. 2015, 45, 279–296. [Google Scholar] [CrossRef]
- Silva, J.K.T.N.F.; Menêses, A.L.; Parmenter, B.J.; Ritti-Dias, R.M.; Farah, B.Q. Effects of Resistance Training on Endothelial Function: A Systematic Review and Meta-Analysis. Atherosclerosis 2021, 333, 91–99. [Google Scholar] [CrossRef]
- Green, D.J.; Maiorana, A.; O’Driscoll, G.; Taylor, R. Effect of Exercise Training on Endothelium-Derived Nitric Oxide Function in Humans. J. Physiol. 2004, 561, 1–25. [Google Scholar] [CrossRef]
- Tinken, T.M.; Thijssen, D.H.; Hopkins, N.; Dawson, E.A.; Cable, N.T.; Green, D.J. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 2010, 55, 312–318. [Google Scholar] [CrossRef]
- de Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simões, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef]
- Ribeiro, F.; Ribeiro, I.P.; Gonçalves, A.C.; Alves, A.J.; Melo, E.; Fernandes, R.; Costa, R.; Sarmento-Ribeiro, A.B.; Duarte, J.A.; Carreira, I.M.; et al. Effects of Resistance Exercise on Endothelial Progenitor Cell Mobilization in Women. Sci. Rep. 2017, 7, 17880. [Google Scholar] [CrossRef]
- Azar, J.T.; Ravasi, A.A.; Soori, R.; Akbarnejad, A.; Nafar, M.H. The Effect of 8 Weeks Aerobic Training on Angiogenesis (VEGF) and Angiostatic (ES) Factors in Sedentary Women. Urmia Med. J. 2017, 27, 1032–1040. [Google Scholar] [CrossRef]
- Pospieszna, B.; Karolkiewicz, J.; Tarnas, J.; Lewandowski, J.; Laurentowska, M.; Pilaczyńska-Szcześniak, Ł. Influence of 12-Week Nordic Walking Training on Biomarkers of Endothelial Function in Healthy Postmenopausal Women. J. Sports Med. Phys. Fit. 2017, 57, 1178–1185. [Google Scholar] [CrossRef]
- Ratajczak, M.; Skrypnik, D.; Krutki, P.; Karolkiewicz, J. Effects of an Indoor Cycling Program on Cardiometabolic Factors in Women with Obesity vs. Normal Body Weight. Int. J. Environ. Res. Public Health 2020, 17, 8718. [Google Scholar] [CrossRef]
- Ratajczak, M.; Skrypnik, D.; Bogdański, P.; Mądry, E.; Walkowiak, J.; Szulińska, M.; Maciaszek, J.; Kręgielska-Narożna, M.; Karolkiewicz, J. Effects of Endurance and Endurance-Strength Training on Endothelial Function in Women with Obesity: A Randomized Trial. Int. J. Environ. Res. Public Health 2019, 16, 4291. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal Muscle Mass and Distribution in 468 Men and Women Aged 18–88 Year. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Sex Differences in Exercise Metabolism and the Role of 17-Beta Estradiol. Med. Sci. Sports Exerc. 2008, 40, 648–654. [Google Scholar] [CrossRef]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Newby, L.K.; Piña, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women–2011 Update: A Guideline from the American Heart Association. Circulation 2011, 123, 1243–1262. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- van Heuvelen, M.J.G.; Rittweger, J.; Judex, S.; Sañudo, B.; Seixas, A.; Fuermaier, A.B.M.; Tucha, O.; Nyakas, C.; Marín, P.J.; Taiar, R.; et al. Reporting Guidelines for Whole-Body Vibration Studies in Humans, Animals and Cell Cultures: A Consensus Statement from an International Group of Experts. Biology 2021, 10, 965. [Google Scholar] [CrossRef]
