Microbiological Profiles after Out-of-Hospital Cardiac Arrest: Exploring the Relationship between Infection, Inflammation, and the Potential Effects of Mechanical Circulatory Support
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort and Study Design
2.2. Statistical Analysis
2.3. Ethics
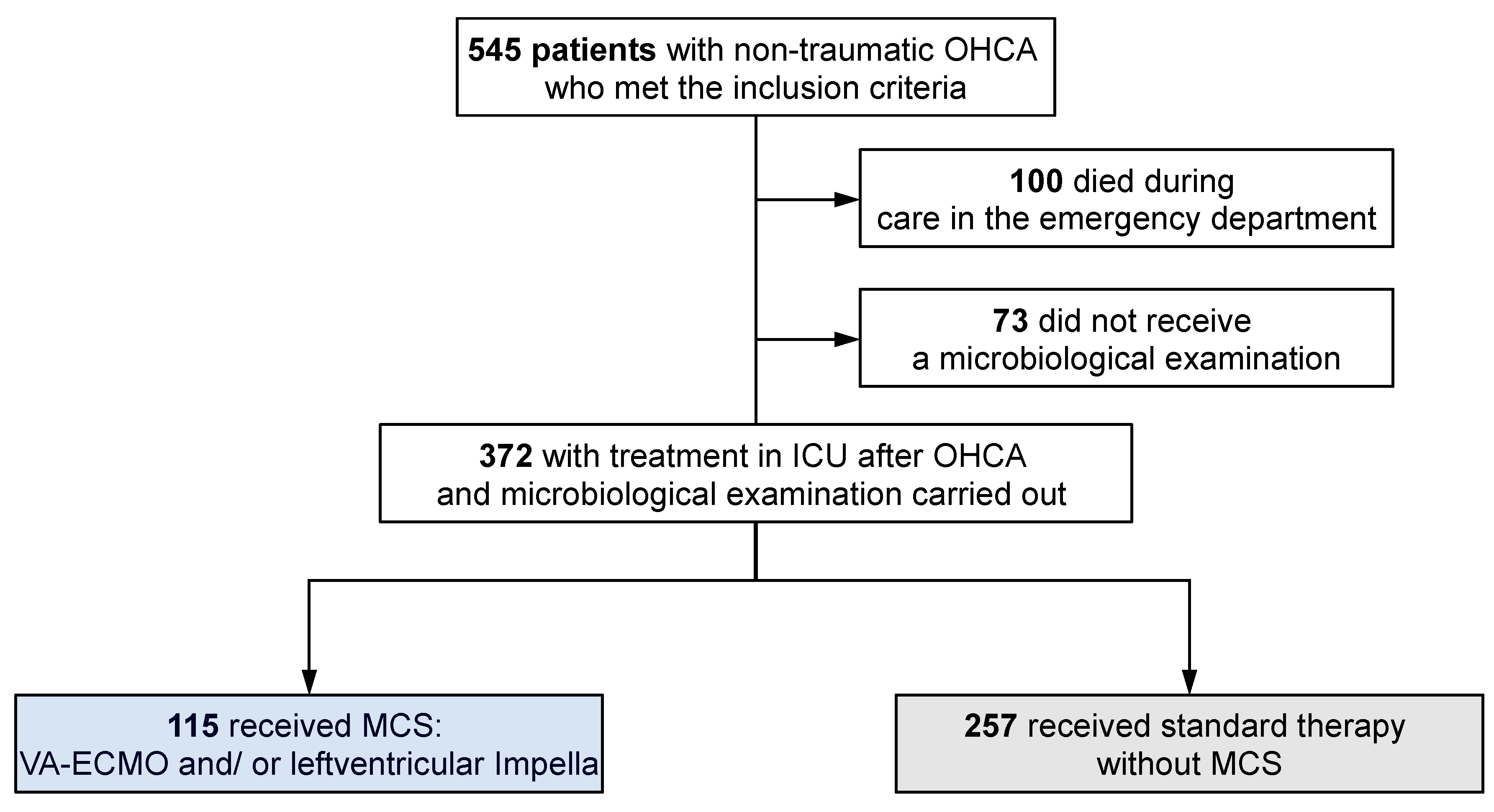
3. Results
3.1. Study Cohort
3.2. Comparison of Patients with Positive and Negative Microbiology Test Results
3.3. Comparison of the Microbiology Test Results in the Overall Cohort and MCS vs. Non-MCS Patients
3.3.1. Results from Blood Cultures
3.3.2. Results from Urine Cultures
3.3.3. Results from Tracheal Secretions
3.3.4. Results from Bronchoalveolar Lavage (BAL)
3.3.5. Results from Pleural or Ascitic Fluid Samples
3.4. Correlation between Microbiological Findings and Length of ICU Stay
3.5. Correlation between Microbiological Findings and Survival
3.6. Correlation between the Levels of Inflammatory Markers and Survival
3.7. Correlation between SCAI Stage, rCAST Score and Microbiological Findings
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021, 47, 369–421. [Google Scholar] [CrossRef] [PubMed]
- Lazzarin, T.; Tonon, C.R.; Martins, D.; Fávero, E.L.; Baumgratz, T.D.; Pereira, F.W.L.; Pinheiro, V.R.; Ballarin, R.S.; Queiroz, D.A.R.; Azevedo, P.S.; et al. Post-Cardiac Arrest: Mechanisms, Management, and Future Perspectives. J. Clin. Med. 2022, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Katsandres, S.C.; Hall, J.; Danielson, K.; Sakr, S.; Dean, S.G.; Carlbom, D.J.; Wurfel, M.M.; Bhatraju, P.K.; Hippensteel, J.A.; Schmidt, E.P.; et al. Inflammation, endothelial injury, and the acute respiratory distress syndrome after out-of-hospital cardiac arrest. Resusc. Plus 2024, 17, 100590. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.M.; Schrage, B.; Sinning, C.R.; Schmack, B.; Fluschnik, N.; Schwarzl, M.; Waldeyer, C.; Lindner, D.; Seiffert, M.; Neumann, J.T.; et al. Venoarterial Extracorporeal Membrane Oxygenation for Cardiopulmonary Support. Circulation 2018, 138, 2298–2300. [Google Scholar] [CrossRef] [PubMed]
- Karatolios, K.; Chatzis, G.; Markus, B.; Luesebrink, U.; Ahrens, H.; Dersch, W.; Betz, S.; Ploeger, B.; Boesl, E.; O’neill, W.; et al. Impella support compared to medical treatment for post-cardiac arrest shock after out of hospital cardiac arrest. Resuscitation 2018, 126, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Lüsebrink, E.; Kellnar, A.; Krieg, K.; Binzenhöfer, L.; Scherer, C.; Zimmer, S.; Schrage, B.; Fichtner, S.; Petzold, T.; Braun, D.; et al. Percutaneous Transvalvular Microaxial Flow Pump Support in Cardiology. Circulation 2022, 145, 1254–1284. [Google Scholar] [CrossRef] [PubMed]
