Microbiological and Salivary Biomarkers Successfully Predict Site-Specific and Whole-Mouth Outcomes of Nonsurgical Periodontal Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion/Exclusion Criteria
2.3. Clinical Parameters and Intervention
2.4. Outcomes
2.5. Sample Size
2.6. Subgingival Biofilm Sampling
2.7. Unstimulated Saliva Collection
2.8. Real-Time PCR Assay for F. nucleatum and P. gingivalis
2.9. Assaying Salivary Proteins
2.10. Statistical Analysis
3. Results
3.1. Basic Demographic and Clinical Variables
3.2. Clinical Outcomes
3.3. Microbiological Outcomes
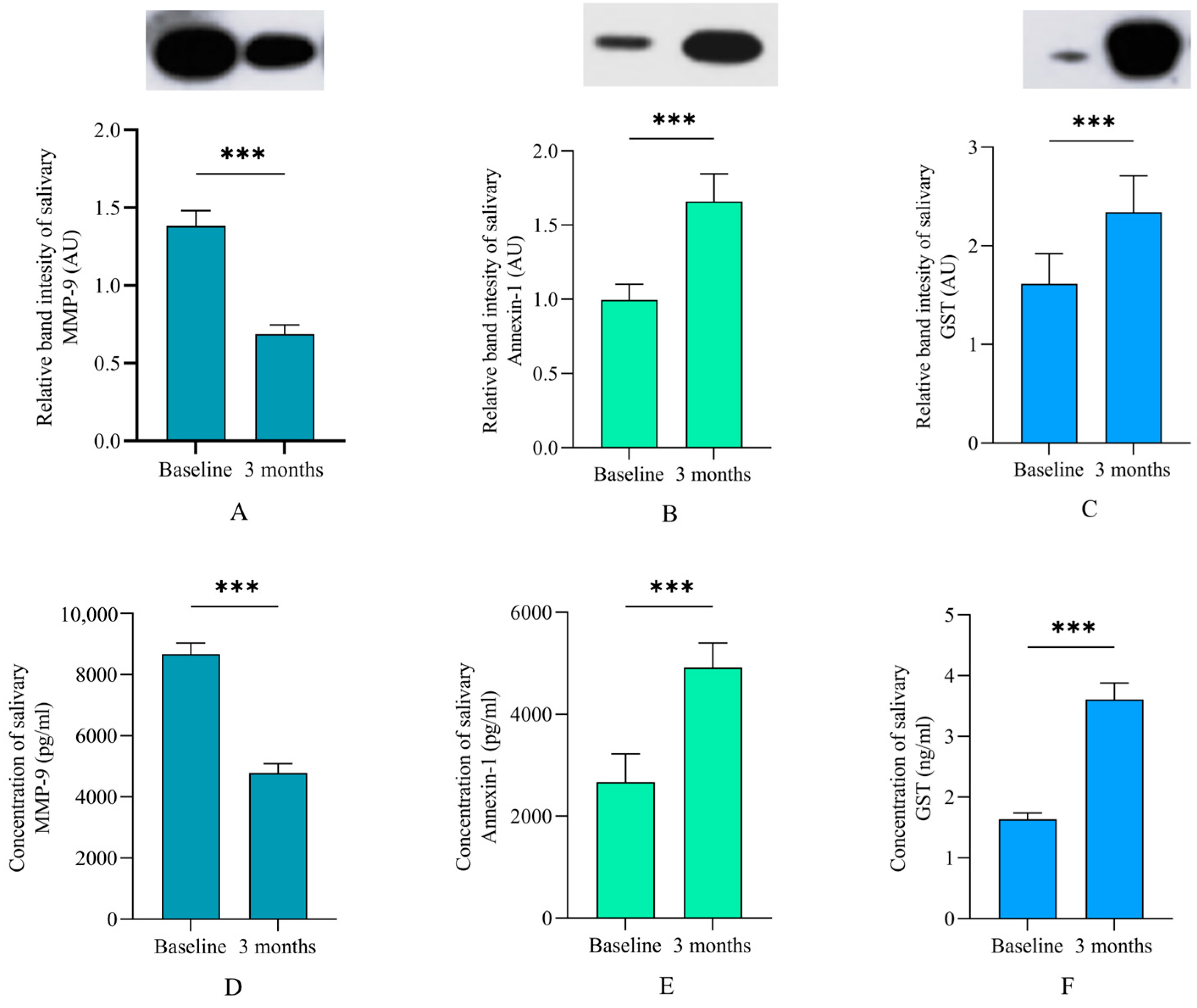
3.4. Levels of Salivary Proteins
3.5. Predictive Potential of Salivary and Bacterial Biomarkers for NSPT
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental plaque-induced gingival conditions. J. Periodontol. 2018, 89 (Suppl. S1), S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol. 2000 2005, 38, 72–122. [Google Scholar] [CrossRef] [PubMed]
- Manoil, D.; Parga, A.; Bostanci, N.; Belibasakis, G.N. Microbial diagnostics in periodontal diseases. Periodontol. 2000 2024. online version of record. [Google Scholar]
- Diaz, P.I.; Zilm, P.S.; Rogers, A.H. Fusobacterium nucleatum supports the growth of Porphyromonas gingivalis in oxygenated and carbon-dioxide-depleted environments. Microbiology 2002, 148, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Ulm, C.; Reischl, H.; Nguyen, P.Q.; Matejka, M.; Rausch-Fan, X. Serum cytokine levels in periodontitis patients in relation to the bacterial load. J. Periodontol. 2011, 82, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, X.; Zhang, H.; Liu, X.; Pan, S.; Li, C. The role of extracellular matrix metalloproteinase inducer glycosylation in regulating matrix metalloproteinases in periodontitis. J. Periodontal Res. 2018, 53, 391–402. [Google Scholar] [CrossRef]
- Araújo, A.A.; Pereira, A.; Medeiros, C.; Brito, G.A.C.; Leitão, R.F.C.; Araújo, L.S.; Guedes, P.M.M.; Hiyari, S.; Pirih, F.Q.; Araújo Júnior, R.F. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS ONE 2017, 12, e0183506. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Abdulkareem, A.A.; Zardawi, F.M.; Gul, S.S. Determination of the Accuracy of Salivary Biomarkers for Periodontal Diagnosis. Diagnostics 2022, 12, 2485. [Google Scholar] [CrossRef] [PubMed]
- Atarchi, A.R. Potential of Salivary Matrix Metalloproteinase 9 to Discriminate Periodontal health and disease. J. Baghdad Coll. Dent. 2022, 34, 74–79. [Google Scholar] [CrossRef]
- Salinas, A.E.; Wong, M.G. Glutathione S-transferases—A review. Curr. Med. Chem. 1999, 6, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Oktay, S.; Ozoner, O.; Alturfan, E.E.; Noyan, U. Determination of Oxidative Stress Parameters and Tissue Factor Activity in the Saliva of Patients with Periodontitis. Eur. J. Biol. 2019, 78, 63–68. [Google Scholar] [CrossRef]