- Zaidell, L.N.; Mileva, K.N.; Sumners, D.P.; Bowtell, J.L. Experimental Evidence of the Tonic Vibration Reflex During Whole-Body Vibration of the Loaded and Unloaded Leg. PLoS ONE 2013, 8, e85247. [Google Scholar] [CrossRef]
- Aydın, T.; Kesiktaş, F.N.; Baskent, A.; Karan, A.; Karacan, I.; Türker, K.S. Cross-training Effect of Chronic Whole-Body Vibration Exercise: A Randomized Controlled Study. Somatosens. Mot. Res. 2020, 37, 51–58. [Google Scholar] [CrossRef]
- Musumeci, G. The Use of Vibration as Physical Exercise and Therapy. J. Funct. Morphol. Kinesiol. 2017, 2, 17. [Google Scholar] [CrossRef]
- Milanese, C.; Cavedon, V.; Sandri, M.; Tam, E.; Piscitelli, F.; Boschi, F.; Zancanaro, C. Metabolic Effect of Bodyweight Whole-Body Vibration in a 20-min Exercise Session: A Crossover Study Using Verified Vibration Stimulus. PLoS ONE 2018, 13, e0192046. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Z.; Li, X.; Lai, Z.; Wang, L. Effect of Whole-Body Vibration on Neuromuscular Activation and Explosive Power of Lower Limb: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0278637. [Google Scholar] [CrossRef]
- Chung, P.; Liu, C.; Wang, H.; Liu, Y.; Chuang, L.; Shiang, T.Y. Various Performance-Enhancing Effects From the Same Intensity of Whole-Body Vibration Training. J. Sport Health Sci. 2017, 6, 333–339. [Google Scholar] [CrossRef]
- Haleva, Y.; Dunsky, A.; Meckel, Y.; Kleinöder, H.; Bar-Eli, M.; Mester, J. The Effect of Long-Term Whole-Body Vibration on Muscular Performance. Braz. J. Health Biomed. Sci. 2021, 19, 100–107. [Google Scholar] [CrossRef]
- Haleva, Y.; Dunsky, A.; Rubinstein, M.; Kleinöder, H.; Mester, J. Muscular Performance is Improved by Long-Term Whole-Body Vibration in Comparison to a Traditional Training Program. J. Athl. Enhanc. 2021, 10, 5. [Google Scholar]
- ElDeeb, A.M.; Abdel-Aziem, A.A. Effect of Whole-Body Vibration Exercise on Power Profile and Bone Mineral Density in Postmenopausal Women with Osteoporosis: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2020, 43, 384–393. [Google Scholar] [CrossRef]
- Sen, E.I.; Esmaeilzadeh, S.; Eskiyurt, N. Effects of Whole-Body Vibration and High Impact exercises on the Bone Metabolism and Funtional Mobility in Postmenopausal Women. J. Bone Miner. Metab. 2020, 38, 392–404. [Google Scholar] [CrossRef]
- Jepsen, D.B.; Ryg, J.; Hansen, S.; Jørgensen, N.R.; Gram, J.; Masud, T. The Combined Effect of Parathyroid Hormone (1-34) and Whole-Body Vibration Exercise in the Treatment of Postmenopausal Osteoporosis (PaVOS Study): A Randomized Controlled Trial. Osteoporos. Int. 2019, 30, 1827–1836. [Google Scholar] [CrossRef]
