Intraoperative Polymerase Chain Reaction from Cardiac Valve Tissue Is Beneficial for Guiding Further Therapy in Patients with Infective Endocarditis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Procedure
2.3. Microbiological Methods
2.4. Statistical Analysis
3. Results
3.1. Study Population and Clinical Data
3.2. Early Clinical Data
4. Discussion
Study Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical Presentation, Etiology, and Outcome of Infective Endocarditis in the 21st Century: The International Collaboration on Endocarditis–Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Delgado, V.; Marsan, N.A.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. ESC Scientific Document Group; 2023 ESC Guidelines for the management of endocarditis: Developed by the task force on the management of endocarditis of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2023, 44, 3966. [Google Scholar] [CrossRef]
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef]
- Prendergast, B.D.; Tornos, P. Surgery for infective endocarditis: Who and when? Circulation 2010, 121, 1141–1152. [Google Scholar] [CrossRef]
- Bohbot, Y.; Peugnet, F.; Lieu, A.; Carbone, A.; Mouhat, B.; Philip, M.; Gouriet, F.; Arregle, F.; Chevalier, F.; Diouf, M.; et al. Characteristics and Prognosis of Patients with Left-Sided Native Bivalvular Infective Endocarditis. Can. J. Cardiol. 2021, 37, 292–299. [Google Scholar] [CrossRef]
- Thuny, F.; Grisoli, D.; Cautela, J.; Riberi, A.; Raoult, D.; Habib, G. Infective endocarditis: Prevention, diagnosis, and management. Can. J. Cardiol. 2014, 30, 1046–1057. [Google Scholar] [CrossRef]
- Godfrey, R.; Curtis, S.; Schilling, W.H.; James, P.R. Blood culture negative endocarditis in the modern era of 16S rRNA sequencing. Clin. Med. 2020, 20, 412–416. [Google Scholar]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Goldenberger, D.; Künzli, A.; Vogt, P.; Zbinden, R.; Altwegg, M. Molecular diagnosis of bacterial endocarditis by broad-range PCR amplification and direct sequencing. J. Clin. Microbiol. 1997, 35, 2733–2739. [Google Scholar] [CrossRef]
- Breitkopf, C.; Hammel, D.; Scheld, H.H.; Peters, G.; Becker, K. Impact of a molecular approach to improve the microbiological diagnosis of infective heart valve endocarditis. Circulation 2005, 111, 1415–1421. [Google Scholar]
