Cell-Free DNA Analysis of Fetal Aneuploidies in Early Pregnancy Loss
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection
2.3. Chromosomal Microarray Analysis of POC Specimens
2.4. Maternal Cell Contamination Analysis
2.5. Cell-Free DNA Analysis
3. Results
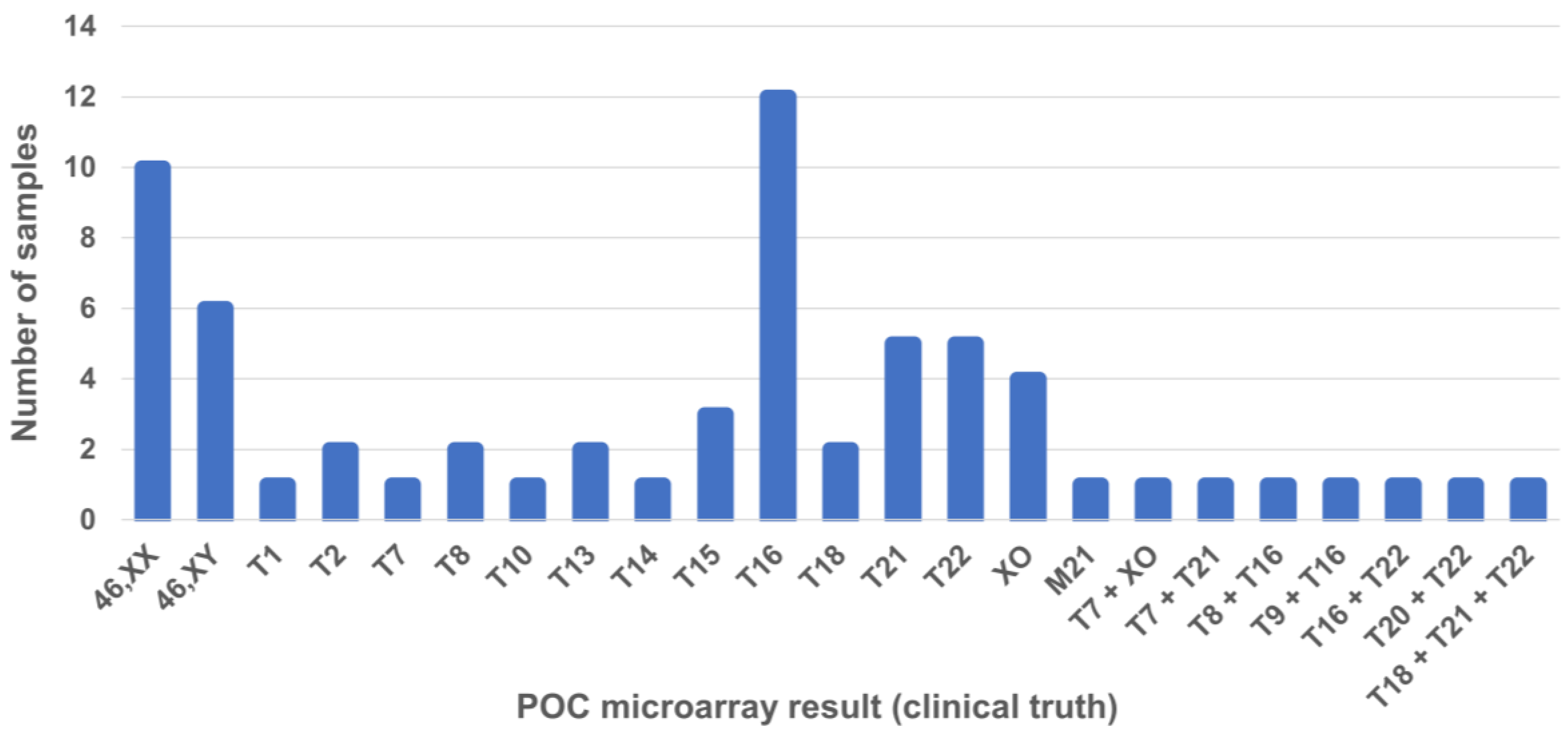
3.1. Sample Details
3.2. Training of the EPL Algorithm
3.3. Performance of the EPL Algorithm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macklon, N.S.; Geraedts, J.P.; Fauser, B.C. Conception to ongoing pregnancy: The ‘black box’ of early pregnancy loss. Hum. Reprod. Update 2002, 8, 333–343. [Google Scholar] [CrossRef]
- Blohm, F.; Fridén, B.; Milsom, I. A prospective longitudinal population-based study of clinical miscarriage in an urban Swedish population. Bjog 2008, 115, 176–182; discussion 183. [Google Scholar] [CrossRef]
- Pauta, M.; Grande, M.; Rodriguez-Revenga, L.; Kolomietz, E.; Borrell, A. Added value of chromosomal microarray analysis over karyotyping in early pregnancy loss: Systematic review and meta-analysis. Ultrasound Obs. Gynecol. 2018, 51, 453–462. [Google Scholar] [CrossRef]
- Finley, J.; Hay, S.; Oldzej, J.; Meredith, M.M.; Dzidic, N.; Slim, R.; Aradhya, S.; Hovanes, K.; Sahoo, T. The genomic basis of sporadic and recurrent pregnancy loss: A comprehensive in-depth analysis of 24,900 miscarriages. Reprod. BioMed. Online 2022, 45, 125–134. [Google Scholar] [CrossRef]
- Hardy, K.; Hardy, P.J.; Jacobs, P.A.; Lewallen, K.; Hassold, T.J. Temporal changes in chromosome abnormalities in human spontaneous abortions: Results of 40 years of analysis. Am. J. Med. Genet. A 2016, 170, 2671–2680. [Google Scholar] [CrossRef]
- Kutteh, W.H.; Papas, R.S.; Maisenbacher, M.K.; Dahdouh, E.M. The role of genetic analysis of products of conception and preimplantation genetic testing in the management of early pregnancy loss. Reprod. BioMed. Online 2023, 49, 103738. [Google Scholar] [CrossRef]
- Schlaikjær Hartwig, T.; Ambye, L.; Gruhn, J.R.; Petersen, J.F.; Wrønding, T.; Amato, L.; Chi-Ho Chan, A.; Ji, B.; Bro-Jørgensen, M.H.; Werge, L.; et al. Cell-free fetal DNA for genetic evaluation in Copenhagen Pregnancy Loss Study (COPL): A prospective cohort study. Lancet 2023, 401, 762–771. [Google Scholar] [CrossRef]