- Betik, A.C.; Parker, L.; Kaur, G.; Wadley, G.D.; Keske, M.A. Whole-Body Vibration Stimulates Microvascular Blood Flow in Skeletal Muscle. Med. Sci. Sports Exerc. 2021, 53, 375–383. [Google Scholar] [CrossRef]
- Lyons, K.D.; Parks, A.G.; Dadematthews, O.D.; McHenry, P.A.; Sefton, J.M. Vertical and Side-Alternating Whole Body Vibration Platform Parameters Influence Lower Extremity Blood Flow and Muscle Oxygenation. Vibration 2022, 5, 557–567. [Google Scholar] [CrossRef]
- Sanni, A.A.; Blanks, A.M.; Derella, C.C.; Horsager, C.; Crandall, R.H.; Looney, J.; Sanchez, S.; Norland, K.; Ye, B.; Thomas, J.; et al. The Effects of Whole-Body Vibration Amplitude on Glucose Metabolism, Inflammation, and Skeletal Muscle Oxygenation. Physiol. Rep. 2022, 10, e15208. [Google Scholar] [CrossRef]
- Blanks, A.M.; Rodriguez-Miguelez, P.; Looney, J.; Tucker, M.A.; Jeong, J.; Thomas, J.; Blackburn, M.; Stepp, D.W.; Weintraub, N.J.; Harris, R.A. Whole Body Vibration Elicits Differential Immune and Metabolic Responses in Obese and Normal Weight Individuals. Brain Behav. Immun. Health 2019, 14, 100011. [Google Scholar] [CrossRef]
- Jawed, Y.; Beli, E.; March, K.; Kaleth, A.; Loghmani, M.T. Whole-Body Vibration Training Increases Stem/Progenitor Cell Circulation Levels and May Attenuate Inflammation. Mil. Med. 2020, 185 (Suppl. S1), 404–412. [Google Scholar] [CrossRef]
- Mohammed, E.; Mawad, A. Impact of Whole Body Vibration Versus Aerobic Exercise Training in Modulation of Inflammatory Markers in Elderly. World J. Med. Sci. 2015, 12, 148–155. [Google Scholar] [CrossRef]
- Andersson, C.; Vasan, R. Epidemiology of Cardiovascular Disease in Young Individuals. Nat. Rev. Cardiol. 2018, 15, 230–240. [Google Scholar] [CrossRef]
- Biernat, E.; Stupnicki, R.; Gajewski, A.K. Międzynarodowy Kwestionariusz Aktywności Fizycznej (IPAQ)—Wersja Polska. Wych. Fiz. I Sport 2007, 51, 47–54. [Google Scholar]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Jarosz, M. Normy Żywienia dla Populacji Polski; Instytut Żywności i Żywienia: Warszawa, Poland, 2017; ISBN 9788386060894. [Google Scholar]
- Tomás, R.; Lee, V.; Going, S. The Use of Vibration Exercise in Clinical Populations. ACSMS Health Fit. J. 2011, 15, 25–31. [Google Scholar] [CrossRef]
- Gattner, H.; Adamiak, J.; Piotrowska, A.; Czerwińska-Ledwig, O.; Mętel, S.; Kępińska-Szyszkowska, M.; Pilch, W. Effect of Whole-Body Vibration Training on Hemorheological Blood Indices in Young, Healthy Women. Int. J. Environ. Res. Public Health 2023, 20, 3232. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Academic Press: New York, NY, USA, 1988; ISBN 978-080-580-283-2. [Google Scholar]
- Sackner, M.A.; Gummels, E.; Adams, J.A. Nitric Oxide is Released into Circulation with Whole-Body, Periodic Acceleration. Chest 2005, 127, 30–39. [Google Scholar] [CrossRef]
