Renal Replacement Therapy in Methylmalonic Aciduria-Related Metabolic Failure: Case Report and Literature Review
Abstract
1. Introduction
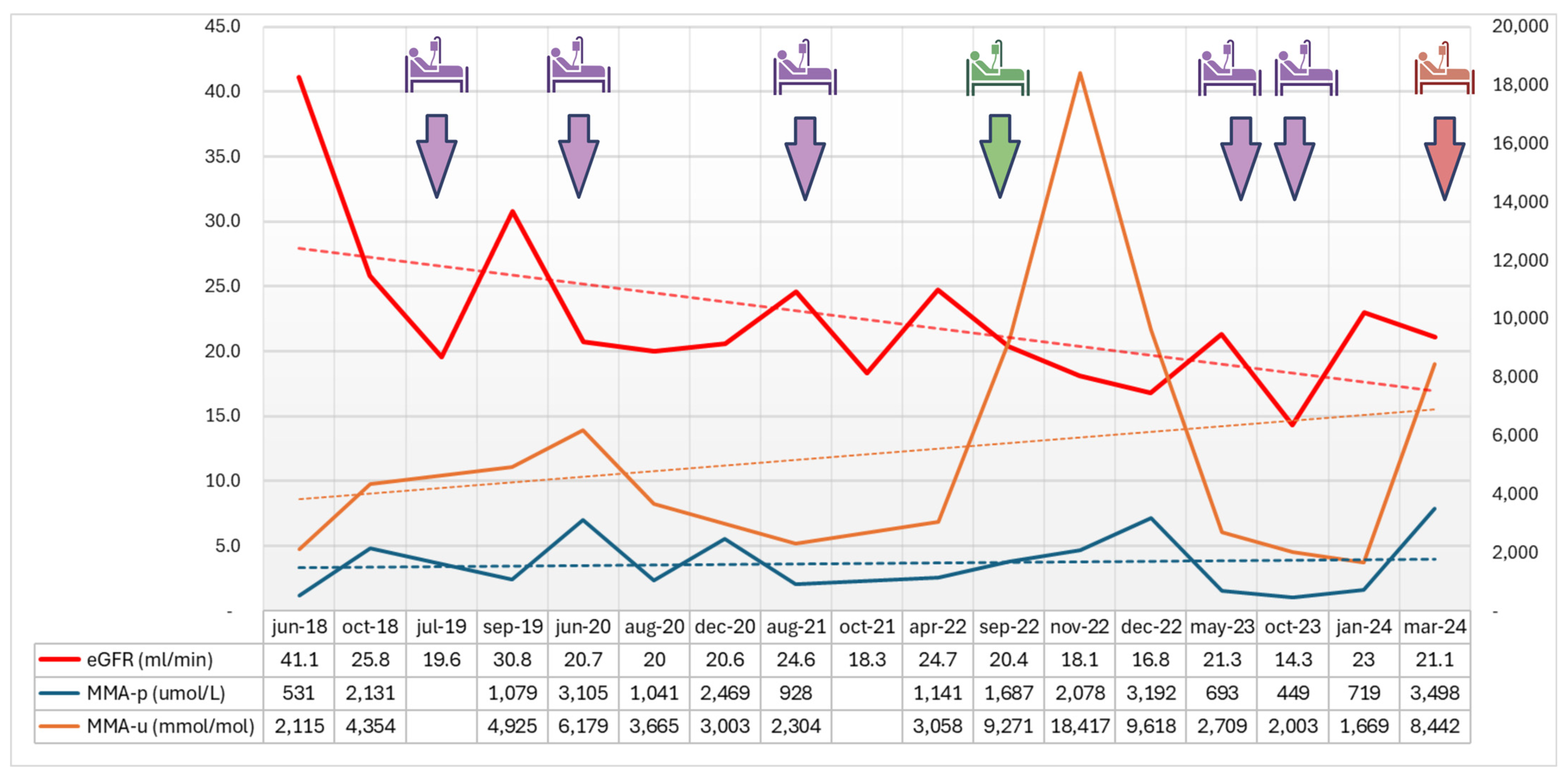
2. Detailed Case Description
3. Discussion
3.1. CKD and ESRD in MA Patients
3.2. Renal Replacement Therapy (RRT) in MA
3.3. Transplantation in MA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ORPHA:293355. Available online: https://www.orpha.net/en/disease/detail/293355 (accessed on 4 May 2024).