- Fournier, P.E.; Thuny, F.; Richet, H.; Lepidi, H.; Casalta, J.P.; Arzouni, J.P.; Maurin, M.; Célard, M.; Mainardi, J.L.; Caus, T.; et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: A prospective study of 819 new cases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010, 51, 131–140. [Google Scholar]
- Vollmer, T.; Piper, C.; Horstkotte, D.; Körfer, R.; Kleesiek, K.; Dreier, J. 23S rDNA real-time polymerase chain reaction of heart valves: A decisive tool in the diagnosis of infective endocarditis. Eur. Heart J. 2010, 31, 1105–1113. [Google Scholar]
- Harris, K.A.; Yam, T.; Jalili, S.; Williams, O.M.; Alshafi, K.; Gouliouris, T.; Munthali, P.; NiRiain, U.; Hartley, J.C. Service evaluation to establish the sensitivity, specificity and additional value of broad-range 16S rDNA PCR for the diagnosis of infective endocarditis from resected endocardial material in patients from eight UK and Ireland hospitals. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2014, 33, 2061–2066. [Google Scholar]
- Miller, R.J.; Chow, B.; Pillai, D.; Church, D. Development and evaluation of a novel fast broad-range 16S ribosomal DNA PCR and sequencing assay for diagnosis of bacterial infective endocarditis: Multi-year experience in a large Canadian healthcare zone and a literature review. BMC Infect. Dis. 2016, 16, 146. [Google Scholar]
- Armstrong, C.; Kuhn, T.C.; Dufner, M.; Ehlermann, P.; Zimmermann, S.; Lichtenstern, C.; Soethoff, J.; Katus, H.A.; Leuschner, F.; Heininger, A.; et al. The diagnostic benefit of 16S rDNA PCR examination of infective endocarditis heart valves: A cohort study of 146 surgical cases confirmed by histopathology. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2020, 110, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iunget, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Geissdorfer, W.; Moos, V.; Moter, A.; Loddenkemper, C.; Jansen, A.; Tandler, R.; Morguet, A.J.; Fenollar, F.; Raoult, D.; Bogdan, C.; et al. High frequency of Tropheryma whipplei in culture-negative endocarditis. J. Clin. Microbiol. 2012, 50, 216–222. [Google Scholar] [CrossRef]