- Humphries, B.; Fenning, A.; Dugan, E.; Guinane, J.; MacRae, K. Whole-Body Vibration Effects on Bone Mineral Density in Women with or Without Resistance Training. Aviat. Space Environ. Med. 2009, 80, 1025–1031. [Google Scholar] [CrossRef]
- Maloney-Hinds, C.; Petrofsky, J.S.; Zimmerman, G.; Hessinger, D.A. The Role of Nitric Oxide in Skin Blood Flow Increases Due to Vibration in Healthy Adults and Adults with Type 2 Diabetes. Diabetes Technol. Ther. 2009, 11, 39–43. [Google Scholar] [CrossRef]
- Beijer, Å.; Degens, H.; Weber, T.; Rosenberger, A.; Gehlert, S.; Herrera, F.; Kohl-Bareis, M.; Zange, J.; Bloch, W.; Rittweger, J. Microcirculation of Skeletal Muscle Adapts Differently to a Resistive Exercise Intervention With and Without Superimposed Whole-Body Vibrations. Clin. Physiol. Funct. Imaging 2015, 35, 425–435. [Google Scholar] [CrossRef]
- Lohman, E.B., III; Petrofsky, J.S.; Maloney-Hinds, C.; Betts-Schwab, H.; Thorpe, D. The Effect of Whole Body Vibration on Lower Extremity Skin Blood Flow in Normal Subjects. Med. Sci. Monit. 2007, 13, 71–76. [Google Scholar]
- Kojda, G.; Hambrecht, R. Molecular Mechanisms of Vascular Adaptations to Exercise. Physical Activity as an Effective Antioxidant Therapy? Cardiovasc. Res. 2005, 67, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Kassab, G.S. Role of Shear Stress and Stretch in Vascular Mechanobiology. J. R. Soc. Interface 2011, 8, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.T.; Thomson, R.L.; Howe, P.R.C.; Buckley, J.D. Effect of Vibration on Muscle Perfusion: A Systematic Review. Clin. Physiol. Funct. Imaging 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Alvarez-Alvarado, S.; Jaime, S.J.; Ormsbee, M.J.; Campbell, J.C.; Post, J.; Pacilio, J.; Figueroa, A. Benefits of Whole-Body Vibration Training on Arterial Function and Muscle Strength in Young Overweight/Obese Women. Hypertens. Res. 2017, 40, 487–492. [Google Scholar] [CrossRef]
- Figueroa, A.; Kalfon, R.; Madzima, T.A.; Wong, A. Whole-Body Vibration Exercise Training Reduces Arterial Stiffness in Postmenopausal Women with Prehypertension and Hypertension. Menopause 2014, 21, 131–136. [Google Scholar] [CrossRef]
- Lai, C.L.; Chen, H.Y.; Tseng, S.Y.; Liao, W.C.; Liu, B.T.; Lee, M.C.; Chen, H.S. Effect of Whole-Body Vibration for 3 Months on Arterial Stiffness in the Middle-Aged and Elderly. Clin. Interv. Aging 2014, 9, 821–828. [Google Scholar] [CrossRef]
- Games, K.E.; Sefton, J.M.; Wilson, A.E. Whole-Body Vibration and Blood Flow and Muscle Oxygenation: A Meta-Analysis. J. Athl. Train. 2015, 50, 542–549. [Google Scholar] [CrossRef]
- Hoier, B.; Hellsten, Y. Exercise-Induced Capillary Growth in Human Skeletal Muscle and the Dynamics of VEGF. Microcirculation 2014, 21, 301–314. [Google Scholar] [CrossRef]
- Ghahramani, M.; Razavi Majd, Z. The Effect of Physical Activity on VEGF and HIF-1 Signaling. J. Clin. Res. Paramed. Sci. 2020, 9, e98493. [Google Scholar] [CrossRef]