- Fowler, B.; Leonard, J.V.; Baumgartner, M.R. Causes of and diagnostic approach to methylmalonic acidurias. J. Inherit. Metab. Dis. 2008, 31, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Morath, M.A.; Hörster, F.; Sauer, S.W. Renal dysfunction in methylmalonic acidurias: Review for the pediatric nephrologist. Pediatr. Nephrol. 2013, 28, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.F.; Watkins, D.; Scott, P.; Rinaldo, P.; Rosenblatt, D.S. Prenatal diagnosis for methylmalonic acidemia and inborn errors of vitamin B12 metabolism and transport. Mol. Genet. Metab. 2005, 86, 160–171. [Google Scholar] [CrossRef]
- Belaramani, K.M.; Chan, T.C.H.; Hau, E.W.L.; Yeung, M.C.W.; Kwok, A.M.K.; Lo, I.F.M.; Law, T.H.F.; Wu, H.; Wong, S.S.N.; Lam, S.W.; et al. Expanded Newborn Screening for Inborn Errors of Metabolism in Hong Kong: Results and Outcome of a 7 Year Journey. Int. J. Neonatal Screen. 2024, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, M.R.; Hörster, F.; Dionisi-Vici, C.; Haliloglu, G.; Karall, D.; Chapman, K.A.; Huemer, M.; Hochuli, M.; Assoun, M.; Ballhausen, D.; et al. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J. Rare Dis. 2014, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Vatanavicharn, N.; Champattanachai, V.; Liammongkolkul, S.; Sawangareetrakul, P.; Keeratichamroen, S.; Ketudat Cairns, J.R.; Srisomsap, C.; Sathienkijkanchai, A.; Shotelersuk, V.; Kamolsilp, M.; et al. Clinical and molecular findings in Thai patients with isolated methylmalonic acidemia. Mol. Genet. Metab. 2012, 106, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Reischl-Hajiabadi, A.T.; Schnabel, E.; Gleich, F.; Mengler, K.; Lindner, M.; Burgard, P.; Posset, R.; Lommer-Steinhoff, S.; Grünert, S.C.; Thimm, E.; et al. Outcomes after newborn screening for propionic and methylmalonic acidemia and homocystinurias. J. Inherit. Metab. Dis. 2024, 47, 674–689. [Google Scholar] [CrossRef]
- Hörster, F.; Hörster, H.; Baumgartner, M.R.; Viardot, C.; Suormala, T.; Burgard, P.; Fowler, B.; Hoffmann, G.F.; Garbade, S.F.; Kölker, S.; et al. Long-Term Outcome in Methylmalonic Acidurias Is Influenced by the Underlying Defect (mut0, mut−, cblA, cblB). Pediatr. Res. 2007, 62, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Ling, S.; Yu, Y.; Shuai, R.; Qiu, W.; Zhang, H.; Shen, L.; Wu, S.; Wei, H.; Chen, Y.; et al. Evaluation of the clinical, biochemical, genotype and prognosis of mut-type methylmalonic acidemia in 365 Chinese cases Genotype-phenotype correlations. J. Med. Genet. 2024, 61, 8–17. [Google Scholar] [CrossRef]
- Head, P.S.E.; Meier, J.L.; Venditti, C.P. New insights into the pathophysiology of methylmalonic acidemia. J. Inherit. Metab. Dis. 2023, 46, 436–449. [Google Scholar] [CrossRef]
