Age as an Effect Modifier of the Effects of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) on Heart Rate Variability in Healthy Subjects
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
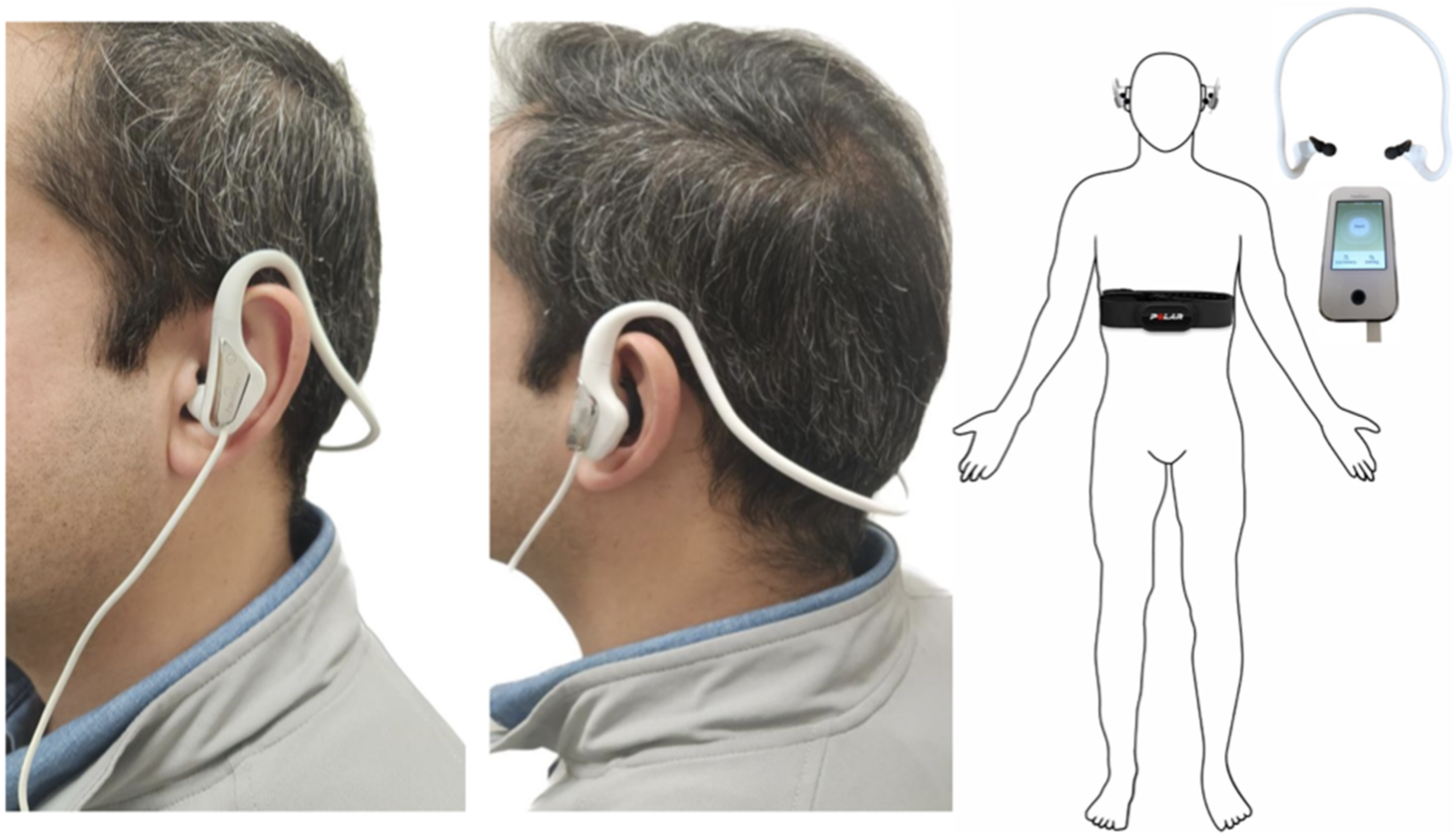
2.3. Intervention
2.4. Outcomes
2.5. Sample Size
2.6. Randomization and Masking
2.7. Statistical Analysis
3. Results
3.1. Healthy Participants
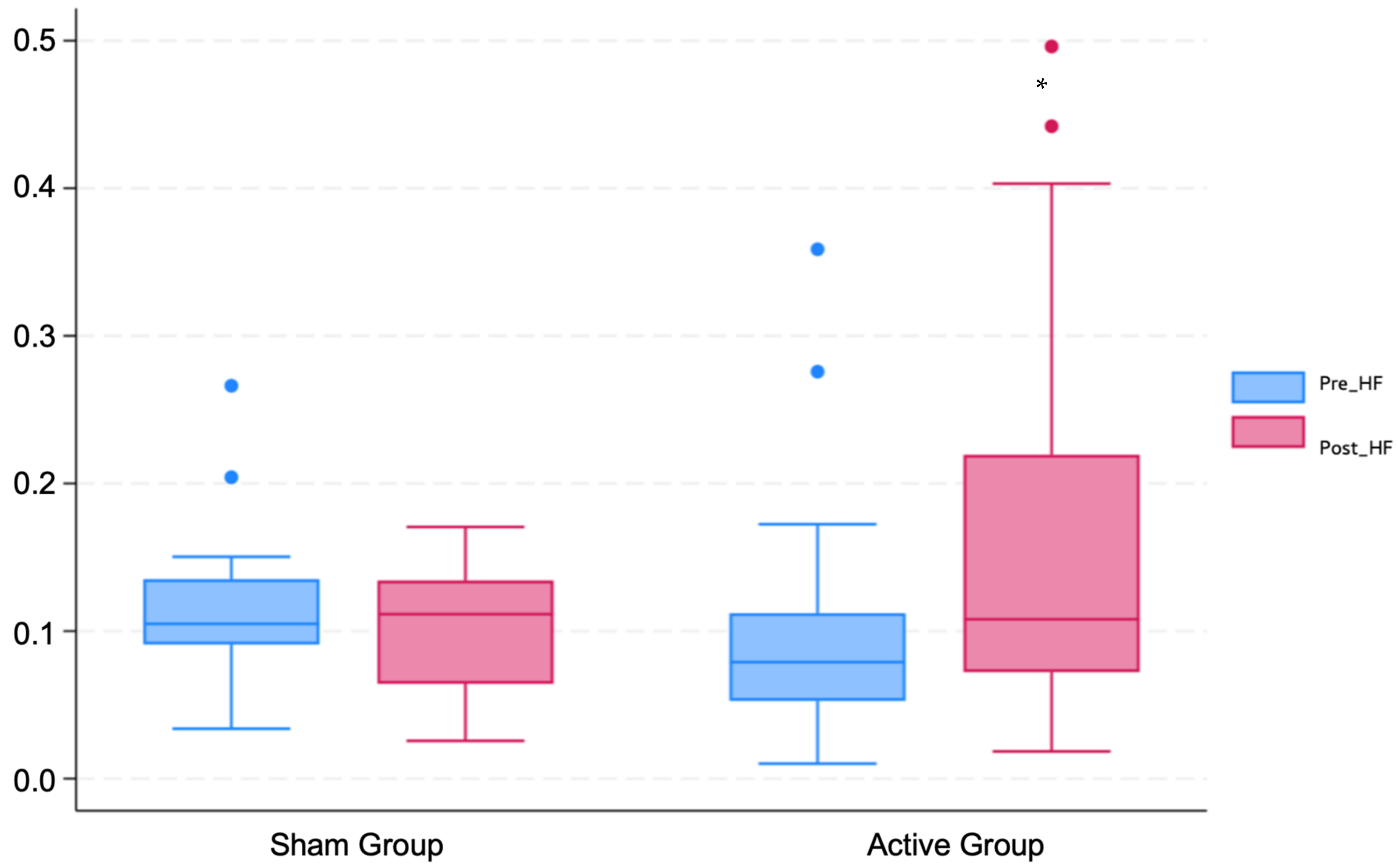
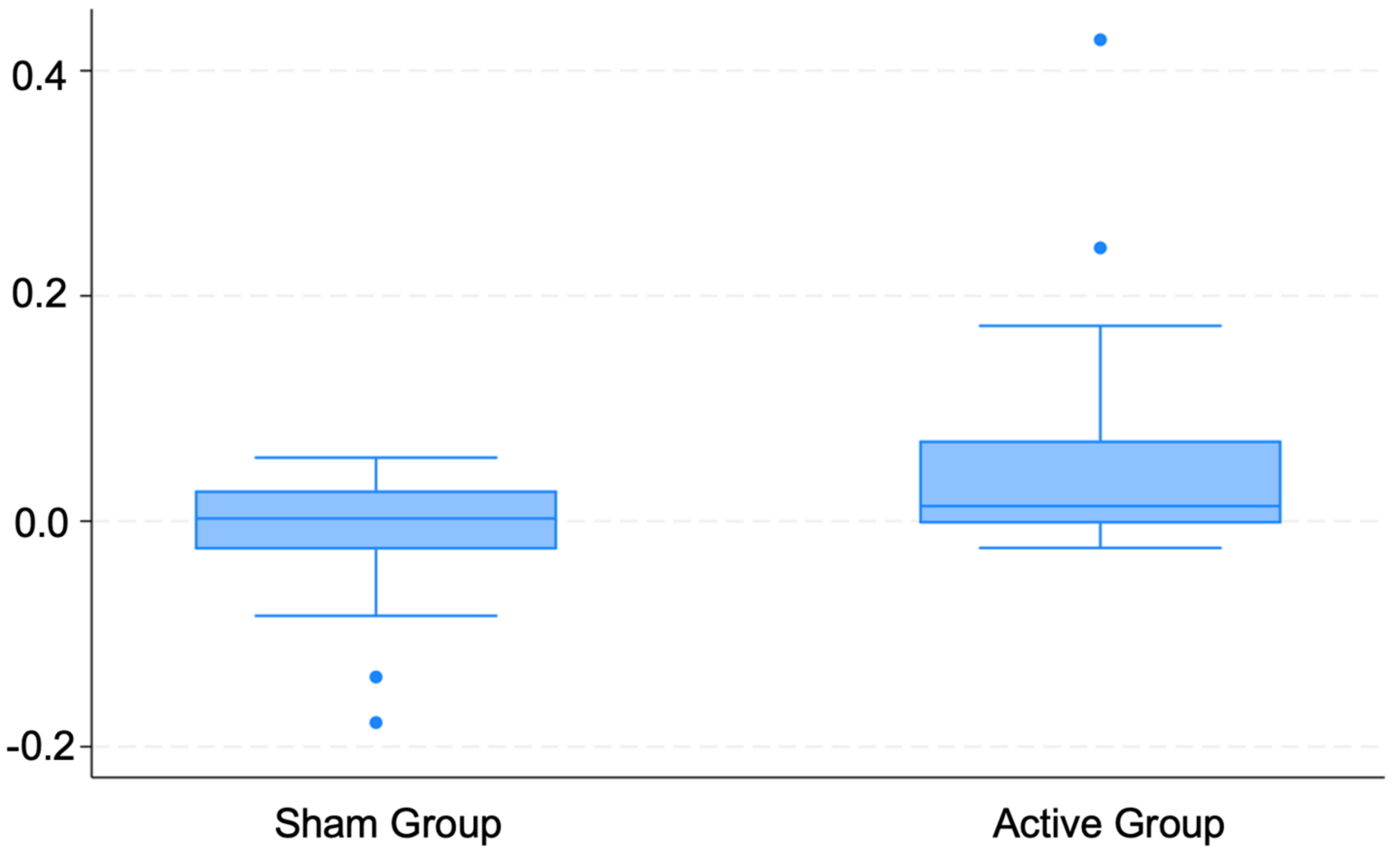
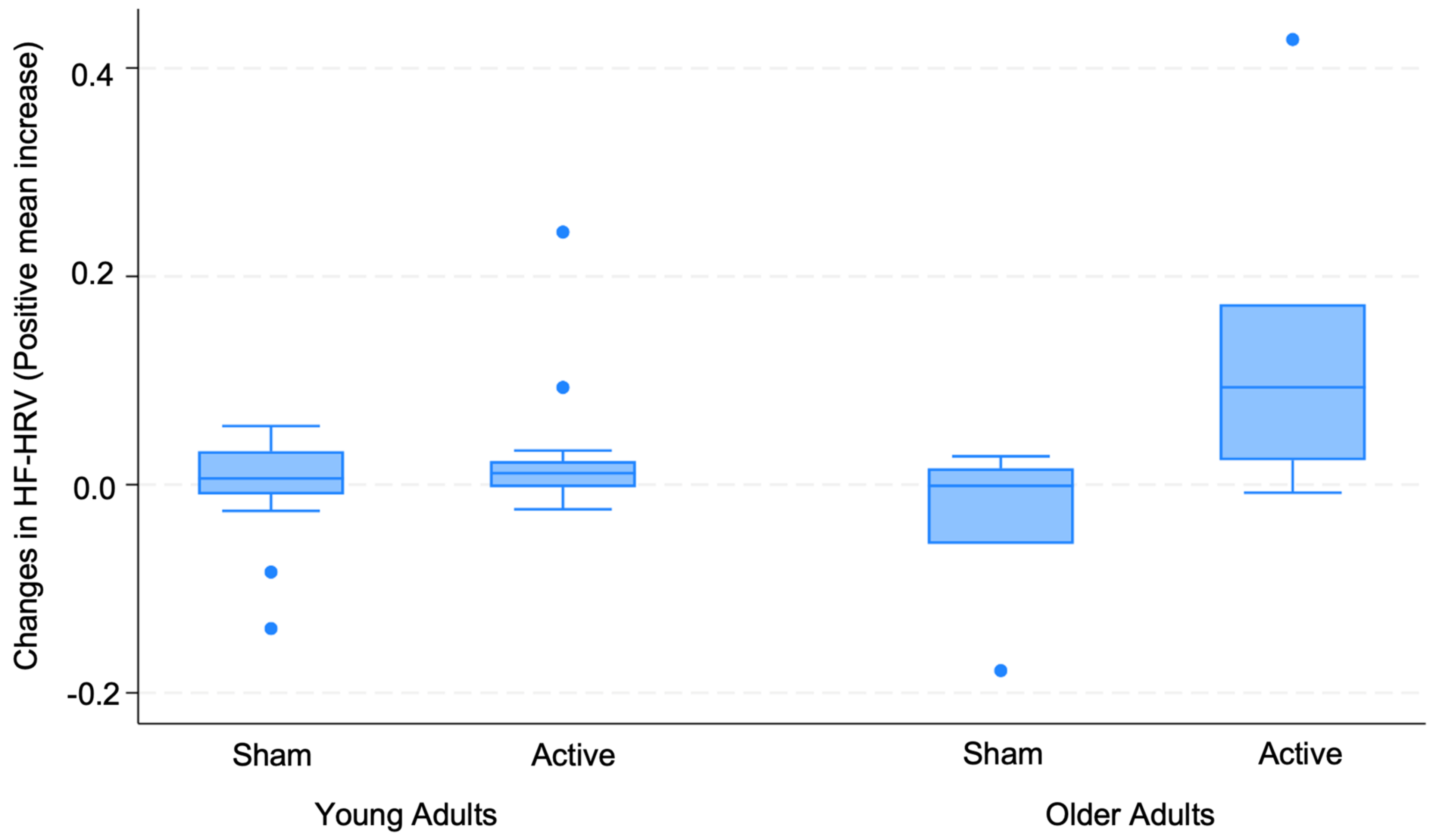
3.2. Changes in HRV
3.3. Regression Analysis
3.4. Correlation
3.5. Adverse Effects
3.6. Successful Blinding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology 1995, 45, 224–230. [Google Scholar] [CrossRef]
- Tan, C.; Qiao, M.; Ma, Y.; Luo, Y.; Fang, J.; Yang, Y. The efficacy and safety of transcutaneous auricular vagus nerve stimulation in the treatment of depressive disorder: A systematic review and meta-analysis of randomized controlled trials. J. Affect. Disord. 2023, 337, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Handforth, A.; DeGiorgio, C.M.; Schachter, S.C.; Uthman, B.M.; Naritoku, D.K.; Tecoma, E.S.; Henry, T.R.; Collins, S.D.; Vaughn, B.V.; Gilmartin, R.C.; et al. Vagus nerve stimulation therapy for partial-onset seizures: A randomized active-control trial. Neurology 1998, 51, 48–55. [Google Scholar] [CrossRef]
- Rush, A.; George, M.S.; A Sackeim, H.; Marangell, L.B.; Husain, M.M.; Giller, C.; Nahas, Z.; Haines, S.; Simpson, R.K.; Goodman, R. Vagus nerve stimulation (VNS) for treatment-resistant depressions: A multicenter study. Biol. Psychiatry 2000, 47, 276–286. [Google Scholar] [CrossRef]
- George, M.S.; Rush, A.J.; Marangell, L.B.; Sackeim, H.A.; Brannan, S.K.; Davis, S.M.; Howland, R.; Kling, M.A.; Moreno, F.; Rittberg, B.; et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol. Psychiatry 2005, 58, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, A.; Urechie, V.; Shiffer, D.; Rigo, S.; Minonzio, M.; Cairo, B.; Smith, E.C.; Okamoto, L.E.; Barbic, F.; Bisoglio, A.; et al. Transdermal auricular vagus stimulation for the treatment of postural tachycardia syndrome. Auton. Neurosci. 2021, 236, 102886. [Google Scholar] [CrossRef] [PubMed]