- Taylor, C.W.; Ingham, S.A.; Hunt, J.E.; Martin, N.R.; Pringle, J.S.; Ferguson, R.A. Exercise Duration-Matched Interval and Continuous Sprint Cycling Induce Similar Increases in AMPK Phosphorylation, PGC-1α and VEGF mRNA Expression in Trained Individuals. Eur. J. Appl. Physiol. 2016, 116, 1445–1454. [Google Scholar] [CrossRef]
- Holloway, T.M.; Snijders, T.; VAN Kranenburg, J.; VAN Loon, L.J.C.; Verdijk, L.B. Temporal Response of Angiogenesis and Hypertrophy to Resistance Training in Young Men. Med. Sci. Sports Exerc. 2018, 50, 36–45. [Google Scholar] [CrossRef]
- Farzanegi, P.; Zamani, M.; Khalili, A.; Dehghani, H.; Fotohi, R.; Ghanbarpour, M.R.; Hosseini, S.A.; Peeri, M.; Rahmati-Ahmadabad, S.; Azarbayjani, M.A. Effects of Upper- and Lower-Extremity Resistance Training on Serum Vascular Endothelial Growth Factor, Myostatin, Endostatin and Follistatin Levels in Sedentary Male Students. Sci. Sports 2021, 36, 139.e1–139.e6. [Google Scholar] [CrossRef]
- Kim, H.B.; Seo, M.W.; Jung, H.C. Effects of Aerobic vs. Resistance Exercise on Vascular Function and Vascular Endothelial Growth Factor in Older Women. Healthcare 2023, 11, 2479. [Google Scholar] [CrossRef]
- Cochrane, D.J. Vibration Exercise: The Potential Benefits. Int. J. Sports Med. 2011, 32, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, P.; Birot, O. Altitude, Exercise, and Skeletal Muscle Angio-Adaptive Responses to Hypoxia: A Complex Story. Front. Physiol. 2021, 12, 735557. [Google Scholar] [CrossRef]
- Lindholm, M.E.; Rundqvist, H. Skeletal Muscle Hypoxia-Inducible Factor-1 and Exercise. Exp. Physiol. 2016, 101, 28–32. [Google Scholar] [CrossRef]
- Chih-Min, W.; Wen-Chyuan, C.; Zong-Yan, C. Effect of Acute Whole-Body Vibration Exercise with Blood Flow Restriction on Vascular Endothelial Growth Factor Response. Kinesiology 2018, 50, 149–156. [Google Scholar] [CrossRef]
- Suhr, F.; Brixius, K.; de Marées, M.; Bölck, B.; Kleinöder, H.; Achtzehn, S.; Bloch, W.; Mester, J. Effects of Short-Term Vibration and Hypoxia During High-Intensity Cycling Exercise on Circulating Levels of Angiogenic Regulators in Humans. J. Appl. Physiol. 2007, 103, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Kut, C.; Mac Gabhann, F.; Popel, A.S. Where is VEGF in the Body? A Meta-Analysis of VEGF Distribution in Cancer. Br. J. Cancer 2007, 97, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Beijer, A.; Rosenberger, A.; Bölck, B.; Suhr, F.; Rittweger, J.; Bloch, W. Whole-Body Vibrations do not Elevate the Angiogenic Stimulus When Applied During Resistance Exercise. PLoS ONE 2013, 8, e80143. [Google Scholar] [CrossRef]
- Theodorou, A.A.; Gerodimos, V.; Karatrantou, K.; Paschalis, V.; Chanou, K.; Jamurtas, A.Z.; Nikolaidis, M.G. Acute and Chronic Whole-Body Vibration Exercise does not Induce Health-Promoting Effects on the Blood Profile. J. Hum. Kinet. 2015, 46, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yabumoto, T.; Shin, S.; Shi, B.; Matsuoka, T. Effect of Short-Term Whole-Body Vibration Training on Metabolic Risk Factors, Inflammatory Markers, and Arterial Stiffness. Adv. Biosci. Biotechnol. 2014, 5, 438–445. [Google Scholar] [CrossRef]
