Identification of Achille’s Tendon Tears: Diagnostic Accuracy of Dual-Energy CT with Respect to MRI
Abstract
:1. Introduction
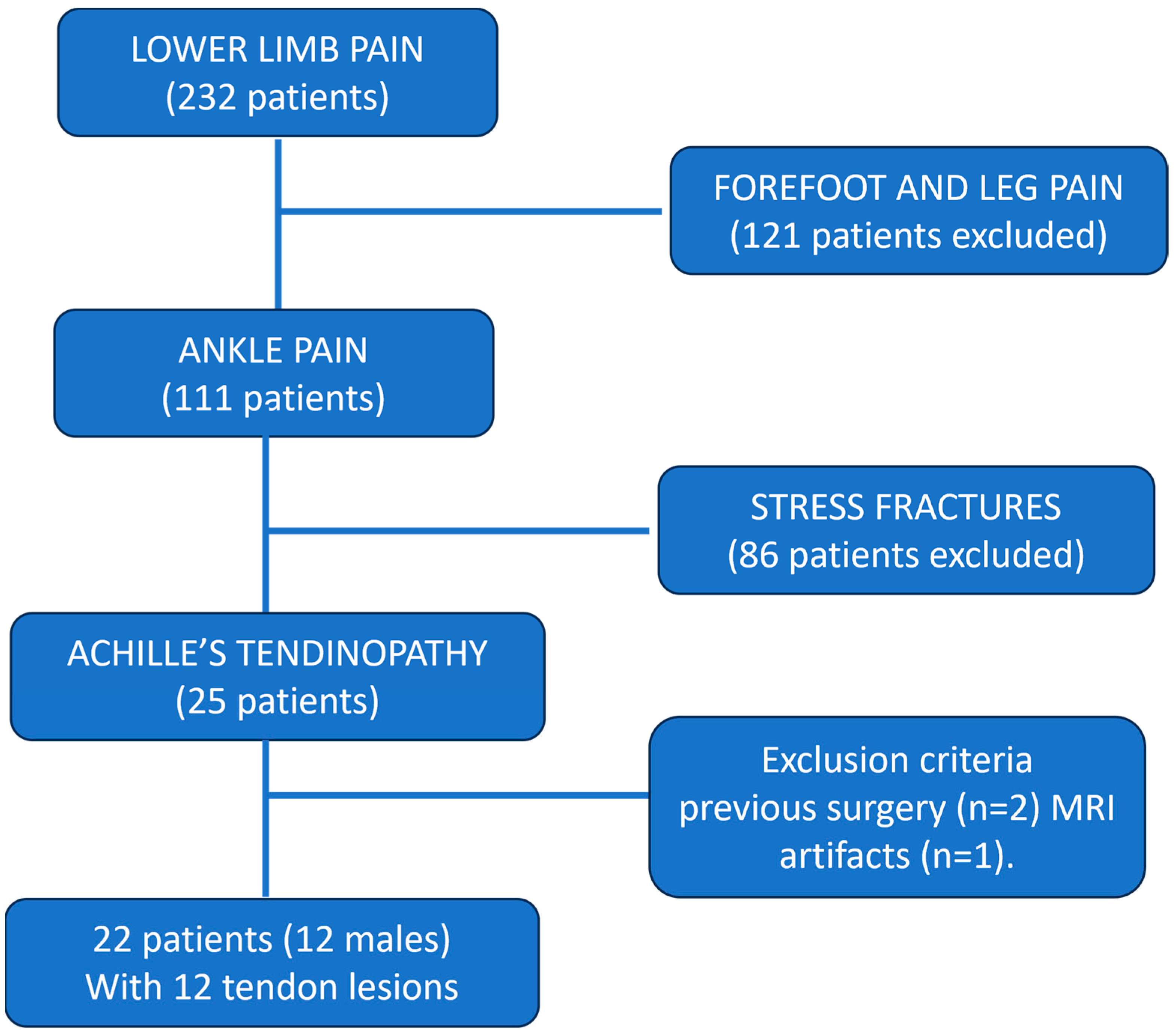
2. Methods
2.1. Participants
2.2. Magnetic Resonance Imaging
2.3. DECT Protocol
2.4. DECT Post-Processing
2.5. Image Analysis
2.6. Statistical Analysis
3. Results
3.1. Patients Results
3.2. MRI Results
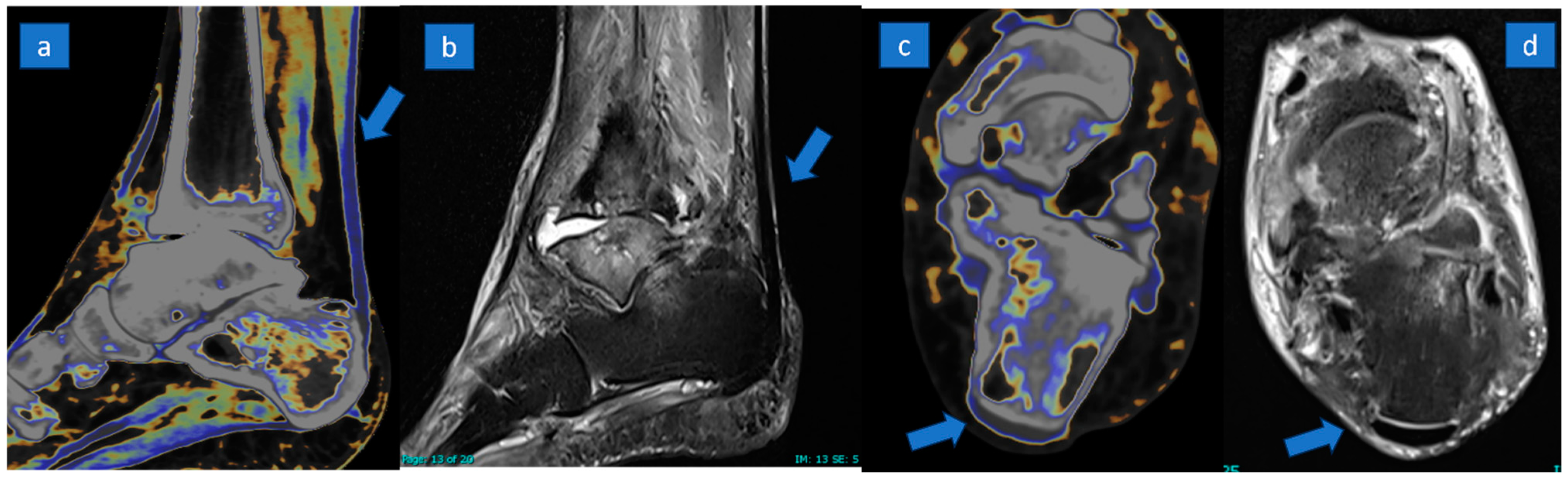
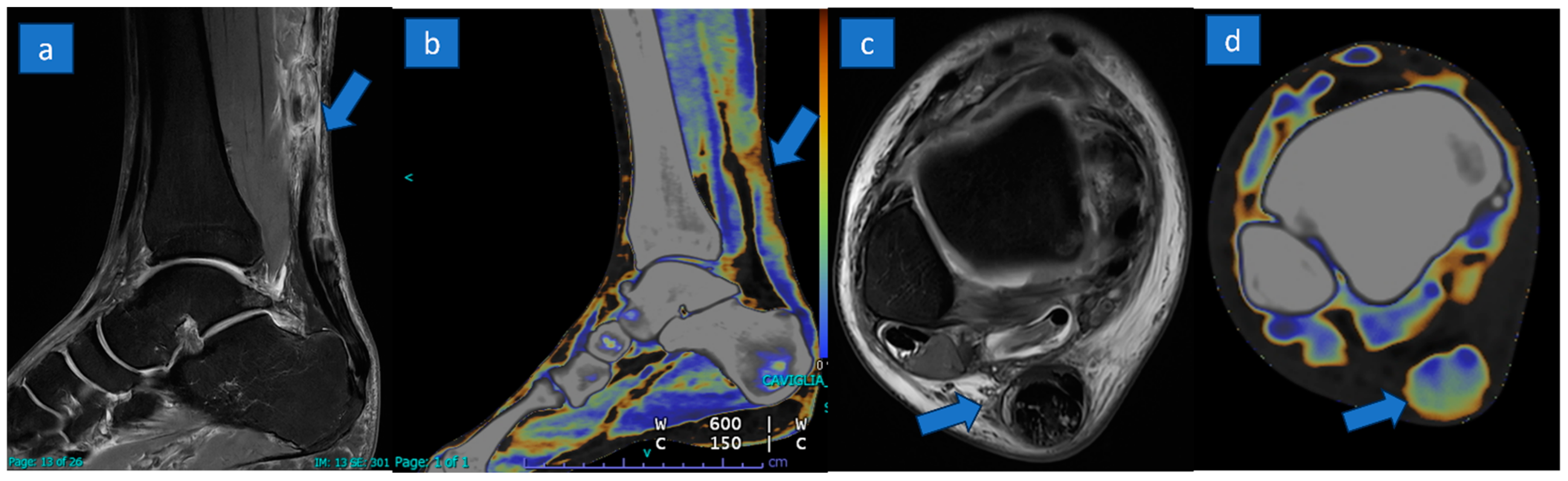
3.3. DECT Results
3.4. DECT Inter-Observer Agreement
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Järvinen, T.A.; Kannus, P.; Maffulli, N.; Khan, K.M. Achilles Tendon Disorders: Etiology and Epidemiology. Foot Ankle Clin. 2005, 10, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.; Kjaer, M.; Eliasson, P. Achilles tendon rupture—Treatment and complications: A systematic review. Scand. J. Med. Sci. Sports 2014, 25, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.-M.E.; Vosseller, J.T. Maximizing Return to Sports After Achilles Tendon Rupture in Athletes. Foot Ankle Clin. 2019, 24, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, M.; Rubinger, D. Functional Rehabilitation for Nonsurgical Treatment of Acute Achilles Tendon Rupture. Foot Ankle Clin. 2019, 24, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Lee, H.S.; Young, K.W.; Seo, S.G. Treatment of Acute Achilles Tendon Rupture. Clin. Orthop. Surg. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Oliva, F.; Bernardi, G.; De Luna, V.; Farsetti, P.; Gasparini, M.; Marsilio, E.; Piccirilli, E.; Tarantino, U.; Rugiero, C.; De Carli, A.; et al. Achilles tendon ruptures guidelines. Muscles Ligaments Tendons J. 2018, 8, 310–363. [Google Scholar] [CrossRef]
- Fornage, B.D. Achilles tendon: US examination. Radiology 1986, 159, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Gervasio, A.; Bollani, P.; Biasio, A. US in mid-portion Achilles tendon injury. J. Ultrasound 2013, 17, 135–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pierre-Jerome, C.; Moncayo, V.; Terk, M.R. MRI of the achilles tendon: A comprehensive review of the anatomy, biomechanics, and imaging of overuse tendinopathies. Acta Radiol. 