- Azeem, T.; Stephens-Lloyd, A.; Spyt, T.; Hartshorne, R.; Gershlick, A.H. Intra-aortic balloon counterpulsation: Variations in use and complications. Int. J. Cardiol. 2004, 94, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Allou, N.; Pinto, H.L.; Persichini, R.; Bouchet, B.; Braunberger, E.; Lugagne, N.; Belmonte, O.; Martinet, O.; Delmas, B.; Dangers, L.; et al. Cannula-Related Infection in Patients Supported by Peripheral ECMO: Clinical and Microbiological Characteristics. ASAIO J. 2019, 65, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, R.R.; Barbaro, R.P.; Rycus, P.T.; Mcmullan, D.M.; Conrad, S.A.; Fortenberry, J.D.; Paden, M.L. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017, 63, 60–67. [Google Scholar] [CrossRef]
- Kuehn, C.; Orszag, P.; Burgwitz, K.; Marsch, G.; Stumpp, N.; Stiesch, M.; Haverich, A. Microbial adhesion on membrane oxygenators in patients requiring extracorporeal life support detected by a universal rDNA PCR test. ASAIO J. 2013, 59, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Diakos, N.A.; Swain, L.; Bhave, S.; Qiao, X.; Libermann, T.; Haywood, J.; Goel, S.; Annamalai, S.; Esposito, M.; Chweich, H.; et al. Circulating Proteome Analysis Identifies Reduced Inflammation After Initiation of Hemodynamic Support with Either Veno-Arterial Extracorporeal Membrane Oxygenation or Impella in Patients with Cardiogenic Shock. J. Cardiovasc. Transl. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 387. [Google Scholar] [CrossRef] [PubMed]
- Frerou, A.; Lesouhaitier, M.; Gregoire, M.; Uhel, F.; Gacouin, A.; Reizine, F.; Moreau, C.; Loirat, A.; Maamar, A.; Nesseler, N.; et al. Venoarterial extracorporeal membrane oxygenation induces early immune alterations. Crit. Care 2021, 25, 9. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-S.; Chiang, W.-C.; Lee, C.-C.; Hsieh, C.-C.; Ko, P.C.-I.; Hsu, C.-Y.; Su, C.-P.; Chen, S.-Y.; Chang, W.-T.; Yuan, A.; et al. Infections in the survivors of out-of-hospital cardiac arrest in the first 7 days. Intensive Care Med. 2005, 31, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Gajic, O.; Festic, E.; Afessa, B. Infectious complications in survivors of cardiac arrest admitted to the medical intensive care unit. Resuscitation 2004, 60, 65–69. [Google Scholar] [CrossRef]
- Mongardon, N.; Perbet, S.; Lemiale, V.; Dumas, F.; Poupet, H.; Charpentier, J.; Péne, F.; Chiche, J.-D.; Mira, J.-P.; Cariou, A. Infectious complications in out-of-hospital cardiac arrest patients in the therapeutic hypothermia era. Crit. Care Med. 2011, 39, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Perbet, S.; Mongardon, N.; Dumas, F.; Bruel, C.; Lemiale, V.; Mourvillier, B.; Carli, P.; Varenne, O.; Mira, J.-P.; Wolff, M.; et al. Early-onset pneumonia after cardiac arrest: Characteristics, risk factors and influence on prognosis. Am. J. Respir. Crit. Care Med. 2011, 184, 1048–1054. [Google Scholar] [CrossRef]