- Borges, I., Jr.; Moreira, E.A.; Filho, D.W.; de Oliveira, T.B.; da Silva, M.B.; Fröde, T.S. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediat. Inflamm. 2007, 2007, 45794. [Google Scholar] [CrossRef] [PubMed]
- Camargo Ortega, V.R.; Bravo López, L.D.; Visoso Salgado, A.; Mejia Sanchez, F.; Castillo Cadena, J. Polymorphisms in Glutathione S-Transferase M1, T1, and P1 in Patients with Chronic Periodontitis: A Pilot Study. Int. Sch. Res. Not. 2014, 2014, 135368. [Google Scholar] [CrossRef]
- Rizal, M.I.; Soeroso, Y.; Sulijaya, B.; Assiddiq, B.F.; Bachtiar, E.W.; Bachtiar, B.M. Proteomics approach for biomarkers and diagnosis of periodontitis: Systematic review. Heliyon 2020, 6, e04022. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Vago, J.P.; Teixeira, M.M.; Sousa, L.P. Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance. J. Immunol. Res. 2016, 2016, 8239258. [Google Scholar] [CrossRef] [PubMed]
- Casarin, R.C.V.; Salmon, C.R.; Stolf, C.S.; Paz, H.E.S.; Rangel, T.P.; Domingues, R.R.; Pauletti, B.A.; Paes-Leme, A.F.; Araújo, C.; Santamaria, M.P.; et al. Salivary annexin A1: A candidate biomarker for periodontitis. J. Clin. Periodontol. 2023, 50, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Heywood, W.; Mills, K.; Parkar, M.; Nibali, L.; Donos, N. Application of label-free absolute quantitative proteomics in human gingival crevicular fluid by LC/MS E (gingival exudatome). J. Proteome Res. 2010, 9, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.N.; Belibasakis, G.N.; Gumus, P.; Öztürk, V.; Emingil, G.; Bostanci, N. Annexin-1 as a salivary biomarker for gingivitis during pregnancy. J. Periodontol. 2018, 89, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Z.; Zhao, Z.; Shen, X.; Feng, J.; Xiong, J. Oral microbiota in periodontitis patients with and without type 2 diabetes mellitus and their shifts after the nonsurgical periodontal therapy. Heliyon 2023, 9, e22110. [Google Scholar] [CrossRef]
- Macín-Cabrera, S.; Sanz-Alonso, M.; Castrillón-Rivera, L.; Palma-Ramos, A.; Noguez-Méndez, N.; Quirino-Barreda, C.; Rubio-Martínez, A. Non surgical periodontal treatment in patients with gingivitis and moderate periodontitis. Biochemical and microbiological response. Rev. Odontológica Mex. 2015, 19, e151–e160. [Google Scholar] [CrossRef]