2010, 51, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Corradin, M.; Macchi, V.; Morra, A.; Porzionato, A.; Biz, C.; De Caro, R. Plantar fascia anatomy and its relationship with Achilles tendon and paratenon. J. Anat. 2013, 223, 665–676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bäcker, H.C.; Wong, T.T.; Vosseller, J.T. MRI Assessment of Degeneration of the Tendon in Achilles Tendon Ruptures. Foot Ankle Int. 2019, 40, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Szaro, P.; Ramirez, W.C.; Borkmann, S.; Bengtsson, A.; Polaczek, M.; Ciszek, B. Distribution of the subtendons in the midportion of the Achilles tendon revealed in vivo on MRI. Sci. Rep. 2020, 10, 16348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, A.; Wong, M.; Kutschera, P.; Lau, K. Dual-energy CT in musculoskeletal trauma. Clin. Radiol. 2020, 76, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.D.; Shah, S.; Murray, N.; Walstra, F.; Khosa, F.; Nicolaou, S. Advanced Musculoskeletal Applications of Dual-Energy Computed Tomography. Radiol. Clin. N. Am. 2018, 56, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.M. Using Dual-Energy CT for Painful Hip Arthroplasties. Radiology 2021, 300, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Omoumi, P.; Becce, F.; Racine, D.; Ott, J.G.; Andreisek, G.; Verdun, F.R. Dual-Energy CT: Basic Principles, Technical Approaches, and Applications in Musculoskeletal Imaging (Part 1). Semin. Musculoskelet. Radiol. 2015, 19, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, I.; Verde, F.; Palumbo, L.; Di Pietto, F.; Puglia, M.; Scaglione, M.; Ragozzino, A.; Romano, S. Dual energy computed tomography evaluation of skeletal traumas. Eur. J. Radiol. 2020, 134, 109456. [Google Scholar] [CrossRef] [PubMed]
- Ficek, K.; Filipek, J.; Ficek, J.; Muzalewska, M.; Humpa, F. Calcaneal CT is a useful tool for identifying Achilles tendon disorders: A pilot study. J. Orthop. Surg. Res. 2017, 12, 139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuo, S.; Desilva, J.M.; Devlin, M.J.; Mcdonald, G.; Morgan, E.F. The Effect of the