- Gould, F.K.; Denning, D.W.; Elliott, T.S.; Foweraker, J.; Perry, J.D.; Prendergast, B.D.; Sandoe, J.A.; Spry, M.J.; Watkin, R.W. Working Party of the British Society for Antimicrobial C. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: A report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2012, 67, 269–289. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Lohmann, C.P.; Linde, H.J.; Reischl, U. Improved detection of microorganisms by polymerase chain reaction in delayed endophthalmitis after cataract surgery. Ophthalmology 2000, 107, 1047–1051. [Google Scholar] [CrossRef]
- Benito, N.; Miro, J.M.; de Lazzari, E.; Cabell, C.H.; del Rio, A.; Altclas, J.; Commerford, P.; Delahaye, F.; Dragulescu, S.; Giamarellou, H.; et al. Health care-associated nativevalve endocarditis: Importance of non-nosocomial acquisition. Ann. Intern. Med. 2009, 150, 586. [Google Scholar] [PubMed]
- Habib, G. Embolic risk in subacute bacterial endocarditis: Role of transesophageal echocardiography. Curr. Cardiol. Rep. 2003, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; di Salvo, G.; Belliard, O.; Avierinos, J.F.; Pergola, V.; Rosenberg, V.; Casalta, J.P.; Gouvernet, J.; Derumeaux, G.; Iarussi, D.; et al. Risk of embolism and death in infective endocarditis: Prognostic value of echocardiography: A prospective multicenter study. Circulation 2005, 112, 73. [Google Scholar]
- Brouqui, P.; Raoult, D. Endocarditis due to rare and fastidious bacteria. Clin. Microbiol. Rev. 2001, 14, 178. [Google Scholar]
- Moreillon, P.; Que, Y.A. Infective endocarditis. Lancet 2004, 363, 144. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Birg, M.L.; la Scola, B.; Fournier, P.E.; Enea, M.; Lepidi, H.; Roux, V.; Piette, J.C.; Vandenesch, F.; Vital-Durand, D.; et al. Cultivation of the bacillus of Whipple’s disease. N. Engl. J. Med. 2000, 342, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Bosshard, P.P. Etiologic Diagnosis of Infective Endocarditis by Broad-Range Polymerase Chain Reaction: A 3-Year Experience. Clin. Infect.Dis. 2003, 37, 170. [Google Scholar]
- Madico, G.E.; Rice, P.A. 16S-Ribosomal DNA to Diagnose Culture-Negative Endocarditis. Curr. Infect. Dis. Rep. 2008, 10, 285. [Google Scholar]
