Facilitating Corticomotor Excitability of the Contralesional Hemisphere Using Non-Invasive Brain Stimulation to Improve Upper Limb Motor Recovery from Stroke—A Scoping Review
Abstract
:1. Introduction
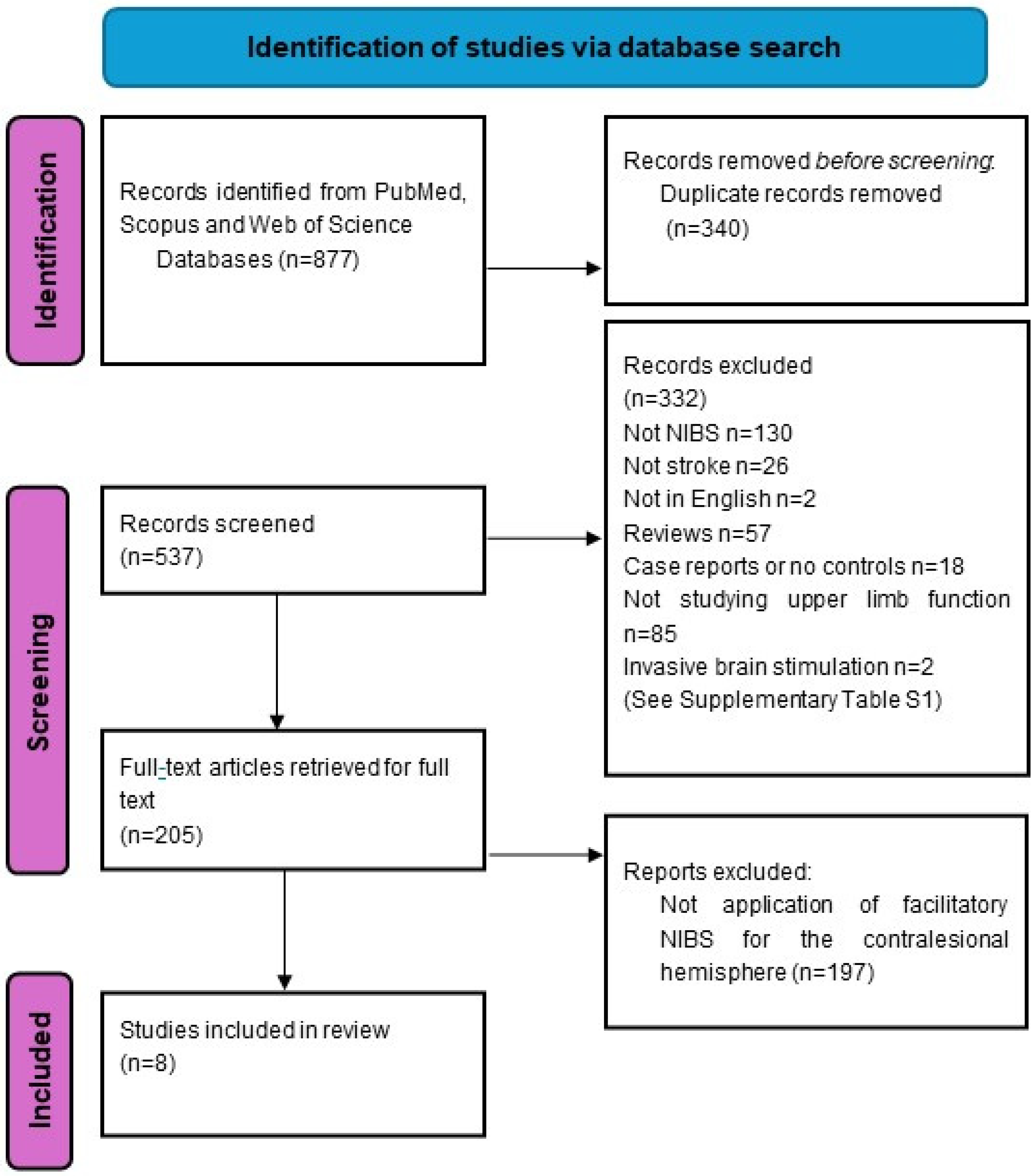
2. Methods
2.1. Study Design
2.2. Search Strategy and Selection Criteria
3. Results
3.1. Study Design and Study Quality
3.2. Stroke Timing, Type, and Lesion Location
3.3. Types of NIBS Interventions and Sites of Stimulation
3.4. Neurophysiological and Behavioral Outcome Measures
3.5. Studies Investigating the Facilitation of Contralesional Primary Motor Cortex
3.6. Studies Investigating the Facilitation of Contralesional Dorsal Premotor Cortex
| Studies Investigating the Facilitation of cM1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study Author, Year (n) | NIBS Type | MEP recorded | Study design/Number of sessions | Stimulation sites | Stimulation protocols | Severity of upper limb impairment Stratification | Stroke characteristics | Summary of Key Results | |
| 1 | Wang et al., 2020 [36] (n = 45) | rTMS | APB and BB of non-paretic hand | Randomized, single-blinded; daily sessions for 14 days | cM1 |
| Severe: -Total FMA 8-38/100 (all subjects were MEP-) No stratification |
|
|
| 2 | Kwon et al., 2016 [43] (n = 20) | tDCS + rTMS | FDI of the non-paretic hand | Randomized, cross-over; single session ×5 | cM1, iM1 bihemispheric concurrent stimulation |
| Mild: all subjects MEP+ and FMA-UE range 39–65 No stratification |
|
|
| 3 | Nemanich et al., 2023 [41] (n = 14) | tDCS | APB and ED of both paretic and non-paretic hand | Randomized; single session over 1 h | MEP+→iM1 MEP-→cM1 |
| Mixed: 42.8% MEP+ Stratification based on MEP status |
(13–14 years)
|
|
| 4 | McCambridge et al., 2018 [39] (n = 10) | tDCS | BB and ECR | Crossover, double-blinded; single sessions ×3 | cM1 |
| Mild-severe: FMA-UE 9-58; 40% subjects MEP- No stratification |
|
|
| 5 | Klomjai et al., 2022 [40] (n = 21) | tDCS | - | Crossover; single sessions × 3 | cM1 |
| Mild to moderate MEP not reported No stratification |
|
|
| 6 | Yao et al., 2015 [42] (n = 9) | tDCS | - | Crossover; single sessions ×3 | iM1 or cM1 |
| FMA-UE range 20–40 No stratification |
|
|
| Studies Investigating the Facilitation of cPMD | |||||||||
| 7 | Sankarasubramanian et al., 2017 [37] (n = 15) | rTMS | EDC in non-paretic hand | Crossover; single sessions | cM1, cPMD and iPMD |
| Mild-severe: FMA-UE 7-63; 60% MEP+ and 40% MEP- Stratification by FMA and mean FA |
| Those with low FMA-UE and low mean FA on MRI had greater improvement in paretic arm reaching time with HF rTMS to cPMD compared to LF rTMS to cM1. Facilitation of cPMD but not cM1 improved reaching time with reduction in iSP to the lesioned cortex. A decision tree for stratifying responders was proposed. |
| 8 | Liao et al., 2019 [38] (n = 14) | rTMS | BB in paretic and non-paretic upper limb | Cross-over; two sessions each ×2 | cPMD iM1 |
| Mild-moderate: FMA-UE 20-65 and MEP+ Stratification based on FMA, ipsilateral silent period, and MEP ratio after rTMS |
| cPMD responders (n = 4) (improved interlimb force coordination and intermuscular coherence) had lower FMA-UE and higher iSP inhibition to the affected arm. iM1 responders (n = 10) had higher FMA-UE and lower iSP inhibition. cPMD responders showed a decrease in iSP inhibition with rTMS. |
4. Discussion
4.1. The Role of cM1 and cPMD in Stroke Recovery