- Soler, A.; Morales, C.; Mademont-Soler, I.; Margarit, E.; Borrell, A.; Borobio, V.; Muñoz, M.; Sánchez, A. Overview of Chromosome Abnormalities in First Trimester Miscarriages: A Series of 1,011 Consecutive Chorionic Villi Sample Karyotypes. Cytogenet. Genome Res. 2017, 152, 81–89. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2012, 98, 1103–1111. [Google Scholar] [CrossRef]
- Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; Vermeulen, N.; et al. ESHRE guideline: Recurrent pregnancy loss. Hum. Reprod. Open 2018, 2018, hoy004. [Google Scholar] [CrossRef]
- Popescu, F.; Jaslow, C.R.; Kutteh, W.H. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum. Reprod. 2018, 33, 579–587. [Google Scholar] [CrossRef]
- Jaslow, C.R.; Carney, J.L.; Kutteh, W.H. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil. Steril. 2010, 93, 1234–1243. [Google Scholar] [CrossRef]
- Tunç, E.; Tanrıverdi, N.; Demirhan, O.; Süleymanova, D.; Çetinel, N. Chromosomal analyses of 1510 couples who have experienced recurrent spontaneous abortions. Reprod. BioMed. Online 2016, 32, 414–419. [Google Scholar] [CrossRef]
- Farren, J.; Mitchell-Jones, N.; Verbakel, J.Y.; Timmerman, D.; Jalmbrant, M.; Bourne, T. The psychological impact of early pregnancy loss. Hum. Reprod. Update 2018, 24, 731–749. [Google Scholar] [CrossRef]
- Lee, L.; Ma, W.; Davies, S.; Kammers, M. Toward Optimal Emotional Care During the Experience of Miscarriage: An Integrative Review of the Perspectives of Women, Partners, and Health Care Providers. J. Midwifery Womens Health 2023, 68, 52–61. [Google Scholar] [CrossRef]
- Musik, T.; Grimm, J.; Juhasz-Böss, I.; Bäz, E. Treatment Options After a Diagnosis of Early Miscarriage: Expectant, Medical, and Surgical. Dtsch. Arztebl. Int. 2021, 118, 789–794. [Google Scholar] [CrossRef]
- Lathi, R.B.; Gustin, S.L.; Keller, J.; Maisenbacher, M.K.; Sigurjonsson, S.; Tao, R.; Demko, Z. Reliability of 46,XX results on miscarriage specimens: A review of 1,222 first-trimester miscarriage specimens. Fertil. Steril. 2014, 101, 178–182. [Google Scholar] [CrossRef]
- Yaron, Y.; Pauta, M.; Badenas, C.; Soler, A.; Borobio, V.; Illanes, C.; Paz, Y.M.F.; Martinez-Portilla, R.; Borrell, A. Maternal plasma genome-wide cell-free DNA can detect fetal aneuploidy in early and recurrent pregnancy loss and can be used to direct further workup. Hum. Reprod. 2020, 35, 1222–1229. [Google Scholar] [CrossRef]