- Parente, J.; Gianlorenco, A.C.; Rebello-Sanchez, I.; Kim, M.; Prati, J.M.; Kim, C.K.; Choi, H.; Song, J.-J.; Fregni, F. Neural, Anti-inflammatory, and Clinical Effects of Transauricular Vagus Nerve Stimulation in Major Depressive Disorder: A Systematic Review. Int. J. Neuropsychopharmacol. 2023, 27, pyad058. [Google Scholar] [CrossRef]
- Yamamoto, T. Clinical Application of Transcutaneous Auricular Vagus Nerve Stimulation. Brain Nerve 2022, 74, 991–995. [Google Scholar]
- Zannad, F.; De Ferrari, G.M.; Tuinenburg, A.E.; Wright, D.; Brugada, J.; Butter, C.; Klein, H.; Stolen, C.; Meyer, S.; Stein, K.M.; et al. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: Results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) randomized controlled trial. Eur. Heart J. 2015, 36, 425–433. [Google Scholar] [CrossRef]
- Yang, H.; Shi, W.; Fan, J.; Wang, X.; Song, Y.; Lian, Y.; Shan, W.; Wang, Q. Transcutaneous Auricular Vagus Nerve Stimulation (ta-VNS) for Treatment of Drug-Resistant Epilepsy: A Randomized, Double-Blind Clinical Trial. Neurotherapeutics 2023, 20, 870–880. [Google Scholar] [CrossRef]
- Li, S.; Rong, P.; Wang, Y.; Jin, G.; Hou, X.; Li, S.; Xiao, X.; Zhou, W.; Wu, Y.; Liu, Y.; et al. Comparative Effectiveness of Transcutaneous Auricular Vagus Nerve Stimulation vs. Citalopram for Major Depressive Disorder: A Randomized Trial. Neuromodulation 2022, 25, 450–460. [Google Scholar] [CrossRef]
- Kong, J.; Fang, J.; Park, J.; Li, S.; Rong, P. Treating Depression with Transcutaneous Auricular Vagus Nerve Stimulation: State of the Art and Future Perspectives. Front. Psychiatry 2018, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Hu, L.; Zhang, Z.; Hu, Y. Changes of spontaneous oscillatory activity to tonic heat pain. PLoS ONE 2014, 9, e91052. [Google Scholar] [CrossRef]