4.2. Possible Mechanisms by Which Facilitation of the Contralesional Hemisphere Improves Function
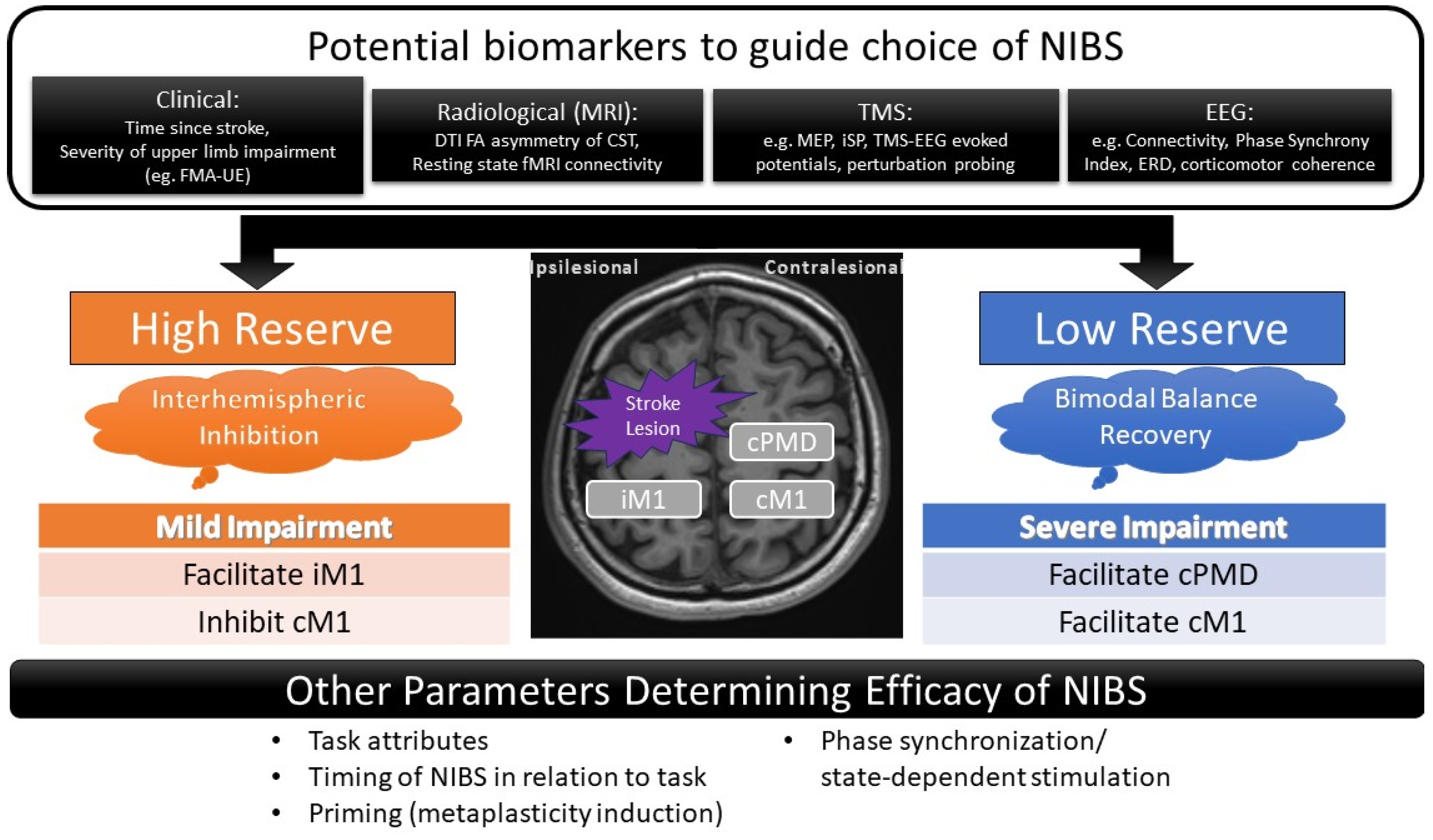
4.3. Predictors of Response to Contralesional Hemispheric Facilitation
4.4. Study Limitations and Future Directions
5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
References
- Krishnamurthi, R.V.; Feigin, V.L.; Forouzanfar, M.H.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.M.; Truelsen, T.; et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet Glob. Health 2013, 1, e259–e281. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef]
- Ovbiagele, B.; Goldstein, L.B.; Higashida, R.T.; Howard, V.J.; Johnston, S.C.; Khavjou, O.A.; Lackland, D.T.; Lichtman, J.H.; Mohl, S.; Sacco, R.L.; et al. Forecasting the future of stroke in the United States: A policy statement from the American Heart Association and American Stroke Association. Stroke 2013, 44, 2361–2375. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Kollen, B.J.; van der Grond, J.; Prevo, A.J. Probability of Regaining Dexterity in the Flaccid Upper Limb. Stroke 2003, 34, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, M.; La Porta, F.; Agosti, M.; Massucci, M. Is health-related-quality of life of stroke patients influenced by neurological impairments at one year after stroke? Eur. J. Phys. Rehabil. Med. 2010, 46, 389–399. [Google Scholar] [PubMed]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; Van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, CD010820. [Google Scholar] [CrossRef]
- Ahmed, I.; Mustafaoglu, R.; Benkhalifa, N.; Yakhoub, Y.H. Does noninvasive brain stimulation combined with other therapies improve upper extremity motor impairment, functional performance, and participation in activities of daily living after stroke? A systematic review and meta-analysis of randomized controlled trial. Top. Stroke Rehabil. 2023, 30, 213–234. [Google Scholar] [PubMed]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Hofmeijer, J.; Ham, F.; Kwakkel, G. Evidence of rTMS for Motor or Cognitive Stroke Recovery: Hype or Hope? Stroke 2023, 54, 2500–2511. [Google Scholar] [CrossRef]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.L.; Edwards, D.; Dunning, K.; Fregni, F.; Stein, J.; Laine, J.; Rogers, L.M.; Vox, F.; Durand-Sanchez, A.; Bockbrader, M.; et al. Randomized Sham-Controlled Trial of Navigated Repetitive Transcranial Magnetic Stimulation for Motor Recovery in Stroke. Stroke 2018, 49, 2138–2146. [Google Scholar] [CrossRef]
- Cohen, S.L.; Bikson, M.; Badran, B.W.; George, M.S. A visual and narrative timeline of US FDA milestones for Transcranial Magnetic Stimulation (TMS) devices. Brain Stimul. 2022, 15, 73–75. [Google Scholar] [CrossRef]
- Fregni, F.; Nitsche, M.A.; Loo, C.K.; Brunoni, A.R.; Marangolo, P.; Leite, J.; Carvalho, S.; Bolognini, N.; Caumo, W.; Paik, N.J.; et al. Regulatory Considerations for the Clinical and Research Use of Transcranial Direct Current Stimulation (tDCS): Review and recommendations from an expert panel. Clin. Res. Regul. Aff. 2015, 32, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capone, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C.; et al. Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nat. Rev. Neurol. 2014, 10, 597–608. [Google Scholar] [CrossRef]
- Ameli, M.; Grefkes, C.; Kemper, F.; Riegg, F.P.; Rehme, A.K.; Karbe, H.; Fink, G.R.; Nowak, D.A. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann. Neurol. 2009, 66, 298–309. [Google Scholar] [CrossRef]