- Dao, M.; Arnoux, J.B.; Bienaimé, F.; Brassier, A.; Brazier, F.; Benoist, J.F.; Pontoizeau, C.; Ottolenghi, C.; Krug, P.; Boyer, O.; et al. Long-term renal outcome in methylmalonic acidemia in adolescents and adults. Orphanet J. Rare Dis. 2021, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Jorge-Finnigan, A.; Gámez, A.; Pérez, B.; Ugarte, M.; Richard, E. Different altered pattern expression of genes related to apoptosis in isolated methylmalonic aciduria cblB type and combined with homocystinuria cblC type. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Schumann, A.; Brutsche, M.; Havermans, M.; Grünert, S.C.; Kölker, S.; Groß, O.; Hannibal, L.; Spiekerkoetter, U. The impact of metabolic stressors on mitochondrial homeostasis in a renal epithelial cell model of methylmalonic aciduria. Sci. Rep. 2023, 13, 7677. [Google Scholar] [CrossRef] [PubMed]
- Luciani, A.; Schumann, A.; Berquez, M.; Chen, Z.; Nieri, D.; Failli, M.; Debaix, H.; Festa, B.P.; Tokonami, N.; Raimondi, A.; et al. Impaired mitophagy links mitochondrial disease to epithelial stress in methylmalonyl-CoA mutase deficiency. Nat. Commun. 2020, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, T.; Schumann, A.; Gröne, H.J.; Okun, J.G.; Kölker, S.; Morath, M.A.; Sauer, S.W. Molecular and biochemical alterations in tubular epithelial cells of patients with isolated methylmalonic aciduria. Hum. Mol. Genet. 2015, 24, 7049–7059. [Google Scholar] [CrossRef] [PubMed]
- Tarçın, G.; Ahmadzada, S.; Saygılı, S.; Kaya, A.; Aktuğlu Zeybek, A.Ç.; Ercan, O. Evaluating renin and aldosterone levels in children with organic acidemia—Therapeutic experience with fludrocortisone. Eur. J. Pediatr. 2023, 182, 5447–5453. [Google Scholar] [CrossRef] [PubMed]
- Arhip, L.; Brox-Torrecilla, N.; Romero, I.; Motilla, M.; Serrano-Moreno, C.; Miguélez, M.; Cuerda, C. Late-onset methylmalonic acidemia and homocysteinemia (cblC disease): Systematic review. Orphanet J. Rare Dis. 2024, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, D.; Dotta, A.; Massella, L.; Picca, S.; Di Pede, A.; Boenzi, S.; Aiello, C.; Dionisi-Vici, C. Cobalamin C defect presenting as severe neonatal hyperammonemia. Eur. J. Pediatr. 2011, 170, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, M.; François, A.; Grangé, S.; Rabant, M.; Châtelet, V.; Cassiman, D.; Cornec-Le Gall, E.; Ambrosetti, D.; Deschênes, G.; Benoist, J.F.; et al. Cobalamin C Deficiency Induces a Typical Histopathological Pattern of Renal Arteriolar and Glomerular Thrombotic Microangiopathy. Kidney Int. Rep. 2018, 3, 1153–1162. [Google Scholar] [CrossRef]
- Barlas, U.K.; Kıhtır, H.S.; Goknar, N.; Ersoy, M.; Akcay, N.; Sevketoglu, E. Hemolytic uremic syndrome with dual caution in an infant: Cobalamin C defect and complement dysregulation successfully treated with eculizumab. Pediatr. Nephrol. 2018, 33, 1093–1096. [Google Scholar] [CrossRef]
- Cohen, J.J.; Harrington, J.T.; Kassirer, J.P. Methylmalonic acidemia. Kidney Int. 1979, 15, 311–320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moreno-Vega, A.I.; Govantes, J.M. Methylmalonic acidemia treated by continuous peritoneal dialysis. N. Engl. J. Med. 1985, 312, 1641–1642. [Google Scholar] [PubMed]