Achilles Tendon on Trabecular Structure in the Primate Calcaneus. Anat. Rec. 2013, 296, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Sun, C.; Liu, C.; Ma, R. Initial experience with visualizing hand and foot tendons by dual-energy computed tomography. Clin. Imaging 2009, 33, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Hickle, J.; Walstra, F.; Duggan, P.; Ouellette, H.; Munk, P.; Mallinson, P. Dual-energy CT characterization of winter sports injuries. Br. J. Radiol. 2020, 93, 20190620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joshi, R.; LeBedis, C.; Dao, K.; Qureshi, M.; Gupta, A. Dual energy CT angiography for lower extremity trauma: Comparison with conventional CT. Emerg. Radiol. 2022, 29, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Umezawa, Y.; Asahina, A.; Nakagawa, H.; Furuya, K.; Fukuda, K. Dual energy CT iodine map for delineating inflammation of inflammatory arthritis. Eur. Radiol. 2017, 27, 5034–5040. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Henes, J.C.; Fuld, M.K.; Fishman, E.K.; Horger, M.S. Dual-Energy Computed Tomography of the Knee, Ankle, and Foot: Noninvasive Diagnosis of Gout and Quantification of Monosodium Urate in Tendons and Ligaments. Semin. Musculoskelet. Radiol. 2016, 20, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Guggenberger, R.; Gnannt, R.; Hodler, J.; Krauss, B.; Wanner, G.A.; Csuka, E.; Payne, B.; Frauenfelder, T.; Andreisek, G.; Alkadhi, H. Diagnostic Performance of Dual-Energy CT for the Detection of Traumatic Bone Marrow Lesions in the Ankle: Comparison with MR Imaging. Radiology 2012, 264, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Catania, M.; Caia, S.; Romano, L.; Beltramello, A.; Zorzi, C.; Carbognin, G. Identification of bone marrow edema of the ankle: Diagnostic accuracy of dual-energy CT in comparison with MRI. La Radiol. Medica 2019, 124, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Guerriero, M.; Faccioli, N.; Fighera, A.; Romano, L.; Zorzi, C.; Carbognin, G. Identification of bone marrow edema around the ankle joint in non-traumatic patients: Diagnostic accuracy of dual-energy computed