- Clark-Ganheart, C.A.; Fries, M.H.; Leifheit, K.M.; Jensen, T.J.; Moreno-Ruiz, N.L.; Ye, P.P.; Jennings, J.M.; Driggers, R.W. Use of cell-free DNA in the investigation of intrauterine fetal demise and miscarriage. Obs. Gynecol. 2015, 125, 1321–1329. [Google Scholar] [CrossRef]
- Colley, E.; Devall, A.J.; Williams, H.; Hamilton, S.; Smith, P.; Morgan, N.V.; Quenby, S.; Coomarasamy, A.; Allen, S. Cell-Free DNA in the Investigation of Miscarriage. J. Clin. Med. 2020, 9, 3428. [Google Scholar] [CrossRef]
- D’Ippolito, S.; Longo, G.; Orteschi, D.; Busnelli, A.; Di Simone, N.; Pulcinelli, E.; Schettini, G.; Scambia, G.; Zollino, M. Investigating the “Fetal Side” in Recurrent Pregnancy Loss: Reliability of Cell-Free DNA Testing in Detecting Chromosomal Abnormalities of Miscarriage Tissue. J. Clin. Med. 2023, 12, 3898. [Google Scholar] [CrossRef]
- Balaguer, N.; Rodrigo, L.; Mateu-Brull, E.; Campos-Galindo, I.; Castellón, J.A.; Al-Asmar, N.; Rubio, C.; Milán, M. Non-invasive cell-free DNA-based approach for the diagnosis of clinical miscarriage: A retrospective study. BJOG 2023, 131, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.M.; Accurti, V.; Santacruz, B.; Plana, M.N.; Nicolaides, K.H. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: Updated meta-analysis. Ultrasound Obs. Gynecol. 2017, 50, 302–314. [Google Scholar] [CrossRef] [PubMed]
- REDCap: Research Electronic Data Capture. Available online: https://projectredcap.org/ (accessed on 21 June 2024).
- Quest Diagnostics. Maternal Cell Contamination Study, STR Analysis. Available online: https://testdirectory.questdiagnostics.com/test/test-detail/10262/maternal-cell-contamination-study-str-analysis?p=r&cc=MASTER#:~:text=Maternal%20Cell%20Contamination%20Study%2C%20STR%20Analysis%20%2D%20Maternal%20Cell%20Contamination%20Study,influenced%20by%20contaminating%20maternal%20material. (accessed on 14 February 2023).
- Pertile, M.D.; Flowers, N.; Vavrek, D.; Andrews, D.; Kalista, T.; Craig, A.; Deciu, C.; Duenwald, S.; Meier, K.; Bhatt, S. Performance of a Paired-End Sequencing-Based Noninvasive Prenatal Screening Test in the Detection of Genome-Wide Fetal Chromosomal Anomalies. Clin. Chem. 2021, 67, 1210–1219. [Google Scholar] [CrossRef]
- Kort, J.D.; McCoy, R.C.; Demko, Z.; Lathi, R.B. Are blastocyst aneuploidy rates different between fertile and infertile populations? J. Assist. Reprod. Genet. 2018, 35, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Hassold, T.; Merrill, M.; Adkins, K.; Freeman, S.; Sherman, S. Recombination and maternal age-dependent nondisjunction: Molecular studies of trisomy 16. Am. J. Hum. Genet. 1995, 57, 867–874. [Google Scholar]