- Schulz, S.; Stein, J.M.; Schumacher, A.; Kupietz, D.; Yekta-Michael, S.S.; Schittenhelm, F.; Conrads, G.; Schaller, H.G.; Reichert, S. Nonsurgical Periodontal Treatment Options and Their Impact on Subgingival Microbiota. J. Clin. Med. 2022, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Belstrøm, D.; Grande, M.A.; Sembler-Møller, M.L.; Kirkby, N.; Cotton, S.L.; Paster, B.J.; Holmstrup, P. Influence of periodontal treatment on subgingival and salivary microbiotas. J. Periodontol. 2018, 89, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Drisko, C.H. Nonsurgical periodontal therapy. Periodontol. 2000 2001, 25, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Raedel, M.; Noack, B.; Priess, H.W.; Bohm, S.; Walter, M.H. Massive data analyses show negative impact of type 1 and 2 diabetes on the outcome of periodontal treatment. Clin. Oral. Investig. 2021, 25, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Madi, M.; Smith, S.; Alshehri, S.; Zakaria, O.; Almas, K. Influence of Smoking on Periodontal and Implant Therapy: A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 5368. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.G., Jr. Compliance and its role in periodontal therapy. Periodontol. 2000 1996, 12, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, A.; Abdulbaqi, H.; Gul, S.; Milward, M.; Chasib, N.; Alhashimi, R. Classic vs. Novel Antibacterial Approaches for Eradicating Dental Biofilm as Adjunct to Periodontal Debridement: An Evidence-Based Overview. Antibiotics 2021, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.S.; Zardawi, F.M.; Abdulkareem, A.A.; Shaikh, M.S.; Al-Rawi, N.H.; Zafar, M.S. Efficacy of MMP-8 Level in Gingival Crevicular Fluid to Predict the Outcome of Nonsurgical Periodontal Treatment: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 3131. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Quirynen, M.; De Soete, M.; Boschmans, G.; Pauwels, M.; Coucke, W.; Teughels, W.; van Steenberghe, D. Benefit of “one-stage full-mouth disinfection” is explained by disinfection and root planing within 24 hours: A randomized controlled trial. J. Clin. Periodontol. 2006, 33, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; Needleman, I. Endpoints of active periodontal therapy. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 61–71. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Non-surgical pocket therapy: Mechanical. Ann. Periodontol. 1996, 1, 443–490. [Google Scholar] [CrossRef]
- Werner, N.; Heck, K.; Walter, E.; Ern, C.; Bumm, C.V.; Folwaczny, M. Probing pocket depth reduction after non-surgical periodontal therapy: Tooth-related factors. J. Periodontol. 2023, 95, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wei, Y.; Hu, W.; Nie, Y.; Wu, X.; Lu, R. The Subgingival Microbiome of Periodontal Pockets With Different Probing Depths in Chronic and Aggressive Periodontitis: A Pilot Study. Front. Cell. Infect. Microbiol. 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol. 2000 2018, 76, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, G.A.; Timmerman, M.F. A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 55–71; discussion 90–51. [Google Scholar]