- Nair, R.M.; Kumar, S.; Saleem, T.; Chawla, S.; Vural, A.; Abdelghaffar, B.; Lee, R.; Higgins, A.; Cremer, P.; Rampersad, P.; et al. Characteristics and Impact of Bloodstream Infections in Cardiogenic Shock Patients on Temporary Mechanical Circulatory Support. JACC Cardiovasc. Interv. 2022, 15, 2110–2112. [Google Scholar] [CrossRef] [PubMed]
- Hellenkamp, K.; Onimischewski, S.; Kruppa, J.; Faßhauer, M.; Becker, A.; Eiffert, H.; Hünlich, M.; Hasenfuß, G.; Wachter, R. Early pneumonia and timing of antibiotic therapy in patients after nontraumatic out-of-hospital cardiac arrest. Crit. Care 2016, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kitlen, E.; Garcia, G.; Khosla, A.; Miller, P.E.; Johnson, J.; Wira, C.; Greer, D.M.; Gilmore, E.J.; Beekman, R. Validation of the rCAST score and comparison to the PCAC and FOUR scores for prognostication after out-of-hospital cardiac arrest. Resuscitation 2023, 188, 109832. [Google Scholar] [CrossRef] [PubMed]
- Pareek, N.; Dworakowski, R.; Webb, I.; Barash, J.; Emezu, G.; Melikian, N.; Hill, J.; Shah, A.; MacCarthy, P.; Byrne, J. SCAI cardiogenic shock classification after out of hospital cardiac arrest and association with outcome. Catheter. Cardiovasc. Interv. 2021, 97, E288–E297. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; van Diepen, S.; Barsness, G.W.; Henry, T.D.; Menon, V.; Rihal, C.S.; Naidu, S.S.; Baran, D.A. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2019, 74, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, E.M.; Satola, S.; Gupta, D.; Daneshmand, M.; Pouch, S. Management and outcomes of heart transplant candidates with bloodstream infection on temporary mechanical circulatory support. J. Heart Lung Transplant. 2023, 42, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Harmon, M.B.; Hodiamont, C.; Dankiewicz, J.; Nielsen, N.; Schultz, M.J.; Horn, J.; Friberg, H.; Juffermans, N.P. Microbiological profile of nosocomial infections following cardiac arrest: Insights from the targeted temperature management (TTM) trial. Resuscitation 2020, 148, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Spillmann, F.; Van Linthout, S.; Schmidt, G.; Klein, O.; Hamdani, N.; Mairinger, T.; Krackhardt, F.; Maroski, B.; Schlabs, T.; Soltani, S.; et al. Mode-of-action of the PROPELLA concept in fulminant myocarditis. Eur. Heart J. 2019, 40, 2164–2169. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Liu, P.P.; Mann, D.L. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 2015, 15, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Kachanova, O.; Lobov, A.; Malashicheva, A. The Role of the Notch Signaling Pathway in Recovery of Cardiac Function after Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 12509. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Botta, L.; Verde, A.; Milazzo, F.; Vecchi, I.; Trivella, M.G.; Martinelli, L.; Paino, R.; Frigerio, M.; Parodi, O. Relationship between pre-implant interleukin-6 levels, inflammatory response, and early outcome in patients supported by left ventricular assist device: A prospective study. PLoS ONE 2014, 9, e90802. [Google Scholar] [CrossRef] [PubMed]