- Porta, F.; Peruzzi, L.; Bonaudo, R.; Pieretti, S.; Busso, M.; Cocchi, E.; Conio, A.; Pagliardini, V.; Spada, M. Differential response to renal replacement therapy in neonatal-onset inborn errors of metabolism. Nephrology 2018, 23, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Saito, O.; Maeda, T.; Ito, C.; Ando, Y.; Yamagata, T.; Muto, S.; Momoi, M.; Kusano, E. Metabolic and Hemodynamic Advantages of an Acetate-Free Citrate Dialysate in a Uremic Case of Congenital Methylmalonic Acidemia. Am. J. Kidney Dis. 2009, 54, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Treacy, E.; Arbour, L.; Chessex, P.; Graham, G.; Kasprzak, L.; Casey, K.; Bell, L.; Mamer, O.; Scriver, C.R. Glutathione deficiency as a complication of methylmalonic acidemia: Response to high doses of ascorbate. J. Pediatr. 1996, 129, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hoff, W.G.; Dixon, M.; Taylor, J.; Mistry, P.; Rolles, K.; Rees, L.; Leonard, J.V. Combined liver-kidney transplantation in methylmalonic acidemia. J. Pediatr. 1998, 132, 1043–1044. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H.J.; Sperati, C.J.; King, J.D.; Poretti, A.; Miller, N.R.; Sloan, J.L.; Cameron, A.M.; Myers, D.; Venditti, C.P.; Valle, D. A detailed analysis of methylmalonic acid kinetics during hemodialysis and after combined liver/kidney transplantation in a patient with mut (0) methylmalonic acidemia. J. Inherit. Metab. Dis. 2014, 37, 899–907. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tsai, T.C.; Lee, W.J.; Chen, H.C. Continuous hemodiafiltration in the treatment of hyperammonemia due to methylmalonic acidemia. Ren. Fail. 2007, 29, 751–754. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kido, J.; Matsumoto, S.; Sawada, T.; Endo, F.; Nakamura, K. Rhabdomyolysis in organic acidemia patients manifesting with metabolic decompensation. Hemodial. Int. 2019, 23, E115–E119. [Google Scholar] [CrossRef]
- Tsai, I.J.; Hwu, W.L.; Huang, S.C.; Lee, N.C.; Wu, E.T.; Chien, Y.H.; Tsau, Y.K. Efficacy and safety of intermittent hemodialysis in infants and young children with inborn errors of metabolism. Pediatr. Nephrol. 2014, 29, 111–116. [Google Scholar] [CrossRef]
- Etuwewe, B.; Jones, C.A.; Mathur, S.; Wright, K.P.; Morris, A.A.M. Peritoneal dialysis for chronic renal failure in a patient with methylmalonic acidaemia. Pediatr. Nephrol. 2009, 24, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Wattanasirichaigoon, D.; Cortina, G.; Liu, Y.; Shu, J.; Cui, X.; Li, N.; Xue, H.; Zhang, F. Case report: Is exchange transfusion a possible treatment for metabolic decompensation in neonates with methylmalonic aciduria in the setting of limited resources? Front. Pediatr. 2022, 10, 926793. [Google Scholar]