- Levy, R.M.; Harvey, R.L.; Kissela, B.M.; Winstein, C.J.; Lutsep, H.L.; Parrish, T.B.; Cramer, S.C.; Venkatesan, L. Epidural Electrical Stimulation for Stroke Rehabilitation: Results of the Prospective, Multicenter, Randomized, Single-Blinded Everest Trial. Neurorehabil. Neural Repair 2016, 30, 107–119. [Google Scholar] [CrossRef]
- Boddington, L.; Reynolds, J. Targeting interhemispheric inhibition with neuromodulation to enhance stroke rehabilitation. Brain Stimul. 2017, 10, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, C.; Maxfield, L. Varieties of interhemispheric inhibition, or how to keep a good hemisphere down. Brain Cogn. 1996, 30, 81–108. [Google Scholar] [CrossRef]
- Cassidy, J.M.; Cramer, S.C. Spontaneous and Therapeutic-Induced Mechanisms of Functional Recovery after Stroke. Transl. Stroke Res. 2017, 8, 33–46. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, Y.; Cao, N.; Jiang, C. Promotion of Poststroke Motor-Function Recovery with Repetitive Transcranial Magnetic Stimulation by Regulating the Interhemispheric Imbalance. Brain Sci. 2020, 10, 648. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.N.; Stinear, C.M. TMS measures of motor cortex function after stroke: A meta-analysis. Brain Stimul. 2017, 10, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Branscheidt, M.; Schambra, H.; Steiner, L.; Widmer, M.; Diedrichsen, J.; Goldsmith, J.; Lindquist, M.; Kitago, T.; Luft, A.R.; et al. Rethinking interhemispheric imbalance as a target for stroke neurorehabilitation. Ann. Neurol. 2019, 85, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Gerges, A.N.; Hordacre, B.; Di Pietro, F.; Moseley, G.L.; Berryman, C. Do Adults with Stroke have Altered Interhemispheric Inhibition? A Systematic Review with Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2022, 31, 106494. [Google Scholar] [CrossRef] [PubMed]
- Plow, E.B.; Sankarasubramanian, V.; Cunningham, D.A.; Potter-Baker, K.; Varnerin, N.; Cohen, L.G.; Sterr, A.; Conforto, A.B.; Machado, A.G. Models to Tailor Brain Stimulation Therapies in Stroke. Neural Plast. 2016, 2016, 4071620. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.S.; Omlor, W.; Rubio, J.C.; Chen, J.L.; Zheng, H.; Schröter, A.; Gullo, M.; Weinmann, O.; Kobayashi, K.; Helmchen, F.; et al. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science 2014, 344, 1250–1255. [Google Scholar] [CrossRef]
- Okabe, N.; Narita, K.; Miyamoto, O. Axonal remodeling in the corticospinal tract after stroke: How does rehabilitative training modulate it? Neural Regen. Res. 2017, 12, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Touvykine, B.; Mansoori, B.K.; Jean-Charles, L.; Deffeyes, J.; Quessy, S.; Dancause, N. The Effect of Lesion Size on the Organization of the Ipsilesional and Contralesional Motor Cortex. Neurorehabil. Neural Repair 2016, 30, 280–292. [Google Scholar] [CrossRef]
- Ward, N.S.; Brown, M.M.; Thompson, A.J.; Frackowiak, R.S.J. Neural correlates of outcome after stroke: A cross-sectional fMRI study. Brain 2003, 126 Pt 6, 1430–1448. [Google Scholar] [CrossRef]
- Ward, N.S.; Newton, J.M.; Swayne, O.B.C.; Lee, L.; Thompson, A.J.; Greenwood, R.J.; Rothwell, J.C.; Frackowiak, R.S.J. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 2006, 129 Pt 3, 809–819. [Google Scholar] [CrossRef]
- Caramia, M.; Palmieri, M.; Giacomini, P.; Iani, C.; Dally, L.; Silvestrini, M. Ipsilateral activation of the unaffected motor cortex in patients with hemiparetic stroke. Clin. Neurophysiol. 2000, 111, 1990–1996. [Google Scholar] [CrossRef]