- Diakos, N.A.; Thayer, K.; Swain, L.; Goud, M.; Jain, P.; Kapur, N.K. Systemic Inflammatory Burden Correlates with Severity and Predicts Outcomes in Patients with Cardiogenic Shock Supported by a Percutaneous Mechanical Assist Device. J. Cardiovasc. Transl. Res. 2021, 14, 476–483. [Google Scholar] [CrossRef]
- Gagnon, D.J.; Ryzhov, S.V.; May, M.A.; Riker, R.R.; Geller, B.; May, T.L.; Bockian, S.; Dekay, J.T.; Eldridge, A.; Van der Kloot, T.; et al. Ceftriaxone to PRevent pneumOnia and inflammaTion aftEr Cardiac arresT (PROTECT): Study protocol for a randomized, placebo-controlled trial. Trials 2022, 23, 197. [Google Scholar] [CrossRef] [PubMed]
- Yetmar, Z.A.; Khodadadi, R.B.; Go, J.R.; Chesdachai, S.; Abu Saleh, O.M. Post-treatment outcomes of ceftriaxone versus antistaphylococcal penicillins or cefazolin for definitive therapy of methicillin-susceptible Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Pan, X.; Pei, Z.; Wang, W.; Qiu, W.; Shi, Z.; Xiao, G. The beta-lactam antibiotic, ceftriaxone, provides neuroprotective potential via anti-excitotoxicity and anti-inflammation response in a rat model of traumatic brain injury. J. Trauma Acute Care Surg. 2012, 73, 654–660. [Google Scholar] [CrossRef] [PubMed]

| n= | Overall Cohort | MCS | Non-MCS | p-Value | |
|---|---|---|---|---|---|
| Number of patients 1 | 372 | 115 (30.9) | 257 (69.1) | ||
| Age (years) 2 | 372 | 65.0 (±13.9) | 58.3 (±13.1) | 68.0 (±13.2) | <0.001 |
| Male sex 1 | 372 | 278 (74.7) | 91 (79.1) | 187 (72.8) | 0.192 |
| BMI (kg/m2) 2 | 266 | 28.4 (±5.8) | 29.2 (±7.1) | 27.9 (±5.0) | 0.119 |
| MI in the past/CHD 1 | 361 | 59 (15.9) | 14 (13.1) | 45 (17.7) | 0.277 |
| Vitium of aortic/mitral valve (grade 2/3) 1 | 361 | 25 (6.7) | 3 (2.8) | 22 (8.7) | 0.045 |
| Heart failure ≥ NYHA 3 1 | 361 | 24 (6.5) | 4 (3.7) | 20 (7.9) | 0.150 |
| Atrial fibrillation 1 | 361 | 45 (12.1) | 6 (5.6) | 39 (15.4) | 0.011 |
| Pacemaker 1 | 361 | 12 (3.2) | 3 (2.8) | 9 (3.5) | 0.720 |
| Arterial hypertension 1 | 361 | 180 (4.8) | 43 (40.2) | 137 (53.9) | 0.017 |
| Hyperlipidemia 1 | 361 | 57 (15.3) | 11 (10.3) | 46 (18.1) | 0.062 |
| Diabetes mellitus 1 | 361 | 63 (16.9) | 11 (10.3) | 52 (20.5) | 0.020 |
| Nicotine abuse (>5py) 1 | 361 | 91 (24.5) | 27 (25.2) | 64 (25.2) | 0.994 |
| Alcohol abuse 1 | 361 | 25 (6.7) | 7 (6.5) | 18 (7.1) | 0.852 |
| Chronic renal failure KDIGO ≥ stage 3 1 | 369 | 42 (11.3) | 8 (7.0) | 34 (13.4) | 0.072 |