- Aikoh, H.; Sasaki, M.; Sugai, K.; Yoshida, H.; Sakuragawa, N. Effective immunoglobulin therapy for brief tonic seizures in methylmalonic acidemia. Brain Dev. 1997, 19, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Nyhan, W.L.; Gargus, J.J.; Boyle, K.; Selby, R.; Koch, R. Progressive neurologic disability in methylmalonic acidemia despite transplantation of the liver. Eur. J. Pediatr. 2002, 161, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Kamei, K.; Ito, S.; Shigeta, T.; Sakamoto, S.; Fukuda, A.; Horikawa, R.; Saito, O.; Muguruma, T.; Nakagawa, S.; Iijima, K.; et al. Preoperative Dialysis for Liver Transplantation in Methylmalonic Acidemia. Ther. Apher. Dial. 2011, 15, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Foglia, E.; Ferrario, L.; Schettini, F.; Pagani, M.B.; Dalla Bona, M.; Porazzi, E. COVID-19 and hospital management costs: The Italian experience. BMC Health Serv. Res. 2022, 22, 991. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, A.; Stojanovic, J.; Vara, R.; De Nictolis, F.; Spada, M.; Dionisi-Vici, C. Safety, efficacy, and timing of transplantation(s) in propionic and methylmalonic aciduria. J. Inherit. Metab. Dis. 2023, 46, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Burrage, L.C.; Chapman, K.A.; Ginevic, I.; Mazariegos, G.V.; Graham, B.H. Solid organ transplantation in methylmalonic acidemia and propionic acidemia: A points to consider statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2023, 25, 100337. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.; Vara, R.; Morais, A. Post-transplantation Outcomes in Patients with PA or MMA: A Review of the Literature. Adv. Ther. 2020, 37, 1866–1896. [Google Scholar] [CrossRef]
- Dello Strologo, L.; Spada, M.; Vici, C.D.; Atti, M.C.D.; Rheault, M.; Bjerre, A.K.; Boyer, O.; Calvo, P.L.; D’Antiga, L.; Harshman, L.A.; et al. Renal outcome and plasma methylmalonic acid levels after isolated or combined liver or kidney transplantation in patients with methylmalonic acidemia: A multicenter analysis. Mol. Genet. Metab. 2022, 137, 265–272. [Google Scholar] [CrossRef]
- Lin, N.C.; Tsai, H.L.; Chen, C.Y.; Yeh, Y.T.; Lei, H.J.; Chou, S.C.; Chung, M.H.; Yang, C.F.; Niu, D.M.; Loong, C.C.; et al. Safety and long-term outcomes of early liver transplantation for pediatric methylmalonic acidemia patients. Pediatr. Transplant. 2022, 26, e14228. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Z.; Zhou, G.P.; Wei, L.; Qu, W.; Zeng, Z.G.; Liu, Y.; Tan, Y.L.; Wang, J.; Zhu, Z.J.; Sun, L.Y. Long-term clinical outcomes and health-related quality of life in patients with isolated methylmalonic acidemia after liver transplantation: Experience from the largest cohort study in China. World J. Pediatr. 2024, 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Noone, D.; Riedl, M.; Atkison, P.; Avitzur, Y.; Sharma, A.P.; Filler, G.; Siriwardena, K.; Prasad, C. Kidney disease and organ transplantation in methylmalonic acidaemia. Pediatr. Transplant. 2019, 23, e13407. [Google Scholar] [CrossRef] [PubMed]
- Brassier, A.; Krug, P.; Lacaille, F.; Pontoizeau, C.; Krid, S.; Sissaoui, S.; Servais, A.; Arnoux, J.B.; Legendre, C.; Charbit, M.; et al. Long-term outcome of methylmalonic aciduria after kidney, liver, or combined liver-kidney transplantation: The French experience. J. Inherit. Metab. Dis. 2020, 43, 234–243. [Google Scholar] [CrossRef]