tomography. Clin. Imaging 2020, 69, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Mallinson, P.I.; Stevens, C.; Reisinger, C.; Nicolaou, S.; Munk, P.L.; Ouellette, H. Achilles Tendinopathy and Partial Tear Diagnosis Using Dual-Energy Computed Tomography Collagen Material Decomposition Application. J. Comput. Assist. Tomogr. 2013, 37, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.Y.; Lee, S.-W.; Jeong, Y.M.; Yu, S. The utility of dual-energy CT collagen material decomposition technique for the visualization of tendon grafts after knee ligament reconstruction. Eur. J. Radiol. 2019, 116, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, P.; Cai, Z.-J.; Lu, W.-H.; Pan, L.-Y.; Liu, X.; Peng, X.-J.; Li, Y.-S.; Xiao, W.-F. Valid and reliable diagnostic performance of dual-energy CT in anterior cruciate ligament rupture. Eur. Radiol. 2023, 33, 7769–7778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fickert, S.; Niks, M.; Dinter, D.J.; Hammer, M.; Weckbach, S.; Schoenberg, S.O.; Lehmann, L.; Jochum, S. Assessment of the diagnostic value of dual-energy CT and MRI in the detection of iatrogenically induced injuries of anterior cruciate ligament in a porcine model. Skelet. Radiol. 2012, 42, 411–417. [Google Scholar] [CrossRef] [PubMed]
- De Vulder, N.; Chen, M.; Huysse, W.; Herregods, N.; Verstraete, K.; Jans, L. Case Series: Dual-Energy CT in Extra-Articular Manifestations of Gout. J. Belg. Soc. Radiol. 2020, 104, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalbeth, N.; Kalluru, R.; Aati, O.; Horne, A.; Doyle, A.J.; McQueen, F.M. Tendon involvement in the feet of patients with gout: A dual-energy CT study. Ann. Rheum. Dis. 2013, 72, 1545–1548. [Google Scholar] [CrossRef] [PubMed]

| Sequences | T1w FSE | T2w FSE | PDw FSE FS |
|---|---|---|---|
| Parameter | |||
| Repetition time (ms) | 515 | 6700 | 2895 |
| Echo time (ms) | 8 | 79 | 34 |
| Matrix size | 374 × 416 | 374 × 416 | 288 × 320 |
| Bandwidth (Hz/pixel) | 420 | 300 | 200 |
| NSA | 1 | 1 | 1 |
| Echo train length | 3 | 20 | 7 |
| Slice thickness (mm) | 2.5 | 2.5 | 2.5 |
| Characteristics | No. of Participants (n = 22) |