- Paccione, C.E.; Stubhaug, A.; Diep, L.M.; Rosseland, L.A.; Jacobsen, H.B. Meditative-based diaphragmatic breathing vs. vagus nerve stimulation in the treatment of fibromyalgia—A randomized controlled trial: Body vs. machine. Front. Neurol. 2022, 13, 1030927. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.J.; Arfianti, L.; Pawana, I.P.; Melaniani, S. Does transcutaneous Vagus Nerve Stimulation (tVNS) reduce pain intensity in chronic low back pain patients? A randomized controlled pilot study. Bali Med. J. 2023, 12, 423–428. [Google Scholar] [CrossRef]
- Baig, S.S.; Kamarova, M.; Ali, A.; Su, L.; Dawson, J.; Redgrave, J.N.; Majid, A. Transcutaneous vagus nerve stimulation (tVNS) in stroke: The evidence, challenges and future directions. Auton. Neurosci. 2022, 237, 102909. [Google Scholar] [CrossRef] [PubMed]
- Badran, B.W.; Dowdle, L.T.; Mithoefer, O.J.; LaBate, N.T.; Coatsworth, J.; Brown, J.C.; DeVries, W.H.; Austelle, C.W.; McTeague, L.M.; George, M.S. Neurophysiologic Effects of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) via Electrical Stimulation of the Tragus: A Concurrent taVNS/fMRI Study and Review. FOCUS 2022, 20, 80–89. [Google Scholar] [CrossRef]
- Frangos, E.; Ellrich, J.; Komisaruk, B.R. Non-Invasive Access to the Vagus Nerve Central Projections via Electrical Stimulation of the External Ear: fMRI Evidence in Humans. Brain Stimul. 2015, 8, 624–636. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Garrido, M.V.; Prada, M. KDEF-PT: Valence, Emotional Intensity, Familiarity and Attractiveness Ratings of Angry, Neutral, and Happy Faces. Front. Psychol. 2017, 8, 2181. [Google Scholar] [CrossRef]
- Haensel, A.; Mills, P.J.; Nelesen, R.A.; Ziegler, M.G.; Dimsdale, J.E. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology 2008, 33, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Liu, X.; Wang, Y.; Wang, J. The effect of transcutaneous auricular vagus nerve stimulation on HRV in healthy young people. PLoS ONE 2022, 17, e0263833. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.A.; Mary, D.A.; Witte, K.K.; Greenwood, J.P.; Deuchars, S.A.; Deuchars, J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014, 7, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.A.; Taylor, J.A. Short-term cardiovascular oscillations in man: Measuring and modelling the physiologies. J. Physiol. 2002, 542 Pt 3, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Eckberg, D.L. The human respiratory gate. J. Physiol. 2003, 548 Pt 2, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Huikuri, H.V.; Perkiömäki, J.S.; Maestri, R.; Pinna, G.D. Philosophical transactions of the Royal Society of London. Ser. A Math. Phys. Eng. Sci. 2009, 367, 1223–1238. [Google Scholar]
- Hohenschurz-Schmidt, D.J.; Calcagnini, G.; Dipasquale, O.; Jackson, J.B.; Medina, S.; O’daly, O.; O’muircheartaigh, J.; Rubio, A.d.L.; Williams, S.C.R.; McMahon, S.B.; et al. Linking Pain Sensation to the Autonomic Nervous System: The Role of the Anterior Cingulate and Periaqueductal Gray Resting-State Networks. Front. Neurosci. 2020, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Santos, M.A.; Barreto-Filho, J.A.; Oliveira, J.L.; Reis, F.P.; da Cunha Oliveira, C.C.; Sousa, A.C. Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch. Gerontol. Geriatr. 2016, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Grässler, B.; Dordevic, M.; Darius, S.; Vogelmann, L.; Herold, F.; Langhans, C.; Halfpaap, N.; Böckelmann, I.; Müller, N.G.; Hökelmann, A. Age-Related Differences in Cardiac Autonomic Control at Resting State and in Response to Mental Stress. Diagnostics 2021, 11, 2218. [Google Scholar] [CrossRef]
- Umetani, K.; Singer, D.H.; McCraty, R.; Atkinson, M. Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. J. Am. Coll. Cardiol. 1998, 31, 593–601. [Google Scholar] [CrossRef]
- Altini, M.; Amft, O. (Eds.) HRV4Training: Large-scale longitudinal training load analysis in unconstrained free-living settings using a smartphone application. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar]
- Altini, M.; Hoof, C.V.; Amft, O. (Eds.) Relation between estimated cardiorespiratory fitness and running performance in free-living: An analysis of HRV4Training data. In Proceedings of the 2017 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Orlando, FL, USA, 16–19 February 2017. [Google Scholar]