- Bradnam, L.V.; Stinear, C.M.; Byblow, W.D. Ipsilateral motor pathways after stroke: Implications for non-invasive brain stimulation. Front. Hum. Neurosci. 2013, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Smith, M.-C.; Byblow, W.D.; Stinear, C.M. Applications of Repetitive Transcranial Magnetic Stimulation to Improve Upper Limb Motor Performance After Stroke: A Systematic Review. Neurorehabil. Neural Repair 2023, 37, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Johansen-Berg, H.; Rushworth, M.F.S.; Bogdanovic, M.D.; Kischka, U.; Wimalaratna, S.; Matthews, P.M. The role of ipsilateral premotor cortex in hand movement after stroke. Proc. Natl. Acad. Sci. USA 2002, 99, 14518–14523. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, D.; Zhao, Y.-Y.; Hai, H.; Ma, Y.-W. Effects of high-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex on motor recovery in severe hemiplegic stroke: A randomized clinical trial. Brain Stimul. 2020, 13, 979–986. [Google Scholar] [CrossRef]
- Sankarasubramanian, V.; Machado, A.G.; Conforto, A.B.; Potter-Baker, K.A.; Cunningham, D.A.; Varnerin, N.M.; Wang, X.; Sakaie, K.; Plow, E.B. Inhibition versus facilitation of contralesional motor cortices in stroke: Deriving a model to tailor brain stimulation. Clin. Neurophysiol. 2017, 128, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-W.; Whitall, J.; Wittenberg, G.F.; Barton, J.E.; Waller, S.M. Not all brain regions are created equal for improving bimanual coordination in individuals with chronic stroke. Clin. Neurophysiol. 2019, 130, 1218–1230. [Google Scholar] [CrossRef]
- McCambridge, A.B.; Stinear, J.W.; Byblow, W.D. Revisiting interhemispheric imbalance in chronic stroke: A tDCS study. Clin. Neurophysiol. 2018, 129, 42–50. [Google Scholar] [CrossRef]
- Klomjai, W.; Giron, A.; Mounir El Mendili, M.; Aymard, C.; Pradat-Diehl, P.; Roche, N.; Katz, R.; Bayen, E.; Lackmy-Vallee, A. Anodal tDCS of contralesional hemisphere modulates ipsilateral control of spinal motor networks targeting the paretic arm post-stroke. Clin. Neurophysiol. 2022, 136, 1–12. [Google Scholar] [CrossRef]
- Nemanich, S.T.; Lench, D.H.; Sutter, E.N.; Kowalski, J.L.; Francis, S.M.; Meekins, G.D.; Krach, L.E.; Feyma, T.; Gillick, B.T. Safety and feasibility of transcranial direct current stimulation stratified by corticospinal organization in children with hemiparesis. Eur. J. Paediatr. Neurol. 2023, 43, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Drogos, J.; Veltink, F.; Anderson, C.; Zaa, J.C.U.; Hanson, L.I.; Dewald, J.P.A. The effect of transcranial direct current stimulation on the expression of the flexor synergy in the paretic arm in chronic stroke is dependent on shoulder abduction loading. Front. Hum. Neurosci. 2015, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.G.; Park, E.; Kang, C.; Chang, W.H.; Kim, Y.-H. The effects of combined repetitive transcranial magnetic stimulation and transcranial direct current stimulation on motor function in patients with stroke. Restor. Neurol. Neurosci. 2016, 34, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Buetefisch, C.M.; Revill, K.P.; Shuster, L.; Hines, B.; Parsons, M. Motor demand-dependent activation of ipsilateral motor cortex. J. Neurophysiol. 2014, 112, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Calautti, C.; Baron, J.C. Functional neuroimaging studies of motor recovery after stroke in adults: A review. Stroke 2003, 34, 1553–1566. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Dricot, L.; Laloux, P.; Gradkowski, W.; Desfontaines, P.; Evrard, F.; Peeters, A.; Jamart, J.; Vandermeeren, Y. Neural substrates underlying stimulation-enhanced motor skill learning after stroke. Brain 2014, 138, 149–163. [Google Scholar] [CrossRef]