- Rodriguez-Miguelez, P.; Fernandez-Gonzalo, R.; Collado, P.S.; Almar, M.; Martinez-Florez, S.; de Paz, J.A.; González-Gallego, J.; Cuevas, M.J. Whole-Body Vibration Improves the Anti-Inflammatory Status in Elderly Subjects Through Toll-Like Receptor 2 and 4 Signaling Pathways. Mech. Ageing 2015, 150, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Marconi, E.; Teixeira-Silva, Y.; Meirelles, A.G.; Melo-Oliveira, M.E.S.; Santos, A.C.G.; Reis-Silva, A.; Paineiras-Domingos, L.L.; Seixas, A.; Dionello, C.D.F.; Sá-Caputo, D.D.C.; et al. Inflammatory Biomarker Responses to Whole-Body Vibration in Subjects with Different Clinical Status: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 14853. [Google Scholar] [CrossRef]
- Oroszi, T.; van Heuvelen, M.; Nyakas, C.; van der Zee, E.A. Vibration Detection: Its function and Recent Advances in Medical Applications. F1000Research 2020, 9, F1000 Faculty Rev-619. [Google Scholar] [CrossRef]



| M | SD | 95% CI | 95% CI | Min | Max | p Values (η2) | ||
|---|---|---|---|---|---|---|---|---|
| Age [years] | EVG | 21.65 | 1.80 | 20.72 | 22.57 | 19.00 | 25.00 | 0.064 (0.097) |
| EXG | 20.17 | 1.75 | 19.06 | 21.28 | 19.00 | 25.00 | ||
| CON | 19.53 | 0.72 | 19.16 | 19.90 | 19.00 | 21.00 | ||
| total | 20.48 | 1.72 | 19.97 | 20.99 | 19.00 | 25.00 | ||
| BH [cm] | EVG | 162.76 | 7.51 | 158.90 | 166.63 | 151.00 | 173.00 | 0.187 (0.095) |
| EXG | 164.67 | 5.94 | 160.89 | 168.44 | 157.00 | 173.00 | ||
| CON | 167.24 | 4.56 | 164.89 | 169.58 | 160.00 | 173.00 | ||
| total | 164.91 | 6.32 | 163.04 | 166.79 | 151.00 | 173.00 | ||
| BM [kg] | EVG | 56.57 | 7.18 | 52.88 | 60.26 | 41.90 | 70.40 | 0.029 (0.137) |
| EXG | 59.43 | 6.04 | 55.59 | 63.26 | 50.20 | 69.10 | ||
| CON | 63.29 * | 8.71 | 58.81 | 67.77 | 51.20 | 77.90 | ||
| total | 59.80 | 7.92 | 57.44 | 62.15 | 41.90 | 77.90 | ||
| BMI [kg/cm2] | EVG | 21.31 | 1.87 | 20.35 | 22.28 | 18.40 | 25.20 | 0.351 (0.052) |
| EXG | 22.02 | 2.91 | 20.17 | 23.87 | 18.20 | 26.80 | ||
| CON | 22.57 | 2.44 | 21.32 | 23.83 | 19.50 | 28.60 | ||
| total | 21.96 | 2.40 | 21.25 | 22.67 | 18.20 | 28.60 | ||
| PBF [%] | EVG | 23.04 | 6.11 | 19.89 | 26.18 | 9.90 | 31.00 | 0.351 (0.068) |
| EXG | 25.62 | 4.14 | 22.99 | 28.25 | 18.50 | 31.50 | ||
| CON | 26.25 | 5.76 | 23.29 | 29.22 | 18.10 | 35.90 | ||
| total | 24.90 | 5.61 | 23.23 | 26.56 | 9.90 | 35.90 | ||
| FM [kg] | EVG | 13.34 | 4.54 | 11.01 | 15.67 | 4.20 | 20.00 | 0.119 (0.100) |
| EXG | 15.40 | 3.85 | 12.96 | 17.84 | 9.30 | 21.80 | ||
| CON | 17.03 | 5.89 | 14.00 | 20.06 | 9.30 | 27.90 | ||
| total | 15.24 | 5.08 | 13.73 | 16.75 | 4.20 | 27.90 | ||
| FFM [kg] | EVG | 43.24 | 3.81 | 41.28 | 45.19 | 37.80 | 52.20 | 0.013 (0.143) |