- Van der Weijden, G.A.F.; Dekkers, G.J.; Slot, D.E. Success of non-surgical periodontal therapy in adult periodontitis patients: A retrospective analysis. Int. J. Dent. Hyg. 2019, 17, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Zeza, B.; Kuis, D.; Vrazic, D.; Domic, T.; Olszewska-Czyz, I.; Popova, C.; Kotsilkov, K.; Firkova, E.; Dermendzieva, Y.; et al. Treatment of Residual Periodontal Pockets Using a Hyaluronic Acid-Based Gel: A 12 Month Multicenter Randomized Triple-Blinded Clinical Trial. Antibiotics 2021, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Schmid, B.; Rutar, A.; Lang, N.P. Local antibiotic therapy guided by microbiological diagnosis. J. Clin. Periodontol. 2002, 29, 743–749. [Google Scholar] [CrossRef]
- Mombelli, A.; McNabb, H.; Lang, N.P. Black-pigmenting gram-negative bacteria in periodontal disease. I. Topographic distribution in the human dentition. J. Periodontal Res. 1991, 26, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, F.; Orrù, G.; Pilloni, A.; Bartosiewicz, I.; Perricone, C.; Martino, E.; Lucchetti, R.; Fais, S.; Vomero, M.; Olivieri, M.; et al. Porphyromonas gingivalis in the tongue biofilm is associated with clinical outcome in rheumatoid arthritis patients. Clin. Exp. Immunol. 2018, 194, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Schenk, A.; Lungeanu, D.; Reitmeir, P.; Eickholz, P. Nonsurgical and surgical periodontal therapy in single-rooted teeth. Clin. Oral Investig. 2007, 11, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Salhi, L.; Albert, A.; Seidel, L.; Lambert, F. Respective Effects of Oral Hygiene Instructions and Periodontal Nonsurgical Treatment (Debridement) on Clinical Parameters and Patient-Reported Outcome Measures with Respect to Smoking. J. Clin. Med. 2020, 9, 2491. [Google Scholar] [CrossRef]
- Lertpimonchai, A.; Rattanasiri, S.; Arj-Ong Vallibhakara, S.; Attia, J.; Thakkinstian, A. The association between oral hygiene and periodontitis: A systematic review and meta-analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Herz, M.M.; Celebi, N.; Bruckner, T.; Bartha, V. Influence on the patient’s oral hygiene depending on the treatment performed by either one or different pre-graduate practitioners - a randomized, controlled, clinical short-term trial. Clin. Oral Investig. 2022, 26, 5339–5350. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Yin, H.J.; Chang, H.H.; Kao, C.T.; Tu, C.C.; Chen, Y.W. Baseline probing depth and interproximal sites predict treatment outcomes of non-surgical periodontal therapy. J. Dent. Sci. 2020, 15, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.S.; Abdulkareem, A.A.; Sha, A.M.; Rawlinson, A. Diagnostic Accuracy of Oral Fluids Biomarker Profile to Determine the Current and Future Status of Periodontal and Peri-Implant Diseases. Diagnostics 2020, 10, 838. [Google Scholar] [CrossRef]
- Cafiero, C.; Spagnuolo, G.; Marenzi, G.; Martuscelli, R.; Colamaio, M.; Leuci, S. Predictive Periodontitis: The Most Promising Salivary Biomarkers for Early Diagnosis of Periodontitis. J. Clin. Med. 2021, 10, 1488. [Google Scholar] [CrossRef]
- Taba, M., Jr.; Kinney, J.; Kim, A.S.; Giannobile, W.V. Diagnostic biomarkers for oral and periodontal diseases. Dent. Clin. N. Am. 2005, 49, 551–571. [Google Scholar] [CrossRef]
- Binti Badlishah Sham, N.I.; Grant, M.M. Role of Glutathione in Neutrophil Chemotaxis in Periodontitis. Oral 2023, 3, 526–538. [Google Scholar] [CrossRef]
- Sham, N.I.B. 240-Decreased Intracellular Glutathione in Peripheral Neutrophils Decreases Chemotactic Behaviour towards Interleukin 8 and FMLP. Free Radic. Biol. Med. 2016, 100, S109. [Google Scholar] [CrossRef]
- Grant, M.M.; Brock, G.R.; Matthews, J.B.; Chapple, I.L. Crevicular fluid glutathione levels in periodontitis and the effect of non-surgical therapy. J. Clin. Periodontol. 2010, 37, 17–23. [Google Scholar] [CrossRef]
- Brock, G.R.; Butterworth, C.J.; Matthews, J.B.; Chapple, I.L. Local and systemic total antioxidant capacity in periodontitis and health. J. Clin. Periodontol. 2004, 31, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Sculley, D.V.; Langley-Evans, S.C. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin. Sci. 2003, 105, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef]
- Marcaccini, A.M.; Meschiari, C.A.; Zuardi, L.R.; de Sousa, T.S.; Taba, M., Jr.; Teofilo, J.M.; Jacob-Ferreira, A.L.; Tanus-Santos, J.E.; Novaes, A.B., Jr.; Gerlach, R.F. Gingival crevicular fluid levels of MMP-8, MMP-9, TIMP-2, and MPO decrease after periodontal therapy. J. Clin. Periodontol. 2010, 37, 180–190. [Google Scholar] [CrossRef]
- Baelum, V.; López, R. Defining and predicting outcomes of non-surgical periodontal treatment: A 1-yr follow-up study. Eur. J. Oral. Sci. 2016, 124, 33–44. [Google Scholar] [CrossRef]
- Imran, N.K.; Abdulbaqi, H.R.; Milward, M. The prevalence of periodontitis in an Iraqi population using the 2017 classification. J. Bagh. Coll. Dent. 2024, 36, 1–10. [Google Scholar] [CrossRef]