- Schulz, R.; Buchholz, A.; Frey, B.M.; Bönstrup, M.; Cheng, B.; Thomalla, G.; Hummel, F.C.; Gerloff, C. Enhanced Effective Connectivity between Primary Motor Cortex and Intraparietal Sulcus in Well-Recovered Stroke Patients. Stroke 2016, 47, 482–489. [Google Scholar] [CrossRef]
- Buma, F.E.; van Kordelaar, J.; Raemaekers, M.; van Wegen, E.E.H.; Ramsey, N.F.; Kwakkel, G. Brain activation is related to smoothness of upper limb movements after stroke. Exp. Brain Res. 2016, 234, 2077–2089. [Google Scholar] [CrossRef]
- Schaechter, J.D.; Perdue, K.L. Enhanced cortical activation in the contralesional hemisphere of chronic stroke patients in response to motor skill challenge. Cereb. Cortex 2008, 18, 638–647. [Google Scholar] [CrossRef]
- Hensel, L.; Lange, F.; Tscherpel, C.; Viswanathan, S.; Freytag, J.; Volz, L.J.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Recovered grasping performance after stroke depends on interhemispheric frontoparietal connectivity. Brain 2022, 146, 1006–1020. [Google Scholar] [CrossRef]
- Bütefisch, C.M.; Netz, J.; Weßling, M.; Seitz, R.J.; Hömberg, V. Remote changes in cortical excitability after stroke. Brain 2003, 126 Pt 2, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Rehme, A.K.; Fink, G.R.; von Cramon, D.Y.; Grefkes, C. The Role of the Contralesional Motor Cortex for Motor Recovery in the Early Days after Stroke Assessed with Longitudinal fMRI. Cereb. Cortex 2010, 21, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.S.; Newton, J.M.; Swayne, O.B.C.; Lee, L.; Frackowiak, R.S.J.; Thompson, A.J.; Greenwood, R.J.; Rothwell, J.C. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur. J. Neurosci. 2007, 25, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Harrington, R.; Chan, E.; Dromerick, A.W.; Breceda, E.Y.; Harris-Love, M. Role of contralesional hemisphere in paretic arm reaching in patients with severe arm paresis due to stroke: A preliminary report. Neurosci. Lett. 2016, 617, 52–58. [Google Scholar] [CrossRef]
- Kantak, S.; Luchmee, D. Contralesional motor cortex is causally engaged during more dexterous actions of the paretic hand after stroke—A Preliminary report. Neurosci. Lett. 2020, 720, 134751. [Google Scholar] [CrossRef] [PubMed]
- Tscherpel, C.; Hensel, L.; Lemberg, K.; Vollmer, M.; Volz, L.J.; Fink, G.R.; Grefkes, C. The differential roles of contralesional frontoparietal areas in cortical reorganization after stroke. Brain Stimul. 2020, 13, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-L.; Potter-Baker, K.A.; Cunningham, D.A.; Li, M.; Sankarasubramanian, V.; Lee, J.; Jones, S.; Sakaie, K.; Wang, X.; Machado, A.G.; et al. Stratifying chronic stroke patients based on the influence of contralesional motor cortices: An inter-hemispheric inhibition study. Clin. Neurophysiol. 2020, 131, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.; Wiemer, V.M.; Hensel, L.; Cieslak, M.; Tscherpel, C.; Grefkes, C.; Grafton, S.T.; Fink, G.R.; Volz, L.J. Interhemispheric Structural Connectivity Underlies Motor Recovery after Stroke. Ann. Neurol. 2023, 94, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Hmaied Assadi, S.; Feige Gross-Nevo, R.; Dudkiewicz, I.; Barel, H.; Rand, D. Improvement of the Upper Extremity at the Subacute Stage Poststroke: Does Hand Dominance Play a Role? Neurorehabil. Neural Repair 2020, 34, 1030–1037. [Google Scholar] [CrossRef]