- Plews, D.J.; Scott, B.; Altini, M.; Wood, M.; Kilding, A.E.; Laursen, P.B. Comparison of Heart-Rate-Variability Recording With Smartphone Photoplethysmography, Polar H7 Chest Strap, and Electrocardiography. Int. J. Sports Physiol. Perform. 2017, 12, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Hopf, H.B.; Skyschally, A.; Heusch, G.; Peters, J. Low-frequency spectral power of heart rate variability is not a specific marker of cardiac sympathetic modulation. Anesthesiology 1995, 82, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Saul, J.P.; Di Rienzo, M.; Mancia, G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension 1995, 25, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Burr, R.L. Interpretation of normalized spectral heart rate variability indices in sleep research: A critical review. Sleep 2007, 30, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Vosseler, A.; Zhao, D.; Fritsche, L.; Lehmann, R.; Kantartzis, K.; Small, D.M.; Peter, A.; Häring, H.-U.; Birkenfeld, A.L.; Fritsche, A.; et al. No modulation of postprandial metabolism by transcutaneous auricular vagus nerve stimulation: A cross-over study in 15 healthy men. Sci. Rep. 2020, 10, 20466. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.; Waters, E. Four Assumptions of Multiple Regression That Researchers Should Always Test. Pract. Assess. Res. Eval. 2002, 8, 2. [Google Scholar]
- Yap, B.W.; Sim, C.H. Comparisons of various types of normality tests. J. Stat. Comput. Simul. 2011, 81, 2141–2155. [Google Scholar] [CrossRef]
- Ernst, G. Heart-Rate Variability-More than Heart Beats? Front. Public Health 2017, 5, 240. [Google Scholar] [CrossRef]
- Hilz, M.J. Transcutaneous vagus nerve stimulation—A brief introduction and overview. Auton. Neurosci. 2022, 243, 103038. [Google Scholar] [CrossRef] [PubMed]