- Hussein, H.R.; Abdulkareem, A.A.; Milward, M.R.; Cooper, P.R. Ability of gingival crevicular fluid volume, E-cadherin, and total antioxidant capacity levels for predicting outcomes of nonsurgical periodontal therapy for periodontitis patients. J. Periodontal. Res. 2024, 59, 289–298. [Google Scholar] [CrossRef]
- Mohammed, A.; Fadhil, R.; Mahmood, M.S.; Al-Waeli, H.A. Diagnostic biomarkers for periodontitis (observational case-control study). J. Bagh. Coll. Dent. 2024, 36, 11–19. [Google Scholar] [CrossRef]
- Go, H.; Park, T.; Shin, A.R.; Jung, Y.S.; Amano, A.; Song, K.B.; Choi, Y.H. Validity of a combination of periodontal pathogens and salivary biomarkers as predictors of periodontitis. J. Periodontal Res. 2022, 57, 1083–1092. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; Manzano-Moreno, F.J.; Ruiz, C.; Illescas-Montes, R. Salivary Biomarkers and Their Application in the Diagnosis and Monitoring of the Most Common Oral Pathologies. Int. J. Mol. Sci. 2020, 21, 5173. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, N.; Todorovic, T.; Rakic, M.; Milinkovic, I.; Dozic, I.; Jankovic, S.; Aleksic, Z.; Cakic, S. Salivary antioxidants as periodontal biomarkers in evaluation of tissue status and treatment outcome. J. Periodontal Res. 2014, 49, 129–136. [Google Scholar] [CrossRef]
- Priya, K.L.; Mahendra, J.; Mahendra, L.; Kanakamedala, A.; Alsharif, K.F.; Mugri, M.H.; Varadarajan, S.; Alamoudi, A.; Hassan, A.A.A.; Alnfiai, M.M.; et al. Salivary Biomarkers in Periodontitis Post Scaling and Root Planing. J. Clin. Med. 2022, 11, 7142. [Google Scholar] [CrossRef] [PubMed]
- Kazem, N.M.; Abdulkareem, A.A.; Milward, M.R. Salivary E-cadherin as a biomarker for diagnosis and predicting grade of periodontitis. J. Periodontal Res. 2023, 58, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Lee, C.S.; Cho, H.J.; Jeon, S.; Choi, Y.N.; Kim, S.; Kim, D.; Jin Lee, H.; Vu, H.; Jeong, H.J.; et al. Diagnostic ability of salivary matrix metalloproteinase-9 lateral flow test point-of-care test for periodontitis. J. Clin. Periodontol. 2020, 47, 1354–1361. [Google Scholar] [CrossRef]
- Luchian, I.; Moscalu, M.; Goriuc, A.; Nucci, L.; Tatarciuc, M.; Martu, I.; Covasa, M. Using Salivary MMP-9 to Successfully Quantify Periodontal Inflammation during Orthodontic Treatment. J. Clin. Med. 2021, 10, 379. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, S.; Jeon, S.; Kim, S.J.; Cho, H.J.; Choi, Y.N. Diagnostic and Prognostic ability of salivary MMP-9 and S100A8 for periodontitis. J. Clin. Periodontol. 2020, 47, 1191–1200. [Google Scholar] [CrossRef]
- Veljovic, T.; Djuric, M.; Mirnic, J.; Gusic, I.; Maletin, A.; Ivic, S.; Stojilkovic, M.; Brkic, S. Effect of Nonsurgical Periodontal Treatment on Salivary and Plasma Superoxide Dismutase Levels of Patients Suffering from Periodontitis. J. Clin. Med. 2023, 12, 6688. [Google Scholar] [CrossRef]