- Harris-Love, M.L.; Harrington, R.M. Non-Invasive Brain Stimulation to Enhance Upper Limb Motor Practice Poststroke: A Model for Selection of Cortical Site. Front. Neurol. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Bestmann, S.; Swayne, O.; Blankenburg, F.; Ruff, C.C.; Teo, J.; Weiskopf, N.; Driver, J.; Rothwell, J.C.; Ward, N.S. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J. Neurosci. 2010, 30, 11926–11937. [Google Scholar] [CrossRef] [PubMed]
- Bradnam, L.V.; Stinear, C.M.; Barber, P.A.; Byblow, W.D. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb. Cortex 2012, 22, 2662–2671. [Google Scholar] [CrossRef]
- Chew, E.; Teo, W.P.; Tang, N.; Ang, K.K.; Ng, Y.S.; Zhou, J.H.; Teh, I.; Phua, K.S.; Zhao, L.; Guan, C. Using Transcranial Direct Current Stimulation to Augment the Effect of Motor Imagery-Assisted Brain-Computer Interface Training in Chronic Stroke Patients-Cortical Reorganization Considerations. Front. Neurol. 2020, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Biabani, M.; Aminitehrani, M.; Zoghi, M.; Farrell, M.; Egan, G.; Jaberzadeh, S. The effects of transcranial direct current stimulation on short-interval intracortical inhibition and intracortical facilitation: A systematic review and meta-analysis. Rev. Neurosci. 2018, 29, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Brus-Ramer, M.; Carmel, J.B.; Martin, J.H. Motor Cortex Bilateral Motor Representation Depends on Subcortical and Interhemispheric Interactions. J. Neurosci. 2009, 29, 6196–6206. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, S.; Havton, L.A.; McKay, H.; Yang, H.; Brant, A.; Roberts, J.; Tuszynski, M.H. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: A quantitative study. J. Comp. Neurol. 2004, 473, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Alawieh, A.; Tomlinson, S.; Adkins, D.; Kautz, S.; Feng, W. Preclinical and Clinical Evidence on Ipsilateral Corticospinal Projections: Implication for Motor Recovery. Transl. Stroke Res. 2017, 8, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Eyre, J.A.; Taylor, J.P.; Villagra, F.; Smith, M.; Miller, S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology 2001, 57, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Ishii, K.; Borgheresi, A.; Yaseen, Z.; Battaglia, F.; Hallett, M.; Cincotta, M.; Wassermann, E.M. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J. Physiol. 1999, 518 Pt 3, 895–906. [Google Scholar] [CrossRef]
- Jankowska, E.; Edgley, S.A. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist 2006, 12, 67–79. [Google Scholar] [CrossRef]
- Ferbert, A.; Priori, A.; Rothwell, J.C.; Day, B.L.; Colebatch, J.G.; Marsden, C.D. Interhemispheric inhibition of the human motor cortex. J. Physiol. 1992, 453, 525–546. [Google Scholar] [CrossRef]
- Salehi Dehno, N.; Kamali, F.; Shariat, A.; Jaberzadeh, S. Comparison of Transcallosal Inhibition between Hemispheres and Its Relationship with Motor Behavior in Patients with Severe Upper Extremity Impairment after Subacute Stroke. J. Stroke Cerebrovasc. Dis. 2022, 31, 106469. [Google Scholar] [CrossRef] [PubMed]