| Renal replacement therapy 1 | 369 | 11 (3.0) | 0 | 11 (4.3) | 0.024 |
| COPD ≥ GOLD 2 1 | 361 | 33 (8.9) | 4 (3.7) | 29 (11.4) | 0.021 |
| Bronchial asthma 1 | 361 | 11 (3.0) | 5 (4.7) | 6 (2.4) | 0.243 |
| OSAS 1 | 361 | 14 (3.8) | 3 (2.8) | 11 (4.3) | 0.493 |
| Apoplexy 1 | 361 | 41 (11.0) | 4 (3.7) | 37 (10.6) | 0.033 |
| Thrombosis/PAE 1 | 361 | 9 (2.4) | 3 (2.8) | 6 (2.4) | 0.806 |
| Malignant disease 1 | 361 | 29 (7.8) | 3 (2.8) | 26 (10.2) | 0.018 |
| Peripheral arterial disease ≥ stage 2 1 | 361 | 14 (3.8) | 4 (3.7) | 10 (3.9) | 0.929 |
| Carotid artery stenosis 1 | 361 | 13 (3.5) | 3 (2.8) | 10 (3.9) | 0.598 |
| Hypo- or hyperthyroidism 1 | 361 | 27 (7.3) | 7 (6.5) | 20 (7.9) | 0.660 |
| Overall Cohort n = 372 | MCS n = 115 | Non-MCS n = 257 | p-Value | |
|---|---|---|---|---|
| Initial shockable rhythm (VF/VT) 1 | 171 (46.0) | 65 (56.5) | 106 (41.2) | 0.006 |
| Witnessed cardiac arrest 1 | 259 (69.6) | 77 (67.0) | 182 (70.8) | 0.454 |
| Performed bystander CPR 1 | 224 (60.2) | 71 (61.7) | 153 (59.5) | 0.688 |
| Resuscitation time until ROSC 3 | 20.0 (10.0–35.0) | 47.5 (20.8–94.8) | 15.0 (10.0–25.0) | <0.001 |
| Mechanical CPR (chest compression device) 1 | 70 (18.8) | 59 (51.3) | 11 (4.3) | <0.001 |
| VA-ECMO | ECMELLA | Impella | p-Value | |
|---|---|---|---|---|
| Number of patients | 54 | 27 | 34 | |
| Age (years) 2 | 56.7 (±13.5) | 56.4 (±12.2) | 62.3 (12.6) | 0.212 |
| Initial shockable rhythm (VF/VT) 1 | 19 (35.2) | 17 (63.0) | 29 (85.3) | <0.001 |
| Witnessed cardiac arrest 1 | 38 (70.4) | 17 (63.0) | 22 (64.7) | 0.787 |
| Performed bystander CPR 1 | 33 (61.1) | 15 (55.6) | 23 (67.6) | 0.640 |
| Resuscitation time until ROSC 3 | 89.0 (30.0–104.0) | 76.0 (25.0–97.0) | 25.0 (10.0–41.3) | <0.001 |
| Mechanical CPR (chest compression device) 1 | 36 (66.7) | 18 (66.7) | 5 (14.7) | <0.001 |
| Duration of in-hospital stay (days) 3 | 3.0 (1.0–16.3) | 6.0 (2.0–22.0) | 17.5 (10.0–22.3) | 0.001 |
| Mechanical ventilation (hours) 3 | 64.0 (16.8–277.5) | 159.0 (40.0–455.0) | 239.0 (155.5–402.0) | 0.047 |
| Duration of MCS (days), survivors 3 | 9.0 (8.0–13.0) | 9.5 (7.0–15.5) | 6.0 (5.0–8.0) | 0.003 |
| Duration of MCS (days), non-survivors 3 | 2.0 (1.5–4.5) | 4.0 (2.0–8.0) | 5.5 (1.8–11.0) | 0.032 |
| Survived 1 | 17 (31.5) | 8 (29.6) | 24 (70.6) | <0.001 |
| Overall | MCS | Non-MCS | p-Value | |
|---|---|---|---|---|
| Patients with blood culture (n=) | 340 | 105 | 235 | |
| Positive samples 1 | 106 (31.2) | 41 (39.0) | 65 (27.7) | 0.037 |
| Gram-positive pathogens 1 | 93 (27.4) | 37 (35.2) | 56 (23.8) | 0.029 |
| Staphylococcus species 1 | 76 (22.4) | 27 (25.7) | 49 (20.9) | 0.321 |
| Staphylococcus epidermidis 1 | 54 (15.9) | 22 (21.0) | 32 (13.6) | 0.088 |