- Sheikh, M.H.; Solito, E. Annexin A1: Uncovering the Many Talents of an Old Protein. Int. J. Mol. Sci. 2018, 19, 1045. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Belstrøm, D.; Eick, S.; Gursoy, U.K.; Johansson, A.; Könönen, E. Periodontal microbiology and microbial etiology of periodontal diseases: Historical concepts and contemporary perspectives. Periodontol. 2000 2023. online ahead of print. [Google Scholar]
- Gursoy, U.K.; Könönen, E.; Pussinen, P.J.; Tervahartiala, T.; Hyvärinen, K.; Suominen, A.L.; Uitto, V.J.; Paju, S.; Sorsa, T. Use of host- and bacteria-derived salivary markers in detection of periodontitis: A cumulative approach. Dis. Markers 2011, 30, 299–305. [Google Scholar] [CrossRef] [PubMed]
- O’Brien-Simpson, N.M.; Burgess, K.; Lenzo, J.C.; Brammar, G.C.; Darby, I.B.; Reynolds, E.C. Rapid Chair-Side Test for Detection of Porphyromonas gingivalis. J. Dent. Res. 2017, 96, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Brochut, P.F.; Marin, I.; Baehni, P.; Mombelli, A. Predictive value of clinical and microbiological parameters for the treatment outcome of scaling and root planing. J. Clin. Periodontol. 2005, 32, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.S.; Griffiths, G.S.; Stafford, G.P.; Al-Zubidi, M.I.; Rawlinson, A.; Douglas, C.W.I. Investigation of a Novel Predictive Biomarker Profile for the Outcome of Periodontal Treatment. J. Periodontol. 2017, 88, 1135–1144. [Google Scholar] [CrossRef]
- Mombelli, A.; Almaghlouth, A.; Cionca, N.; Cancela, J.; Courvoisier, D.S.; Giannopoulou, C. Microbiologic Response to Periodontal Therapy and Multivariable Prediction of Clinical Outcome. J. Periodontol. 2017, 88, 1253–1262. [Google Scholar] [CrossRef]


| Variables | |
|---|---|
| Age † | 55.4 ± 6.3 |
| Sex ‡ | |
| Male | 31, 45.6% |
| Female | 37, 54.4% |
| Stage ‡ | |
| 2 | 19, 27.9% |
| 3 | 32, 47.1% |
| 4 | 17, 25.0% |
| Grade ‡ | |
| B | 44, 64.7% |
| C | 24, 35.3% |
| Periodontal pockets (4–6 mm) | |
| Total | 1111, 100.0% |
| ≥5 mm (no BOP) ‡ | 692, 62.3% |
| PPD ≥ 4 mm + BOP ‡ | 419, 37.7% |
| Missing teeth † | 4.2 ± 1.3 |
| Baseline | Endpoint | |
|---|---|---|
| PI † | 45.6 ± 8.2 | 11.2 ± 6.2 * |
| BOP † | 53.7 ± 8.2 | 13.1 ± 5.3 * |
| PPD † | 4.7 ± 0.7 | 3.8 ± 0.6 * |
| CAL † | 3.5 ± 0.6 | 3.23 ± 1.1 * |
| Whole-mouth outcomes ‡ | ||
| Success | 42, 61.8% | |
| Failure | 26, 38.2% | |
| Teeth outcomes ‡ | ||
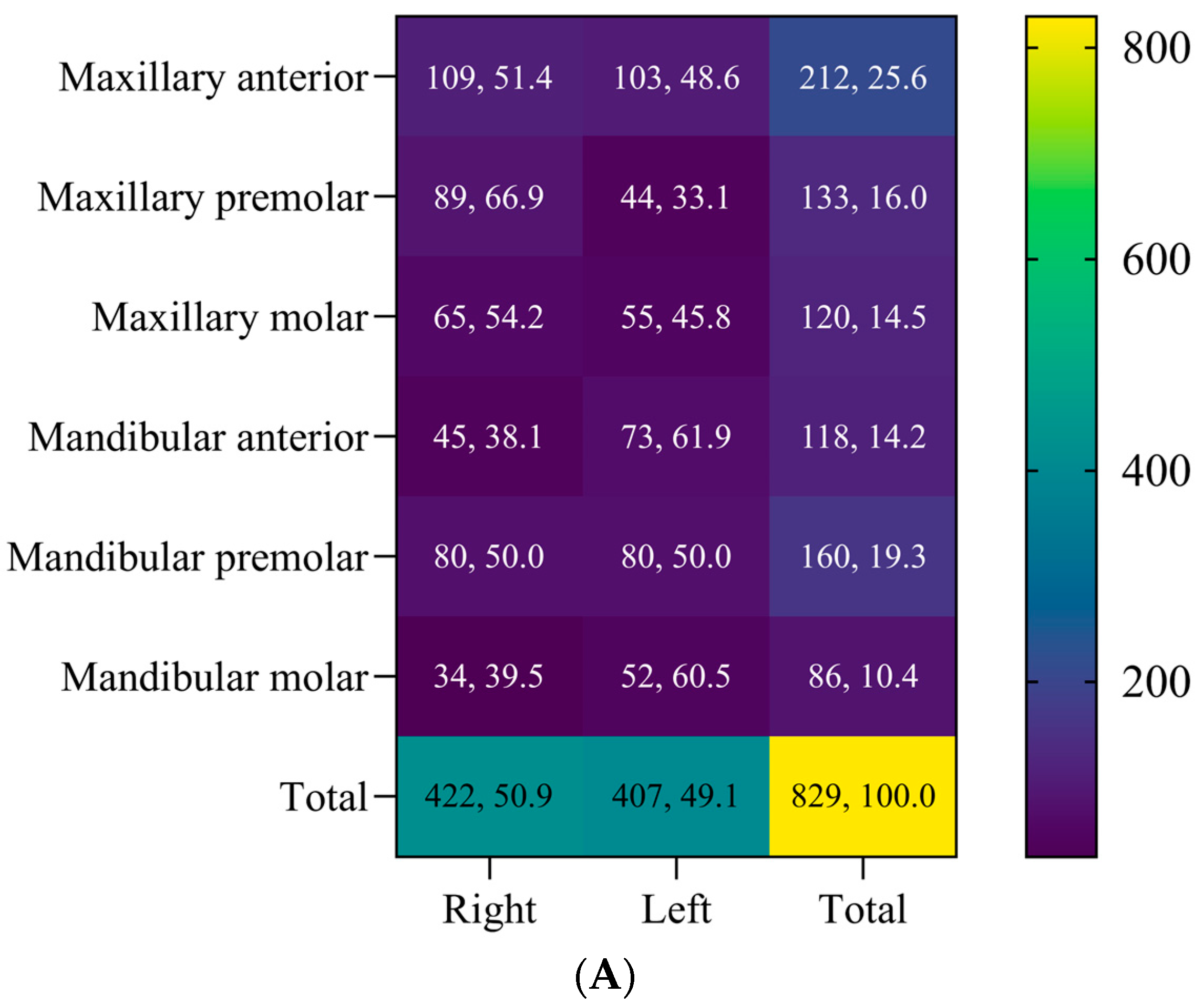
| Maxillary (n = 465) | ||
| Success | 332, 71.4% | |
| Failure | 133, 28.6% | |
| Mandibular (n = 364) | ||
| Success | 249, 68.4% | |
| Failure | 115, 31.6% | |