- Milani, G.; Antonioni, A.; Baroni, A.; Malerba, P.; Straudi, S. Relation between EEG Measures and Upper Limb Motor Recovery in Stroke Patients: A Scoping Review. Brain Topogr. 2022, 35, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Hattori, N.; Uno, Y.; Hatakenaka, M.; Yagura, H.; Fujimoto, H.; Yoshioka, T.; Nagasako, M.; Otomune, H.; Kitajo, K.; et al. Electroencephalographic Phase Synchrony Index as a Biomarker of Poststroke Motor Impairment and Recovery. Neurorehabil. Neural Repair 2020, 34, 711–722. [Google Scholar] [CrossRef]
- Kaiser, V.; Daly, I.; Pichiorri, F.; Mattia, D.; Müller-Putz, G.R.; Neuper, C. Relationship between Electrical Brain Responses to Motor Imagery and Motor Impairment in Stroke. Stroke 2012, 43, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Rossiter, H.E.; Eaves, C.; Davis, E.; Boudrias, M.-H.; Park, C.-H.; Farmer, S.; Barnes, G.; Litvak, V.; Ward, N.S. Changes in the location of cortico-muscular coherence following stroke. Neuroimage Clin. 2012, 2, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, A.; Tabarelli, D.; Belardinelli, P. A New Framework to Interpret Individual Inter-Hemispheric Compensatory Communication after Stroke. J. Pers. Med. 2022, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Zhang, J.J.; Fong, K.N.K. Intracortical and intercortical networks in patients after stroke: A concurrent TMS-EEG study. J. NeuroEng. Rehabil. 2023, 20, 100. [Google Scholar] [CrossRef]
- Jensen, O.; Bahramisharif, A.; Oostenveld, R.; Klanke, S.; Hadjipapas, A.; Okazaki, Y.O.; van Gerven, M.A. Using brain-computer interfaces and brain-state dependent stimulation as tools in cognitive neuroscience. Front. Psychol. 2011, 2, 100. [Google Scholar] [CrossRef]
- Bergmann, T.O. Brain State-Dependent Brain Stimulation. Front. Psychol. 2018, 9, 2108. [Google Scholar] [CrossRef]
- Rehme, A.K.; Grefkes, C. Cerebral network disorders after stroke: Evidence from imaging-based connectivity analyses of active and resting brain states in humans. J. Physiol. 2013, 591, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Hartwigsen, G.; Volz, L.J. Probing rapid network reorganization of motor and language functions via neuromodulation and neuroimaging. Neuroimage 2021, 224, 117449. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M.; Di Iorio, R.; Rossini, P.M.; Park, J.E.; Chen, R.; Celnik, P.; Strafella, A.P.; Matsumoto, H.; Ugawa, Y. Contribution of transcranial magnetic stimulation to assessment of brain connectivity and networks. Clin. Neurophysiol. 2017, 128, 2125–2139. [Google Scholar] [CrossRef]
- Lotze, M.; Markert, J.; Sauseng, P.; Hoppe, J.; Plewnia, C.; Gerloff, C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J. Neurosci. 2006, 26, 6096–6102. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.M.; Chan, E.; Rounds, A.K.; Wutzke, C.J.; Dromerick, A.W.; Turkeltaub, P.E.; Harris-Love, M.L. Roles of Lesioned and Nonlesioned Hemispheres in Reaching Performance Poststroke. Neurorehabil. Neural Repair 2020, 34, 61–71. [Google Scholar] [CrossRef]
- Lowenthal-Raz, J.; Liebermann, D.G.; Friedman, J.; Soroker, N. Kinematic descriptors of arm reaching movement are sensitive to hemisphere-specific immediate neuromodulatory effects of transcranial direct current stimulation post stroke. Sci. Rep. 2024, 14, 11971. [Google Scholar] [CrossRef] [PubMed]
- Mahdy Ibrahim, E.; Ahmed Zaki, M.; Gaber Mahmoud Gabr, M. Effect of high frequency repetitive transcranial magnetic stimulation of the contralesional motor cortex on recovery from post-stroke severe motor impairment. Al-Azhar Med. J. 2020, 49, 651–666. [Google Scholar] [CrossRef]