| Staphylococcus aureus (including MRSA) 1 | 2 (0.6) | 0 (0.0) | 2 (0.9) | 0.344 |
| Other staphylococci 1 | 29 (8.5) | 7 (6.7) | 22 (9.4) | 0.412 |
| Propionibacteriacae 1 | 14 (4.1) | 7 (6.7) | 7 (3.0) | 0.114 |
| Streptococcus species 1 | 5 (1.5) | 3 (2.9) | 2 (0.9) | 0.156 |
| Corynebacteriacae 1 | 3 (0.9) | 1 (1.0) | 2 (0.9) | 0.927 |
| Gram-negative pathogens 1 | 12 (3.5) | 2 (1.9) | 10 (4.3) | 0.279 |
| Klebsiella species 1 | 5 (1.5) | 1 (1.0) | 4 (1.7) | 0.596 |
| Escherichia coli (including 3MRGN) 1 | 4 (1.2) | 1 (1.0) | 3 (1.3) | 0.798 |
| Proteus mirabilis 1 | 2 (0.6) | / | 2 (0.9) | 0.344 |
| Anaerobic pathogens 1 | 8 (2.4) | 5 (4.8) | 3 (1.3) | 0.050 |
| Bacteroides species 1 | 2 (0.6) | 1 (1.0) | 1 (0.4) | 0.523 |
| Overall Cohort | MCS | Non-MCS | p-Value | |
|---|---|---|---|---|
| Patients with urine culture (n=) | 271 | 72 | 199 | |
| Positive samples 1 | 69 (25.5) | 10 (13.9) | 59 (29.6) | 0.009 |
| Gram-positive pathogens 1 | 23 (8.5) | 3 (4.2) | 20 (10.1) | 0.125 |
| Enterococcus species 1 | 15 (5.5) | 2 (2.8) | 13 (6.5) | 0.233 |
| Staphylococcus species 1 | 6 (2.2) | 1 (1.4) | 5 (2.5) | 0.579 |
| Gram-negative pathogens 1 | 45 (16.6) | 7 (9.7) | 38 (19.1) | 0.068 |
| Escherichia coli (including 3MRGN) 1 | 21 (7.7) | 3 (4.2) | 18 (9.0) | 0.185 |
| Gram-negative rods 1 | 17 (6.3) | 4 (5.6) | 13 (6.5) | 0.770 |
| Klebsiella species 1 | 4 (1.5) | / | 4 (2.0) | 0.226 |
| Pseudomonas aeruginosa (including 3MRGN) 1 | 4 (1.5) | / | 4 (2.0) | 0.226 |
| Mycosis 1 | 13 (4.8) | 1 (1.4) | 12 (6.0) | 0.115 |
| Candida species 1 | 7 (2.6) | / | 7 (3.5) | 0.108 |
| Overall Cohort | MCS | Non-MCS | p-Value | |
|---|---|---|---|---|
| Patients with tracheal secretion (n=) | 210 | 62 | 148 | |
| Positive samples 1 | 200 (95.2) | 57 (91.9) | 143 (96.6) | 0.147 |
| Gram-positive pathogens 1 | 161 (76.7) | 47 (75.8) | 114 (77.0) | 0.849 |
| Streptococcus species 1 | 135 (64.3) | 40 (64.5) | 95 (64.2) | 0.964 |
| Staphylococcus species 1 | 73 (34.8) | 16 (25.8) | 57 (38.5) | 0.078 |
| Enterococcus species 1 | 41 (19.5) | 15 (24.2) | 26 (17.6) | 0.270 |
| Gram-negative pathogens 1 | 107 (51.0) | 23 (37.1) | 84 (56.8) | 0.010 |
| Neisseria species 1 | 44 (21.0) | 9 (14.5) | 35 (23.6) | 0.139 |
| Haemophilus species 1 | 26 (12.4) | 7 (11.3) | 19 (12.8) | 0.757 |
| Klebsiella species 1 | 21 (10.0) | 1 (1.6) | 20 (13.5) | 0.009 |
| Mycosis 1 | 123 (58.6) | 33 (53.2) | 90 (60.8) | 0.310 |
| Candida species | 41 (19.5) | 8 (12.9) | 33 (22.3) | 0.118 |
| Candida albicans | 103 (49.0) | 28 (45.2) | 75 (50.7) | 0.467 |
| Candida glabrata | 2 (1.0) | 1 (1.6) | 1 (0.7) | 0.525 |
| Aspergillus niger | 2 (1.0) | / | 2 (1.4) | 0.359 |
| Overall Cohort | MCS | Non-MCS | p-Value | |
|---|---|---|---|---|
| Patients with BAL (n=) | 32 | 11 | 21 | |
| Positive samples 1 | 27 (84.4) | 8 (72.7) | 19 (90.5%) | 0.196 |