| All teeth (n = 829) | ||
| Success | 581, 70.1% | |
| Failure | 248, 29.9% | |
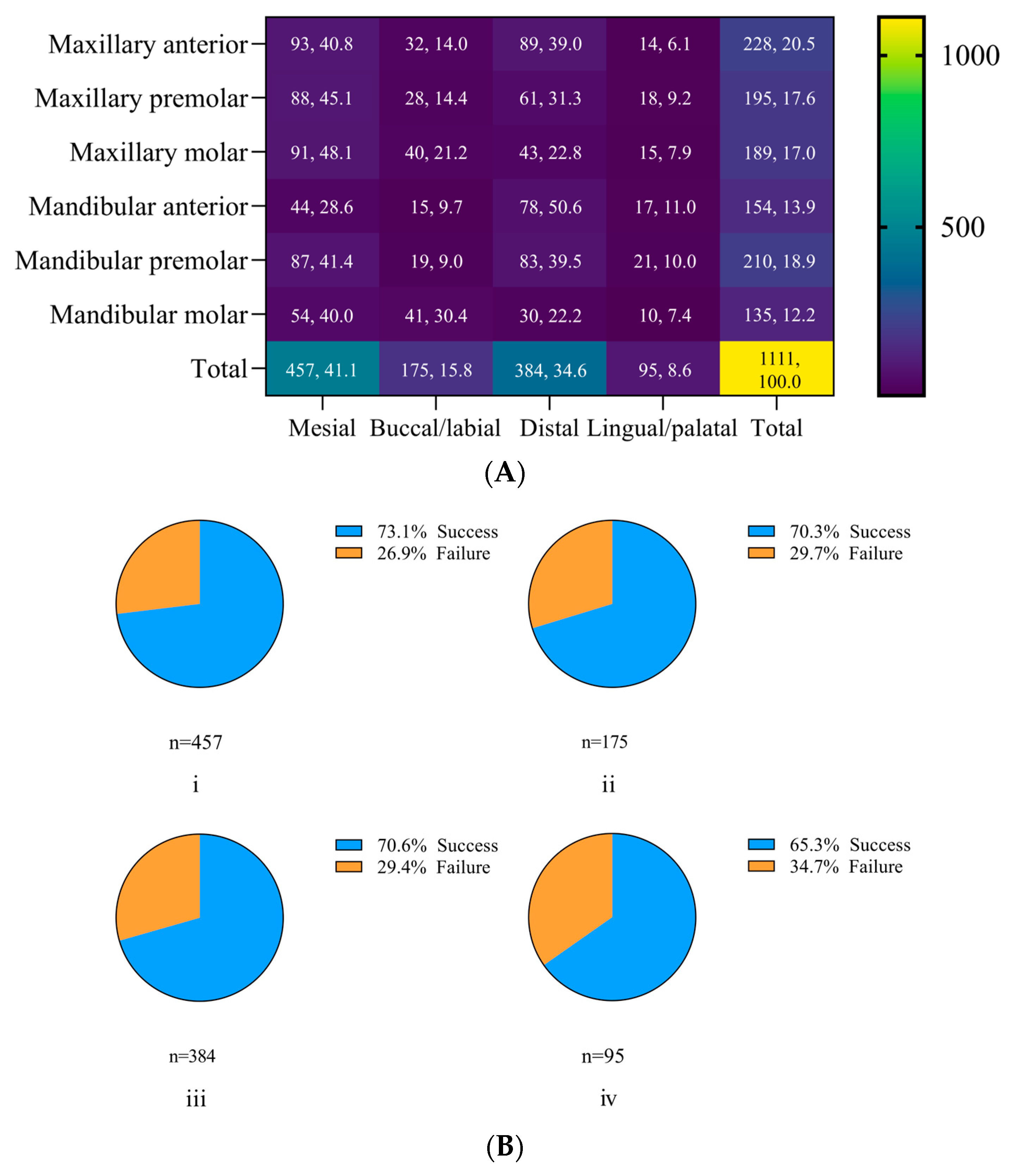
| Site outcomes (n = 1111) ‡ | ||
| Success | 790, 71.1% | |
| Failure | 321, 28.9% | |
| Residual pockets | ||
| ≥5 mm (no BOP) ‡ | 120, 10.8% | |
| PPD ≥ 4 mm +BOP ‡ | 201, 18.1% |
| Baseline | Endpoint | p-Value * | |
|---|---|---|---|
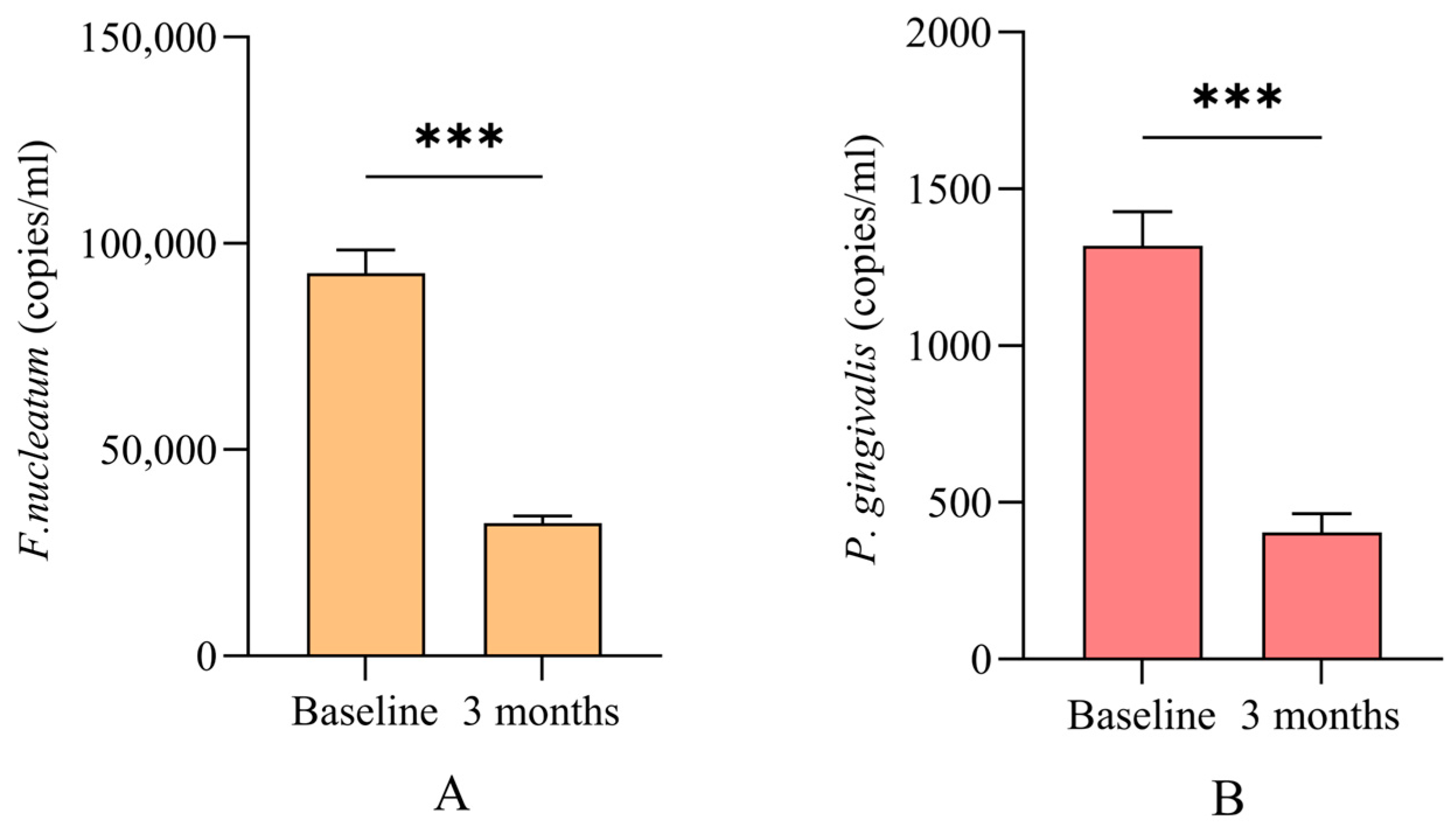
| P. gingivalis † | |||
| + | 250, 22.5% | 187, 16.8% | |
| − | 861, 77.5% | 924, 83.2% | <0.001 |
| F. nucleatum † | |||
| + | 1053, 94.8% | 524, 47.2% | |
| − | 58, 5.2% | 587, 52.8% | <0.001 |
| Unresponsive sites † | |||
| P. gingivalis + | 72, 38.5% | ||
| F. nucleatum + | 142, 27.1% | ||
| Simultaneous presence of bacteria † | |||
| Total sites | 98, 8.8% | ||
| Unresponsive sites | 98, 30.5% |
| Predictors | B | SE | p-Value | Exp (B) | 95% CI for Exp (B) |
|---|---|---|---|---|---|
| Site-specific | |||||
| Tooth type a | |||||
| Premolar | 0.011 | 0.171 | 0.95 | 1.011 | 0.723 to 1.413 |
| Molar | −0.194 | 0.202 | 0.33 | 1.214 | 0.817 to 1.803 |
| Mandible b | −0.130 | 0.142 | 0.34 | 0.878 | 0.665 to 1.160 |
| F. nucleatum | 0.015 | 0.310 | 0.96 | 1.015 | 0.552 to 1.865 |
| P. gingivalis | −0.317 | 0.152 | 0.006 | 0.997 | 0.180 to 0.463 |
| PPD | −0.457 | 0.118 | <0.001 | 0.633 | 0.503 to 0.799 |
| CAL | 0.067 | 0.103 | 0.51 | 1.069 | 0.873 to 1.309 |
| Site c | |||||
| Distal | 0.317 | 0.144 | 0.03 | 1.374 | 1.027 to 1.837 |
| Facial | 0.308 | 0.261 | 0.27 | 1.360 | 0.783 to 2.363 |
| Oral | −0.657 | 0.416 | 0.11 | 0.519 | 0.229 to 1.172 |
| Overall model accuracy | 71.1% | ||||
| Whole-mouth † | |||||
| MMP-9 | −0.208 | 0.102 | 0.04 | 0.798 | 0.799 to 1.000 |
| GST | 0.896 | 0.364 | 0.01 | 2.449 | 1.252 to 5.308 |
| Annexin-1 | 0.750 | 0.241 | 0.002 | 1.071 | 1.261 to 4.275 |
| Overall model accuracy | 79.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sharqi, A.J.; Abdulkareem, A. Microbiological and Salivary Biomarkers Successfully Predict Site-Specific and Whole-Mouth Outcomes of Nonsurgical Periodontal Treatment. J. Clin. Med. 2024, 13, 4256. https://doi.org/10.3390/jcm13144256
Al-Sharqi AJ, Abdulkareem A. Microbiological and Salivary Biomarkers Successfully Predict Site-Specific and Whole-Mouth Outcomes of Nonsurgical Periodontal Treatment. Journal of Clinical Medicine. 2024; 13(14):4256. https://doi.org/10.3390/jcm13144256
Chicago/Turabian StyleAl-Sharqi, Ali JB, and Ali Abdulkareem. 2024. "Microbiological and Salivary Biomarkers Successfully Predict Site-Specific and Whole-Mouth Outcomes of Nonsurgical Periodontal Treatment" Journal of Clinical Medicine 13, no. 14: 4256. https://doi.org/10.3390/jcm13144256