- Plotnicki, L.; Kohl, C.D.; Höcker, B.; Krupka, K.; Rahmel, A.; Pape, L.; Hoyer, P.; Marks, S.D.; Webb, N.J.A.; Söylemezoglu, O.; et al. The CERTAIN Registry: A Novel, Web-Based Registry and Research Platform for Pediatric Renal Transplantation in Europe. Transplant. Proc. 2013, 45, 1414–1417. [Google Scholar] [CrossRef]
| Blood and Urine Tests | Admission | Discharge |
|---|---|---|
| White cells (109/L) | 14.16 | 6.35 |
| Neutrophils (109/L) | 12.35 | 7.04 |
| Red cells (1012/L) | 3.67 | 3.42 |
| Hemoglobin (g/L) | 105 | 97 |
| Hematocrit (L/L) | 0.329 | 0.304 |
| MCV (fL) | 89.6 | 88.9 |
| MCH (pg) | 28.6 | 28.4 |
| MCHC (g/L) | 319 | 319 |
| Platelets (109/L) | 233 | 242 |
| P-carnitine (µmol/L) | 71.9 | |
| Creatinine (µmol/L) | 303 | 424 |
| Urea (mmol/L) | 29.5 | 20.7 |
| Sodium (mmol/L) | 142 | 139 |
| Potassium (mmol/L) | 3.8 | 4.7 |
| Chloride (mmol/L) | 95 | 96 |
| Calcium (mmol/L) | 2.74 | 2.45 |
| Phosphorus (mmol/L) | 1.19 | 1.42 |
| Magnesium (mmol/L) | 0.76 | 0.86 |
| Lactate dehydrogenase (U/L) | 241 | 255 |
| Lactic acid (mmol/L) | 2 | 2 |
| Ammonia (µmol/L) | 32 | 35 |
| C-reactive protein (mg/L) | 14.84 | |
| Procalcitonin (ug/L) | 0.62 | 0.49 |
| Glucose (mmol/L) | 9.9 | 4.2 |
| Intact PTH (ng/L) (n.v. 6.5–36.8) | 123 | |
| Urinary pH | 6 | 7 |
| Urinary proteins (g/L) | 1 | 0.3 |
| Urinary albumin/creatinine (mg/g) | >300 | >300 |
| Venous Blood Gas Analysis | Admission | Day 2 | Day 4 | Discharge |
|---|---|---|---|---|
| pH | 7.34 | 7.39 | 7.39 | 7.43 |
| HCO3− (mmol/L) | 13.1 | 23.2 | 25.5 | 26.9 |
| Base excess (mmol/L) | −11.2 | −1.5 | 0.4 | 2.4 |
| Anion gap (mmol/L) | 33 | 14 | 18 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintus, G.; Vitturi, N.; Carraro, G.; Lenzini, L.; Gugelmo, G.; Fasan, I.; Madinelli, A.; Burlina, A.; Avogaro, A.; Calò, L.A. Renal Replacement Therapy in Methylmalonic Aciduria-Related Metabolic Failure: Case Report and Literature Review. J. Clin. Med. 2024, 13, 4304. https://doi.org/10.3390/jcm13154304
Pintus G, Vitturi N, Carraro G, Lenzini L, Gugelmo G, Fasan I, Madinelli A, Burlina A, Avogaro A, Calò LA. Renal Replacement Therapy in Methylmalonic Aciduria-Related Metabolic Failure: Case Report and Literature Review. Journal of Clinical Medicine. 2024; 13(15):4304. https://doi.org/10.3390/jcm13154304
Chicago/Turabian StylePintus, Giovanni, Nicola Vitturi, Gianni Carraro, Livia Lenzini, Giorgia Gugelmo, Ilaria Fasan, Alberto Madinelli, Alberto Burlina, Angelo Avogaro, and Lorenzo Arcangelo Calò. 2024. "Renal Replacement Therapy in Methylmalonic Aciduria-Related Metabolic Failure: Case Report and Literature Review" Journal of Clinical Medicine 13, no. 15: 4304. https://doi.org/10.3390/jcm13154304
APA StylePintus, G., Vitturi, N., Carraro, G., Lenzini, L., Gugelmo, G., Fasan, I., Madinelli, A., Burlina, A., Avogaro, A., & Calò, L. A. (2024). Renal Replacement Therapy in Methylmalonic Aciduria-Related Metabolic Failure: Case Report and Literature Review. Journal of Clinical Medicine, 13(15), 4304. https://doi.org/10.3390/jcm13154304