| EXG | 44.03 | 2.84 | 42.22 | 45.83 | 40.20 | 48.50 | ||
| CON | 46.25 * | 3.24 | 44.58 | 47.91 | 40.30 | 51.30 | ||
| total | 44.55 | 3.56 | 43.50 | 45.61 | 37.80 | 52.20 |
| M | SD | 95% CI | 95% CI | Min | Max | ||
|---|---|---|---|---|---|---|---|
| VEGF I | EVG | 240.48 | 94.40 | 190.18 | 290.78 | 117.81 | 407.56 |
| EXG | 124.30 | 29.89 | 105.31 | 143.29 | 88.69 | 181.06 | |
| CON | 199.88 | 97.44 | 149.78 | 249.98 | 72.19 | 372.69 | |
| total | 194.16 | 94.01 | 165.92 | 222.41 | 72.19 | 407.56 | |
| VEGF II | EVG | 225.28 | 83.77 | 180.64 | 269.92 | 116.69 | 398.94 |
| EXG | 121.29 | 26.19 | 104.65 | 137.93 | 76.81 | 167.19 | |
| total | 180.71 | 83.21 | 148.45 | 212.98 | 76.81 | 398.94 | |
| VEGF III | EVG | 237.16 | 80.30 | 194.37 | 279.94 | 143.69 | 389.31 |
| EXG | 126.79 | 31.58 | 106.73 | 146.86 | 82.19 | 179.81 | |
| CON | 203.24 | 105.33 | 149.08 | 257.39 | 82.06 | 383.69 | |
| total | 194.91 | 91.78 | 167.34 | 222.49 | 82.06 | 389.31 | |
| VEGF IV | EVG | 229.70 | 77.06 | 188.64 | 270.76 | 144.44 | 389.69 |
| EXG | 129.14 | 35.56 | 106.54 | 151.73 | 81.94 | 201.31 | |
| total | 186.60 | 79.89 | 155.62 | 217.58 | 81.94 | 389.69 | |
| Parameter | I | II | ΔII-I | III | IV | ΔIV-III | ΔIV-II | ANOVA p Values (η2) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Group | G × T | |||||||||
| VEGF [pg/mL] | EVG | 240.48 ± 94.4 ## | 225.28 ± 83.77 ## | −15.2 ± 38.24 | 237.16 ± 80.3 ## | 229.7 ± 77.06 ## | −7.46 ± 11.87 | 4.41 ± 29.85 | 0.440 (0.104) | 0.003 (0.003) | 0.626 (0.069) |
| EXG | 124.3 ± 29.89 | 121.29 ± 26.19 | −3.01 ± 20.33 | 126.79 ± 31.58 | 129.14 ± 35.56 | 2.34 ± 33.9 | 7.84 ± 31.3 | ||||
| eNOS [U/mL] | EVG | 43.46 ± 12.34 | 44.84 ± 12.12 | 1.39 ± 5.12 | 41.97 ± 11.58 | 42.49 ± 10.28 | 0.529 ± 7.191 | −2.349 ± 3.943 | 0.145 (0.072) | 0.315 (0.042) | 0.909 (0.007) |
| EXG | 39.52 ± 7.2 | 41.15 ± 7.16 | 1.64 ± 9.1 | 37.68 ± 6.67 | 40.07 ± 7.57 | 2.39 ± 6.53 | −1.09 ± 5.95 | ||||
| hsCRP [mg/L] | EVG | 0.93 ± 1.07 | 0.91 ± 1.11 | −0.03 ± 0.35 | 0.89 ± 0.76 | 0.9 ± 0.78 | 0.01 ± 0.05 | −0.01 ± 0.77 | 0.044 (0.072) | 0.516 (0.016) | 0.228 (0.056) |
| EXG | 1.05 ± 1.12 | 0.95 ± 0.89 | −0.1 ± 0.41 | 1.3 ± 1.24 | 1.31 ± 1.27 | 0.01 ± 0,04 | 0.36 ± 0.49 | ||||
| Parameter | I | III | Δ III-I | ANOVA p Values (η2) | |||
|---|---|---|---|---|---|---|---|
| Time | Group | G × T | |||||
| VEGF [pg/mL] | EVG | 240.48 ± 94.4 # | 237.16 ± 80.3 # | −3.33 ± 38.84 | 0.874 (0.001) | 0.003 (0.003) | 0.843 0.008 |
| EXG | 124.3 ± 29.89 | 126.79 ± 31.58 | 2.49 ± 28.08 | ||||
| CON | 199.88 ± 97.44 | 203.24 ± 105.33 | 3.36 ± 35.2 | ||||
| eNOS [U/mL] | EVG | 43.46 ± 12.34 | 41.97 ± 11.58 | −1.49 ± 7.47 | 0.275 0.033 | 0.613 0.027 | 0.973 0.002 |
| EXG | 39.52 ± 7.2 | 37.68 ± 6.67 | −1.84 ± 9.27 | ||||
| CON | 43.93 ± 20.21 | 42.86 ± 17.46 | −1.07 ± 8.05 | ||||
| hsCRP [mg/L] | EVG | 0.93 ± 1.07 | 0.89 ± 0.76 | −0.05 ± 0.78 | 0.327 0.023 | 0.183 0.078 | 0.438 0.039 |