- McIntyre, A.; Mirkowski, M.; Thompson, S.; Burhan, A.M.; Miller, T.; Teasell, R. A Systematic Review and Meta-Analysis on the Use of Repetitive Transcranial Magnetic Stimulation for Spasticity Poststroke. PM R 2018, 10, 293–302. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Cambiaghi, M.; Bacigaluppi, M.; Gallizioli, M.; Gaude, E.; Mari, S.; Sandrone, S.; Cursi, M.; Teneud, L.; Comi, G.; et al. Safety and efficacy of transcranial direct current stimulation in acute experimental ischemic stroke. Stroke 2013, 44, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.J.; Oh, B.M.; Kim, D.Y. Functional improvement and neuroplastic effects of anodal transcranial direct current stimulation (tDCS) delivered 1 day vs. 1 week after cerebral ischemia in rats. Brain Res. 2012, 1452, 61–72. [Google Scholar] [CrossRef]
- Delvaux, V.; Alagona, G.; Gérard, P.; De Pasqua, V.; Pennisi, G.; de Noordhout, A.M. Post-stroke reorganization of hand motor area: A 1-year prospective follow-up with focal transcranial magnetic stimulation. Clin. Neurophysiol. 2003, 114, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Hummel, F.C.; Celnik, P.; Pascual-Leone, A.; Fregni, F.; Byblow, W.D.; Buetefisch, C.M.; Rothwell, J.; Cohen, L.G.; Gerloff, C. Controversy: Noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul. 2008, 1, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.; Chen, R.; Cohen, L.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, E.; Semenov, R.; Pavlova-Deb, M.; Guekht, A. Transcranial direct current stimulation of the premotor cortex aimed to improve hand motor function in chronic stroke patients. Brain Res. 2022, 1780, 147790. [Google Scholar] [CrossRef]
- Tatsuno, H.; Hamaguchi, T.; Sasanuma, J.; Kakita, K.; Okamoto, T.; Shimizu, M.; Nakaya, N.; Abo, M. Does a combination treatment of repetitive transcranial magnetic stimulation and occupational therapy improve upper limb muscle paralysis equally in patients with chronic stroke caused by cerebral hemorrhage and infarction?: A retrospective cohort study. Medicine 2021, 100, e26339. [Google Scholar] [CrossRef]
- Koch, G.; Bonnì, S.; Casula, E.P.; Iosa, M.; Paolucci, S.; Pellicciari, M.C.; Cinnera, A.M.; Ponzo, V.; Maiella, M.; Picazio, S.; et al. Effect of Cerebellar Stimulation on Gait and Balance Recovery in Patients with Hemiparetic Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 170–178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tam, P.K.; Oey, N.E.; Tang, N.; Ramamurthy, G.; Chew, E. Facilitating Corticomotor Excitability of the Contralesional Hemisphere Using Non-Invasive Brain Stimulation to Improve Upper Limb Motor Recovery from Stroke—A Scoping Review. J. Clin. Med. 2024, 13, 4420. https://doi.org/10.3390/jcm13154420
Tam PK, Oey NE, Tang N, Ramamurthy G, Chew E. Facilitating Corticomotor Excitability of the Contralesional Hemisphere Using Non-Invasive Brain Stimulation to Improve Upper Limb Motor Recovery from Stroke—A Scoping Review. Journal of Clinical Medicine. 2024; 13(15):4420. https://doi.org/10.3390/jcm13154420
Chicago/Turabian StyleTam, Pui Kit, Nicodemus Edrick Oey, Ning Tang, Guhan Ramamurthy, and Effie Chew. 2024. "Facilitating Corticomotor Excitability of the Contralesional Hemisphere Using Non-Invasive Brain Stimulation to Improve Upper Limb Motor Recovery from Stroke—A Scoping Review" Journal of Clinical Medicine 13, no. 15: 4420. https://doi.org/10.3390/jcm13154420