- Kenny, B.J.; Bordoni, B. Neuroanatomy, Cranial Nerve 10 (Vagus Nerve). In StatPearls; StatPearls Publishin LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Butt, M.F.; Albusoda, A.; Farmer, A.D.; Aziz, Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J. Anat. 2020, 236, 588–611. [Google Scholar] [CrossRef] [PubMed]
- Matusik, P.S.; Zhong, C.; Matusik, P.T.; Alomar, O.; Stein, P.K. Neuroimaging Studies of the Neural Correlates of Heart Rate Variability: A Systematic Review. J. Clin. Med. 2023, 12, 1016. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Kim, A.Y.; Marduy, A.; de Melo, P.S.; Gianlorenco, A.C.; Kim, C.K.; Choi, H.; Song, J.J.; Fregni, F. Safety of transcutaneous auricular vagus nerve stimulation (taVNS): A systematic review and meta-analysis. Sci. Rep. 2022, 12, 22055. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Faes, L.; Bari, V.; Marchi, A.; Bassani, T.; Nollo, G.; Perseguini, N.M.; Milan, J.; Minatel, V.; Borghi-Silva, A.; et al. Effect of age on complexity and causality of the cardiovascular control: Comparison between model-based and model-free approaches. PLoS ONE 2014, 9, e89463. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.K.; Barzilay, J.I.; Chaves, P.H.; Domitrovich, P.P.; Gottdiener, J.S. Heart rate variability and its changes over 5 years in older adults. Age Ageing 2009, 38, 212–218. [Google Scholar] [CrossRef]
- Van der Wall, E.E.; van Gilst, W.H. Neurocardiology: Close interaction between heart and brain. Neth. Heart J. 2013, 21, 51–52. [Google Scholar] [CrossRef]
- Peters, R. Ageing and the brain. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef]
- Yoo, H.J.; Thayer, J.F.; Greening, S.; Lee, T.-H.; Ponzio, A.; Min, J.; Sakaki, M.; Nga, L.; Mather, M.; Koenig, J. Brain structural concomitants of resting state heart rate variability in the young and old: Evidence from two independent samples. Brain Struct. Funct. 2018, 223, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Ask, T.F.; Sütterlin, S. Prefrontally modulated vagal neuroimmunomodulation is associated with telomere length. Front. Neurosci. 2022, 16, 1063162. [Google Scholar] [CrossRef] [PubMed]