- Tak, T.; Shukla, S.K. Molecular Diagnosis of Infective Endocarditis: A Helpful Addition to the Duke Criteria. Clin. Med. Res. 2004, 2, 207. [Google Scholar] [CrossRef]
- Rothman, R.E.; Majmudar, M.D.; Kelen, G.D.; Madico, G.; Gaydos, C.A.; Walker, T.; Quinn, T.C. Detection of bacteremia in emergency department patients at risk for infective endocarditis using universal 16S rRNA primers in a decontaminated polymerase chain reaction assay. J. Infect. Dis. 2002, 186, 1677–1678. [Google Scholar] [PubMed]
- Arvieux, C.; Common, H. New diagnostic tools for prosthetic joint infection. Orthop. Traumatol. Surg. Res. 2019, 105, 23–30. [Google Scholar] [CrossRef]
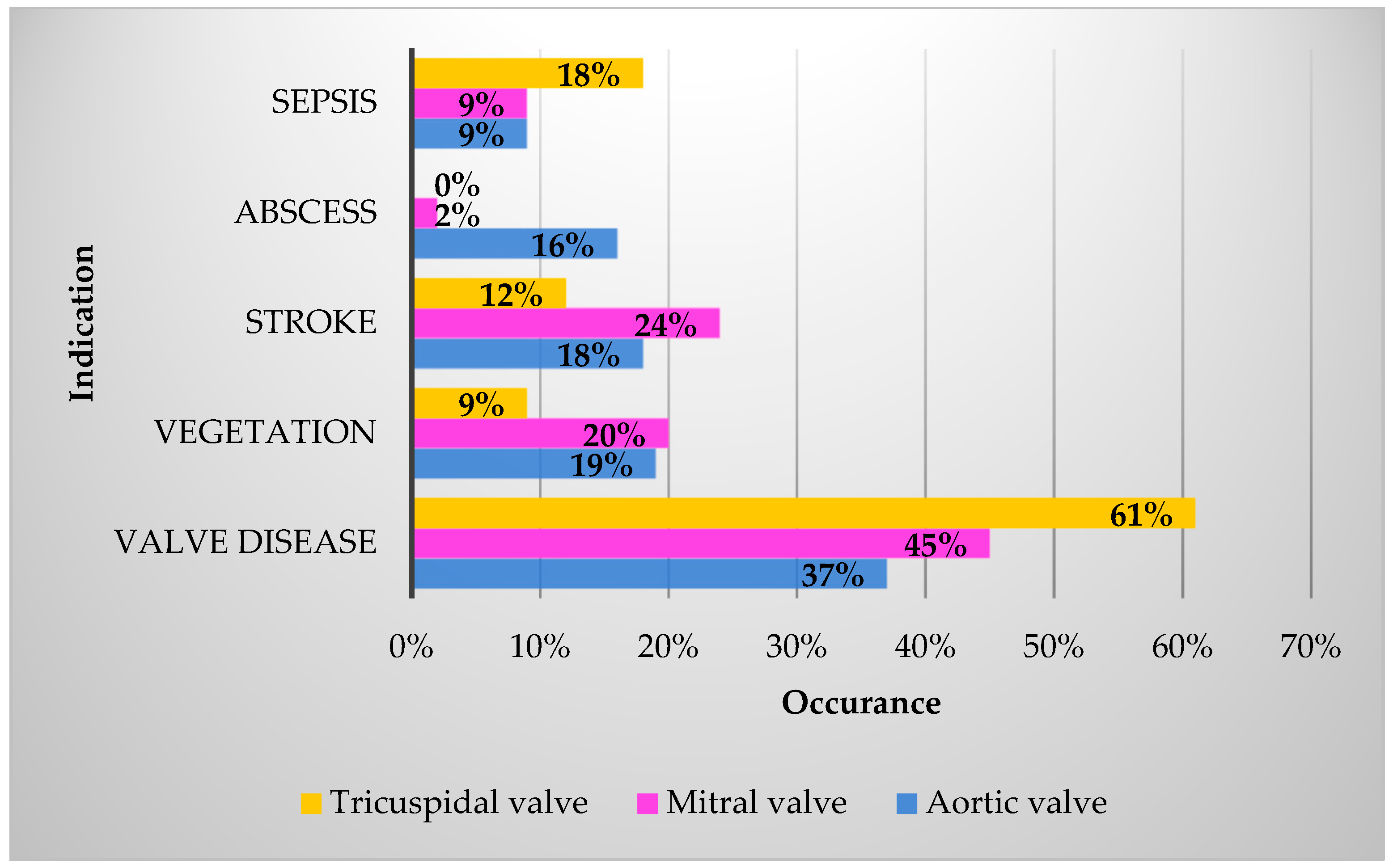
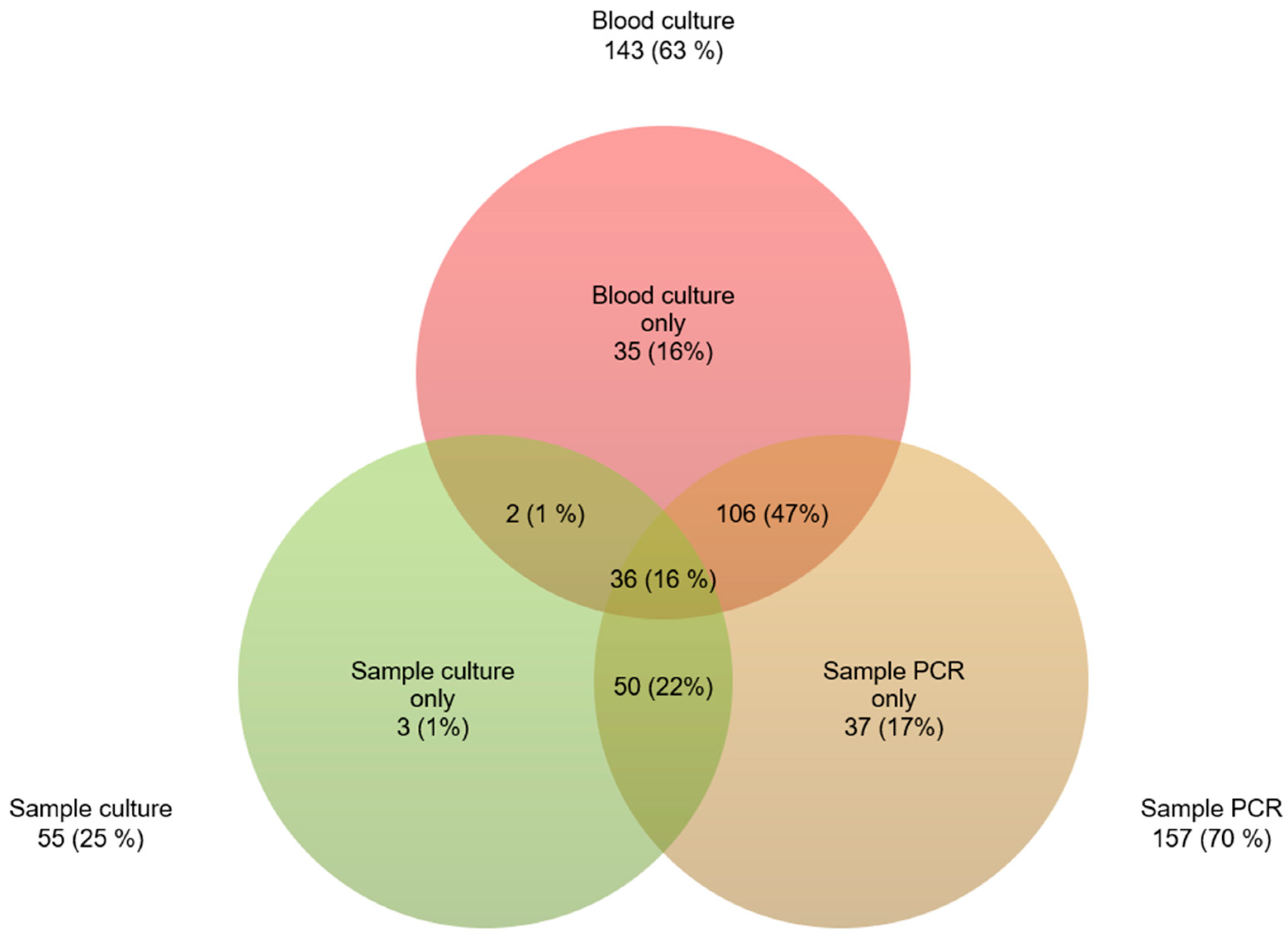
| Demographics | n (%) |
|---|---|
| Number of patients | 224 |
| Age, median (range), years | 63 (23–84) |
| Gender | |
| Male | 160 (71%) |
| Female | 64 (29%) |
| Comorbidities | |
| Stroke | 79 (35%) |
| Coronary artery disease | 56 (25%) |
| Diabetes | 21 (9.45%) |
| NYHA class | |
| III | 104 (46%) |
| IV | 70 (3%) |
| LV ejection fraction | |
| >50% | 155 (69%) |
| 30–50% | 67 (20%) |
| <30% | 2 (1%) |
| Kidney injury (KDIGO) | |
| Stage G1 (GFR ≥90 [mL/min/1.73 m2]) | 67 (30%) |
| Stage G2 (GFR 60–90) | 70 (31%) |
| Stage G3 (GFR 30–60) | 63 (28%) |
| Stage G4 (GFR 15–30) | 16 (7%) |
| Stage G5 (GFR <15) | 8 (4%) |
| Preoperative dialysis | 11 (5%) |
| EuroSCORE II | 8.35 (11.99) |
| Endocarditis | |
| Native | 154 (67%) |
| Prosthetic | 70 (31%) |
| Aortic valve | |
| Insufficiency (grade I–IV) | 140 (63%) |
| Insufficiency ≥ grade II | 102 (46%) |
| Stenosis | 15 (7%) |
| Combined | 21 (9%) |
| Not affected | 48 (21%) |
| Vegetation | 89 (40%) |
| Abscess | 19 (8%) |
| Combined | 29 (13%) |
| Mitral valve | |
| Insufficiency (grade I–IV) | 184 (82%) |
| Insufficiency ≥ grade II | 104 (46%) |
| Stenosis | 5 (2%) |
| Combined | 3 (1%) |
| Not affected | 32 (14%) |
| Vegetation | 90 (40%) |
| Abscess | 2 (1%) |
| Combined | 7 (3%) |
| Tricuspid valve | |
| Insufficiency (grade I–IV) | 101 (45%) |
| Insufficiency ≥ grade 2 | 45 (20%) |
| Stenosis | 3 (1%) |
| Combined | - |
| Not affected | 120 (54%) |
| Vegetation | 18 (8%) |
| Abscess | - |
| Combined | 4 (2%) |
| Abscess | 60 (27%) |