- Benn, P. Trisomy 16 and trisomy 16 Mosaicism: A review. Am. J. Med. Genet. 1998, 79, 121–133. [Google Scholar] [CrossRef]
- Pylyp, L.Y.; Spynenko, L.O.; Verhoglyad, N.V.; Mishenko, A.O.; Mykytenko, D.O.; Zukin, V.D. Chromosomal abnormalities in products of conception of first-trimester miscarriages detected by conventional cytogenetic analysis: A review of 1000 cases. J. Assist. Reprod. Genet. 2018, 35, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Koert, E.; Malling, G.M.H.; Sylvest, R.; Krog, M.C.; Kolte, A.M.; Schmidt, L.; Nielsen, H.S. Recurrent pregnancy loss: Couples’ perspectives on their need for treatment, support and follow up. Hum. Reprod. 2018, 34, 291–296. [Google Scholar] [CrossRef]
- Quenby, S.; Gallos, I.D.; Dhillon-Smith, R.K.; Podesek, M.; Stephenson, M.D.; Fisher, J.; Brosens, J.J.; Brewin, J.; Ramhorst, R.; Lucas, E.S.; et al. Miscarriage matters: The epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 2021, 397, 1658–1667. [Google Scholar] [CrossRef]
- Nikcevic, A.V.; Tunkel, S.A.; Kuczmierczyk, A.R.; Nicolaides, K.H. Investigation of the cause of miscarriage and its influence on women’s psychological distress. Br. J. Obs. Gynaecol. 1999, 106, 808–813. [Google Scholar] [CrossRef]
- Nikčević, A.V.; Nicolaides, K.H. Search for meaning, finding meaning and adjustment in women following miscarriage: A longitudinal study. Psychol. Health 2013, 29, 50–63. [Google Scholar] [CrossRef]
- Zayyad, S.; Liang, R.; Winkel, A.F.; Keefe, D.L.; Quinn, G.P. Can cell-free DNA (cfDNA) testing alleviate psychological distress in early miscarriage? A commentary. J. Assist. Reprod. Genet. 2022, 39, 1219–1224. [Google Scholar] [CrossRef]
- Yu, S.C.; Lee, S.W.; Jiang, P.; Leung, T.Y.; Chan, K.C.; Chiu, R.W.; Lo, Y.M. High-resolution profiling of fetal DNA clearance from maternal plasma by massively parallel sequencing. Clin. Chem. 2013, 59, 1228–1237. [Google Scholar] [CrossRef]

| Variable |
Study Cohort (Total n = 76) |
Final Analysis Samples (Total n = 65) |
|---|---|---|
| Maternal age, yr a | ||
| Mean | 34.9 | 34.9 |
| Median | 35.0 | 35.0 |
| Range | 25.0–44.0 | 25.0–44.0 |
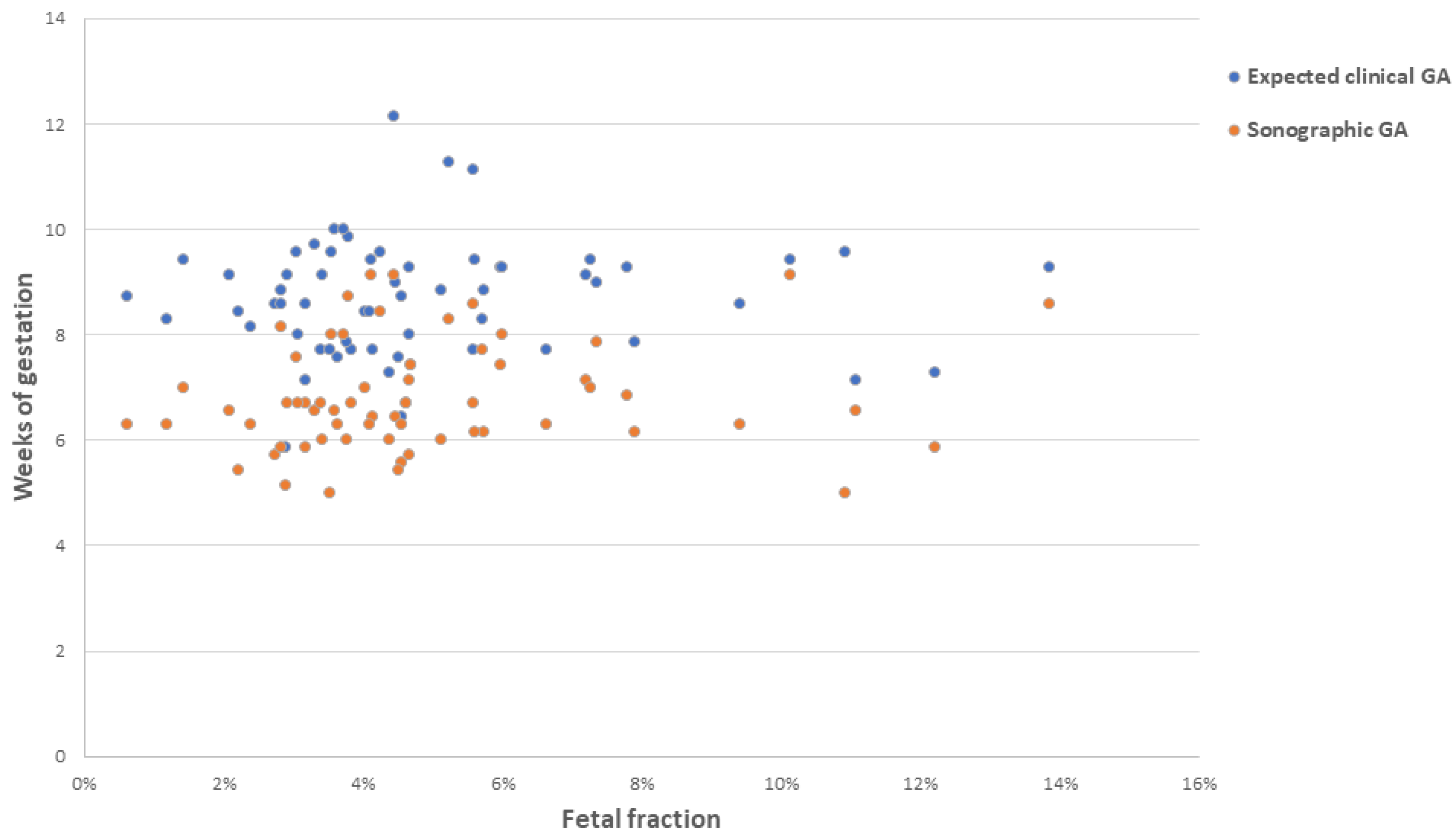