| EXG | 1.05 ± 1.12 | 1.3 ± 1.24 | 0.25 ± 0.5 | ||||
| CON | 0.56 ± 0.51 | 0.62 ± 0.57 | 0.06 ± 0.41 | ||||
| M | SD | 95% CI | 95% CI | Min | Max | ||
|---|---|---|---|---|---|---|---|
| eNOS I | EVG | 43.46 | 12.34 | 36.33 | 50.58 | 27.12 | 65.59 |
| EXG | 39.52 | 7.20 | 34.94 | 44.09 | 31.03 | 54.24 | |
| CON | 43.93 | 20.21 | 31.72 | 56.14 | 26.98 | 96.32 | |
| total | 42.40 | 14.14 | 37.82 | 46.98 | 26.98 | 96.32 | |
| eNOS II | EVG | 44.84 | 12.12 | 37.85 | 51.84 | 28.17 | 61.09 |
| EXG | 41.15 | 7.16 | 36.61 | 45.70 | 29.48 | 55.41 | |
| total | 43.14 | 10.12 | 39.05 | 47.23 | 28.17 | 61.09 | |
| eNOS III | EVG | 41.97 | 11.58 | 35.28 | 48.65 | 25.03 | 59.64 |
| EXG | 37.68 | 6.67 | 33.44 | 41.92 | 25.13 | 46.82 | |
| CON | 42.86 | 17.46 | 32.31 | 53.41 | 26.44 | 83.60 | |
| total | 40.94 | 12.65 | 36.84 | 45.04 | 25.03 | 83.60 | |
| eNOS IV | EVG | 42.49 | 10.28 | 36.56 | 48.43 | 27.39 | 57.32 |
| EXG | 40.07 | 7.57 | 35.26 | 44.88 | 30.72 | 55.20 | |
| total | 41.37 | 9.04 | 37.72 | 45.02 | 27.39 | 57.32 |
| M | SD | 95% CI | 95% CI | Min | Max | ||
|---|---|---|---|---|---|---|---|
| hsCRP I | EVG | 0.93 | 1.07 | 0.36 | 1.50 | 0.20 | 3.90 |
| EXG | 1.05 | 1.12 | 0.34 | 1.76 | 0.22 | 3.47 | |
| CON | 0.56 | 0.51 | 0.30 | 0.82 | 0.15 | 2.11 | |
| total | 0.82 | 0.92 | 0.55 | 1.10 | 0.15 | 3.90 | |
| hsCRP II | EVG | 0.91 | 1.11 | 0.31 | 1.50 | 0.16 | 4.24 |
| EXG | 0.95 | 0.89 | 0.38 | 1.52 | 0.26 | 3.24 | |
| total | 0.93 | 1.01 | 0.54 | 1.31 | 0.16 | 4.24 | |
| hsCRP III | EVG | 0.89 | 0.76 | 0.49 | 1.29 | 0.18 | 2.49 |
| EXG | 1.30 | 1.24 | 0.51 | 2.09 | 0.24 | 4.38 | |
| CON | 0.62 | 0.57 | 0.32 | 0.91 | 0.15 | 2.18 | |
| total | 0.90 | 0.88 | 0.63 | 1.16 | 0.15 | 4.38 | |
| hsCRP IV | EVG | 0.90 | 0.78 | 0.48 | 1.32 | 0.18 | 2.49 |
| EXG | 1.31 | 1.27 | 0.50 | 2.11 | 0.23 | 4.48 | |
| total | 1.07 | 1.02 | 0.68 | 1.47 | 0.18 | 4.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gattner, H.; Adamiak, J.; Czerwińska-Ledwig, O.; Mętel, S.; Kępińska-Szyszkowska, M.; Piotrowska, A. Whole Body Vibration Training Has No Effect on Vascular Endothelial and Inflammatory Markers in Young Healthy Women. J. Clin. Med. 2024, 13, 4228. https://doi.org/10.3390/jcm13144228
Gattner H, Adamiak J, Czerwińska-Ledwig O, Mętel S, Kępińska-Szyszkowska M, Piotrowska A. Whole Body Vibration Training Has No Effect on Vascular Endothelial and Inflammatory Markers in Young Healthy Women. Journal of Clinical Medicine. 2024; 13(14):4228. https://doi.org/10.3390/jcm13144228
Chicago/Turabian StyleGattner, Halina, Justyna Adamiak, Olga Czerwińska-Ledwig, Sylwia Mętel, Magdalena Kępińska-Szyszkowska, and Anna Piotrowska. 2024. "Whole Body Vibration Training Has No Effect on Vascular Endothelial and Inflammatory Markers in Young Healthy Women" Journal of Clinical Medicine 13, no. 14: 4228. https://doi.org/10.3390/jcm13144228