| Double valve endocarditis | 39 (18%) |
| Pathogens | Occurrence |
|---|---|
| Staphylococcus aureus | 72 (32%) |
| Viridans streptococci (S. mutans, S. mitis, S. anginosus, S. sanguinis, S. salivarius, S. gordonii, S. constellatus) | 38 (17%) |
| Enterococcus faecalis | 31 (14%) |
| Staphylococcus epidermidis | 13 (6%) |
| Cutibacterium acnes | 11 (5%) |
| Streptococcus agalactiae | 9 (4%) |
| Granulicatella adiacens | 5 (2%) |
| Haemuphilus influencae | 5 (2%) |
| Staphylococcus lugdunensis | 5 (2%) |
| Streptococcus gallolyticus | 5 (2%) |
| Others | 22 (10%) |
| Abiotrophia defectiva | |
| Bartonella quintana | |
| Bartonella henselae | |
| Cardiobacterium hominis | |
| Citrobacterium koseri | |
| Corynebacterium striatum | |
| Enterococcus gallinarum | |
| Serratia marcescens | |
| Staphylococcus capitis | |
| Moraxella osloensis | |
| Clostridium intestinale | |
| Pseudomonas aeruginosa | |
| Streptococcus pneumoniae | |
| Streptococcus dysgalactiae | |
| Staphylococcus saprophyticus | |
| Staphylococcus haemolyticus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Zeppelin, M.; Gharoony, S.A.; Holubcova, Z.; Salem, R.; Hlavicka, J.; Heyl, S.; Ochs, M.; Wichelhaus, T.A.; Kessel, J.; Moritz, A.; et al. Intraoperative Polymerase Chain Reaction from Cardiac Valve Tissue Is Beneficial for Guiding Further Therapy in Patients with Infective Endocarditis. J. Clin. Med. 2024, 13, 4319. https://doi.org/10.3390/jcm13154319
von Zeppelin M, Gharoony SA, Holubcova Z, Salem R, Hlavicka J, Heyl S, Ochs M, Wichelhaus TA, Kessel J, Moritz A, et al. Intraoperative Polymerase Chain Reaction from Cardiac Valve Tissue Is Beneficial for Guiding Further Therapy in Patients with Infective Endocarditis. Journal of Clinical Medicine. 2024; 13(15):4319. https://doi.org/10.3390/jcm13154319
Chicago/Turabian Stylevon Zeppelin, Mascha, Seyed Arian Gharoony, Zdenka Holubcova, Razan Salem, Jan Hlavicka, Stephan Heyl, Marco Ochs, Thomas A. Wichelhaus, Johanna Kessel, Anton Moritz, and et al. 2024. "Intraoperative Polymerase Chain Reaction from Cardiac Valve Tissue Is Beneficial for Guiding Further Therapy in Patients with Infective Endocarditis" Journal of Clinical Medicine 13, no. 15: 4319. https://doi.org/10.3390/jcm13154319