| Expected clinical GA at time of sample collection, wk b | ||
| Mean | 8.7 | 8.7 |
| Median | 8.7 | 8.7 |
| Range | 5.9–12.1 | 5.9–12.1 |
| Sonographic GA at time of ultrasound confirmation of miscarriage, wk | ||
| Mean | 6.7 | 6.8 |
| Median | 6.4 | 6.6 |
| Range | 5.0–9.1 | 5.0–9.1 |
| BMI | ||
| Mean | 29.8 | 29.7 |
| Median | 27.7 | 27.5 |
| Range | 18.1–47.8 | 18.1–47.8 |
| Previous pregnancies, n (%) c | ||
| 0 | 19 (25) | 16 (25) |
| 1 | 20 (26) | 17 (26) |
| 2 | 16 (21) | 14 (22) |
| ≥3 | 21 (28) | 18 (28) |
| Previous miscarriages, n (%) | ||
| 0 | 41 (54) | 34 (52) |
| 1 | 18 (24) | 17 (26) |
| 2 | 5 (7) | 4 (6) |
| 3 | 7 (9) | 6 (9) |
| 4 | 3 (4) | 2 (3) |
| 5 | 1 (1) | 1 (2) |
| 6 | 1 (1) | 1 (2) |
| Previous pregnancy with a chromosomal abnormality, n (%) d | ||
| Yes | 8 (11) | 8 (12) |
| No | 68 (90) | 57 (88) |
| Use of ART in current pregnancy, n (%) | ||
| Yes | 37 (49) | 30 (46) |
| No | 39 (51) | 35 (54) |
| Sample Level Calculations | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Full concordance a | 69.4 | 100 |
| Partial concordance b | 73.5 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutteh, W.H.; Miller, C.E.; Park, J.K.; Corey, V.; Chavez, M.; Racicot, K.; Alagia, D.P., III; Jinnett, K.N.; Curnow, K.; Dalton, K.; et al. Cell-Free DNA Analysis of Fetal Aneuploidies in Early Pregnancy Loss. J. Clin. Med. 2024, 13, 4283. https://doi.org/10.3390/jcm13154283
Kutteh WH, Miller CE, Park JK, Corey V, Chavez M, Racicot K, Alagia DP III, Jinnett KN, Curnow K, Dalton K, et al. Cell-Free DNA Analysis of Fetal Aneuploidies in Early Pregnancy Loss. Journal of Clinical Medicine. 2024; 13(15):4283. https://doi.org/10.3390/jcm13154283
Chicago/Turabian StyleKutteh, William H., Charles E. Miller, John K. Park, Victoria Corey, Mauro Chavez, Karen Racicot, Damian P. Alagia, III, Kristine N. Jinnett, Kirsten Curnow, Kristin Dalton, and et al. 2024. "Cell-Free DNA Analysis of Fetal Aneuploidies in Early Pregnancy Loss" Journal of Clinical Medicine 13, no. 15: 4283. https://doi.org/10.3390/jcm13154283
APA StyleKutteh, W. H., Miller, C. E., Park, J. K., Corey, V., Chavez, M., Racicot, K., Alagia, D. P., III, Jinnett, K. N., Curnow, K., Dalton, K., Bhatt, S., & Keefe, D. L. (2024). Cell-Free DNA Analysis of Fetal Aneuploidies in Early Pregnancy Loss. Journal of Clinical Medicine, 13(15), 4283. https://doi.org/10.3390/jcm13154283