- Antonino, D.; Teixeira, A.L.; Maia-Lopes, P.M.; Souza, M.C.; Sabino-Carvalho, J.L.; Murray, A.R.; Deuchars, J.; Vianna, L.C. Non-invasive vagus nerve stimulation acutely improves spontaneous cardiac baroreflex sensitivity in healthy young men: A randomized placebo-controlled trial. Brain Stimul. 2017, 10, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Favieri, F.; Leemhuis, E.; De Martino, M.L.; Giannini, A.M.; De Gennaro, L.; Casagrande, M.; Pazzaglia, M. Ear your heart: Transcutaneous auricular vagus nerve stimulation on heart rate variability in healthy young participants. PeerJ 2022, 10, e14447. [Google Scholar] [CrossRef] [PubMed]
- Wolf, V.; Kühnel, A.; Teckentrup, V.; Koenig, J.; Kroemer, N.B. Does transcutaneous auricular vagus nerve stimulation affect vagally mediated heart rate variability? A living and interactive Bayesian meta-analysis. Psychophysiology 2021, 58, e13933. [Google Scholar] [CrossRef]
- Wolf, V.; Kühnel, A.; Teckentrup, V.; Koenig, J.; Kroemer, N.B. International Consensus Based Review and Recommendations for Minimum Reporting Standards in Research on Transcutaneous Vagus Nerve Stimulation (Version 2020). Front. Hum. Neurosci. 2021, 14, 568051. [Google Scholar]
| Variables | Sham Mean or % (SD) | Active Mean or % (SD) | Total Mean or % (SD) | p-Value |
|---|---|---|---|---|
| Sample size | 22 | 22 | 44 | |
| Age (year) | 40.8 (17.8) | 41.7 (22.8) | 41.3 (20.2) | 0.88 |
| Sex (female, %) | 54.5 | 68.2 | 61.3 | 0.53 |
| Ethnicity (%): | 0.22 | |||
| Non-Hispanic | 77.3 | 90.9 | 84.1 | |
| Hispanic | 22.7 | 9.1 | 15.9 | |
| Race (%): | 0.37 | |||
| American Indian or Alaska Native | 0.0 | 0.0 | 0.0 | |
| Asian | 31.8 | 13.6 | 22.7 | |
| Black or African American | 4.5 | 9.1 | 6.8 | |
| White | 50.0 | 63.6 | 56.8 | |
| Other | 9.1 | 13.6 | 11.4 | |
| Unknown or not reported | 4.5 | 0.0 | 2.3 | |
| Education level (%): | 0.55 | |||
| High school | 9.1 | 18.2 | 13.6 | |
| College | 40.9 | 45.5 | 43.2 | |
| Higher than college | 50 | 36.4 | 43.2 | |
| HR | 69.3 (9.5) | 71.1 (9.2) | 70.2 (9.3) | 0.55 |