|---|---|
| Age (y) | 48.5 years (range 26–78 years) [12.3] |
| Number of men | 12 (54.5.0%) |
| Number of women | 10 (45.5%) |
| Normal tendon | 10 (45.5%) |
| Tendon injuries | 12 (54.5%) |
| Complete rupture | 2 (9.0%) |
| Partial tear | 8 (36.4%) Thickness 3.8 (range 1–8 mm) Length 16.2 (range 2–34 mm) |
| Tendinopathy | 2 (9.0%) |
| Tendon’s thickness | |
| Control | 6.5 mm (range 5.5 to 7 mm) |
| Injured tendons | 10 mm (5.5 mm to 13 mm) |
| DECT | R1 | R2 | R3 |
|---|---|---|---|
| Overall assessment (accuracy) | 90.9% (20/22) | 86.4% (19/22) | 81.8% (18/22) |
| [80.6, 95.8] | [76.4, 93.8] | [0.72, 0.92] | |
| Normal tendon | 90% (9/10) | 90% (9/10) | 80% (8/10) |
| [82.0, 96.5] | [82.0, 96.5] | [72.5, 86.5] | |
| Partial tear | 87.5% (7/8) | 87.5% (7/8) | 75% (6/8) |
| [0.83, 0.92] | [0.83, 0.92] | [0.65, 0.85] | |
| Complete tear | 100% (2/2) | 100% (2/2) | 100% (2/2) |
| [90.0, 1.0] | [90.0, 1.0] | [90.0, 1.0] | |
| Inflammatory changes | 100% (2/2) | 50.0% (1/2) | 100% (2/2) |
| [90.0, 1.0] | [0.45, 0.55] | [90.0, 1.0] |
| Fleiss’ Kappa | ||
|---|---|---|
| DECT | MRI * | |
| Overall | 0.88 | 0.92 |
| Tears | 0.94 | 0.97 |
| Inflammatory changes | 082 | 0.88 |
| Thickness | 0.96 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, G.; Bortoli, L.; Tronu, M.; Montefusco, S.; Serra, G.; Filippini, R.; Iacono, V. Identification of Achille’s Tendon Tears: Diagnostic Accuracy of Dual-Energy CT with Respect to MRI. J. Clin. Med. 2024, 13, 4426. https://doi.org/10.3390/jcm13154426
Foti G, Bortoli L, Tronu M, Montefusco S, Serra G, Filippini R, Iacono V. Identification of Achille’s Tendon Tears: Diagnostic Accuracy of Dual-Energy CT with Respect to MRI. Journal of Clinical Medicine. 2024; 13(15):4426. https://doi.org/10.3390/jcm13154426
Chicago/Turabian StyleFoti, Giovanni, Luca Bortoli, Matteo Tronu, Sabrina Montefusco, Gerardo Serra, Roberto Filippini, and Venanzio Iacono. 2024. "Identification of Achille’s Tendon Tears: Diagnostic Accuracy of Dual-Energy CT with Respect to MRI" Journal of Clinical Medicine 13, no. 15: 4426. https://doi.org/10.3390/jcm13154426