| Gram-positive pathogens 1 | 17 (53.1) | 6 (54.5) | 11 (52.4) | 0.909 |
| Streptococcus species 1 | 11 (34.4) | 4 (36.4) | 7 (33.3) | 0.866 |
| Staphylococcus species 1 | 9 (28.1) | 2 (18.2) | 7 (33.3) | 0.373 |
| Enterococcus species 1 | 5 (15.6) | 3 (27.3) | 2 (9.5) | 0.196 |
| Gram-negative pathogens | 8 (25.0) | 1 (9.1) | 7 (33.3) | 0.139 |
| Neisseria species 1 | 3 (9.4) | 1 (9.1) | 2 (9.5) | 0.969 |
| Klebsiella species 1 | 2 (6.3) | / | 2 (9.5) | 0.298 |
| Mycosis 1 | 17 (53.1) | 6 (54.5) | 11 (52.4) | 0.909 |
| Candida species 1 | 5 (15.6) | / | 5 (23.8) | 0.083 |
| Candida albicans 1 | 14 (43.8) | 6 (54.5) | 8 (38.1) | 0.381 |
| Overall Cohort | MCS | Non-MCS | p-Value | |
|---|---|---|---|---|
| Patients with pleural/ascitic fluid samples (n=) | 12 | 5 | 7 | |
| Positive samples 1 | 6 (50.0) | 3 (60.0) | 3 (42.9) | 0.575 |
| Gram-positive pathogens 1 | 3 (25.0) | 2 (40.0) | 1 (14.3) | 0.322 |
| Staphylococcus species 1 | 3 (25.0) | 2 (40.0) | 1 (14.3) | 0.322 |
| Gram-negative pathogens 1 | 2 (16.6) | 1 (20.0) | 1 (14.3) | 0.802 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreutz, J.; Müller, C.; Chatzis, G.; Syntila, S.; Choukeir, M.; Schäfer, A.-C.; Betz, S.; Schieffer, B.; Patsalis, N.; Markus, B. Microbiological Profiles after Out-of-Hospital Cardiac Arrest: Exploring the Relationship between Infection, Inflammation, and the Potential Effects of Mechanical Circulatory Support. J. Clin. Med. 2024, 13, 4297. https://doi.org/10.3390/jcm13154297
Kreutz J, Müller C, Chatzis G, Syntila S, Choukeir M, Schäfer A-C, Betz S, Schieffer B, Patsalis N, Markus B. Microbiological Profiles after Out-of-Hospital Cardiac Arrest: Exploring the Relationship between Infection, Inflammation, and the Potential Effects of Mechanical Circulatory Support. Journal of Clinical Medicine. 2024; 13(15):4297. https://doi.org/10.3390/jcm13154297
Chicago/Turabian StyleKreutz, Julian, Charlotte Müller, Georgios Chatzis, Styliani Syntila, Maryana Choukeir, Ann-Christin Schäfer, Susanne Betz, Bernhard Schieffer, Nikolaos Patsalis, and Birgit Markus. 2024. "Microbiological Profiles after Out-of-Hospital Cardiac Arrest: Exploring the Relationship between Infection, Inflammation, and the Potential Effects of Mechanical Circulatory Support" Journal of Clinical Medicine 13, no. 15: 4297. https://doi.org/10.3390/jcm13154297
APA StyleKreutz, J., Müller, C., Chatzis, G., Syntila, S., Choukeir, M., Schäfer, A.-C., Betz, S., Schieffer, B., Patsalis, N., & Markus, B. (2024). Microbiological Profiles after Out-of-Hospital Cardiac Arrest: Exploring the Relationship between Infection, Inflammation, and the Potential Effects of Mechanical Circulatory Support. Journal of Clinical Medicine, 13(15), 4297. https://doi.org/10.3390/jcm13154297