| LFs | 0.1 (0.1) | 0.1 (0.0) | 0.1 (0.0) | 0.36 |
| HFs | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.1) | 0.55 |
| LF/HF ratio | 0.9 (0.3) | 1.0 (0.7) | 0.9 (0.5) | 0.40 |
| SDNN | 54.2 (27.3) | 44.5 (24.6) | 49.4 (26.1) | 0.24 |
| RMSSD | 39.9 (27.2) | 32.5 (27.0) | 36.2 (27.0) | 0.39 |
| pNN50 | 18.1 (15.5) | 15.6 (21.8) | 16.9 (18.5) | 0.70 |
| - | Sham taVNS (n = 18) (Mean ± SD) | Active taVNS (n = 20) (Mean ± SD) | Unadjusted Effects Coef., 95% CI, p-Value | Adjusted Effects * Coef., 95% CI, p-Value |
|---|---|---|---|---|
| HF difference | 0.1 ± 0.2 | 0.6 ± 0.3 | 1.95, (0.33–3.57), 0.02 | 2.01, (0.33–3.69), 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianlorenço, A.C.; Pacheco-Barrios, K.; Daibes, M.; Camargo, L.; Choi, H.; Song, J.-J.; Fregni, F. Age as an Effect Modifier of the Effects of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) on Heart Rate Variability in Healthy Subjects. J. Clin. Med. 2024, 13, 4267. https://doi.org/10.3390/jcm13144267
Gianlorenço AC, Pacheco-Barrios K, Daibes M, Camargo L, Choi H, Song J-J, Fregni F. Age as an Effect Modifier of the Effects of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) on Heart Rate Variability in Healthy Subjects. Journal of Clinical Medicine. 2024; 13(14):4267. https://doi.org/10.3390/jcm13144267
Chicago/Turabian StyleGianlorenço, Anna Carolyna, Kevin Pacheco-Barrios, Marianna Daibes, Lucas Camargo, Hyuk Choi, Jae-Jun Song, and Felipe Fregni. 2024. "Age as an Effect Modifier of the Effects of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) on Heart Rate Variability in Healthy Subjects" Journal of Clinical Medicine 13, no. 14: 4267. https://doi.org/10.3390/jcm13144267
APA StyleGianlorenço, A. C., Pacheco-Barrios, K., Daibes, M., Camargo, L., Choi, H., Song, J.-J., & Fregni, F. (2024). Age as an Effect Modifier of the Effects of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) on Heart Rate Variability in Healthy Subjects. Journal of Clinical Medicine, 13(14), 4267. https://doi.org/10.3390/jcm13144267







