Macrophages and Mast Cells in the Gastric Mucosa of Patients with Obesity Undergoing Sleeve Gastrectomy
Abstract
:1. Introduction
2. Material and Methods
2.1. Patient Sampling
2.2. Immunohistochemistry
2.3. Morphometrical Assay
2.4. Statistical Analysis
3. Results
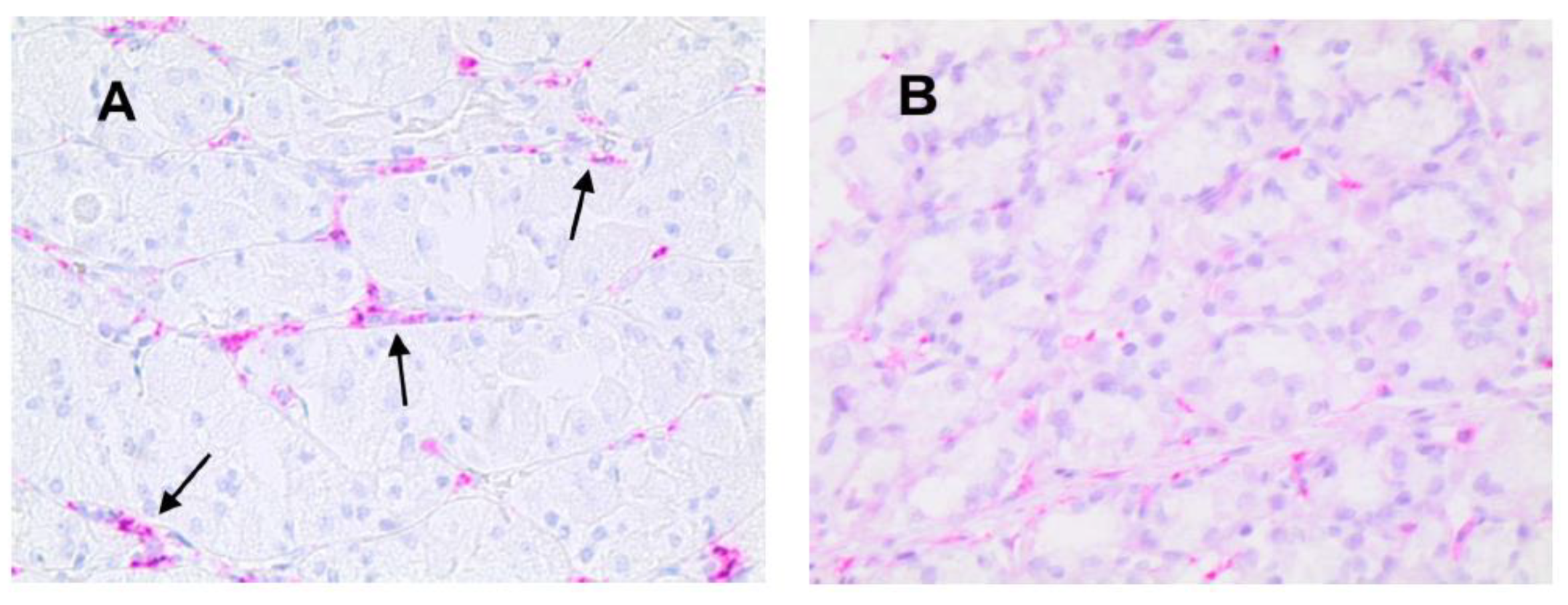
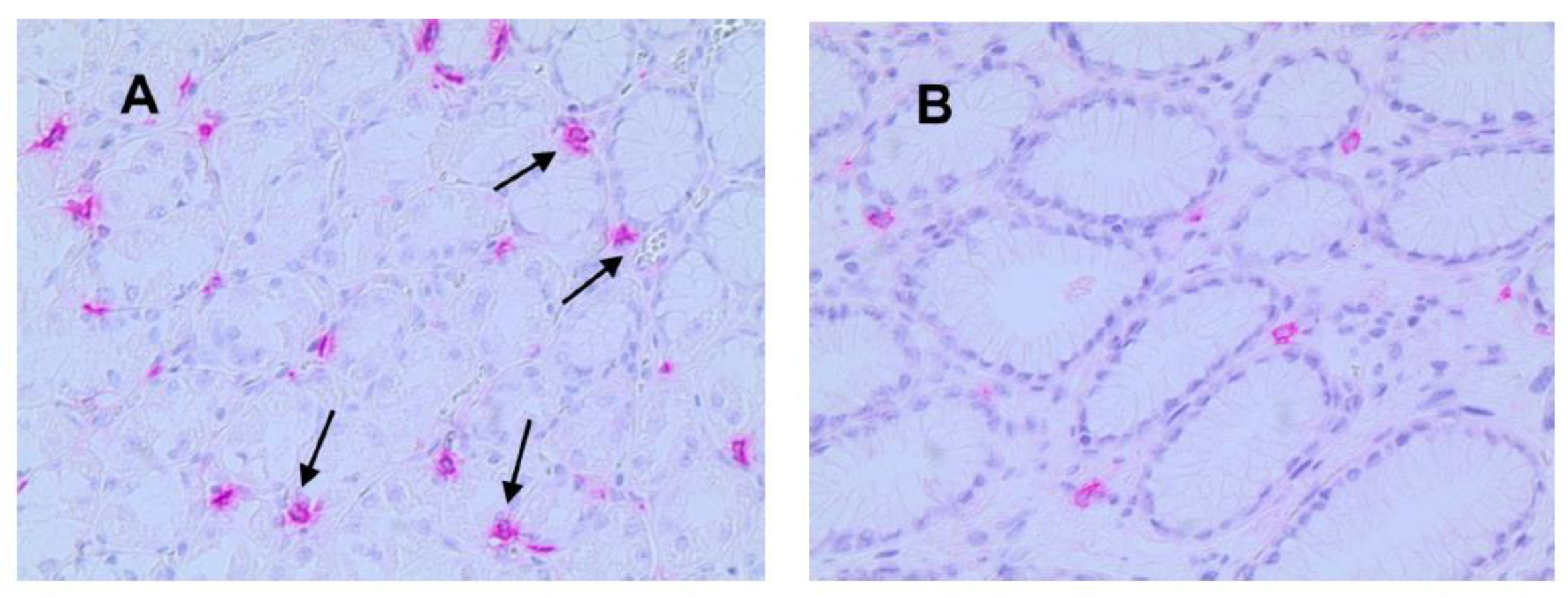
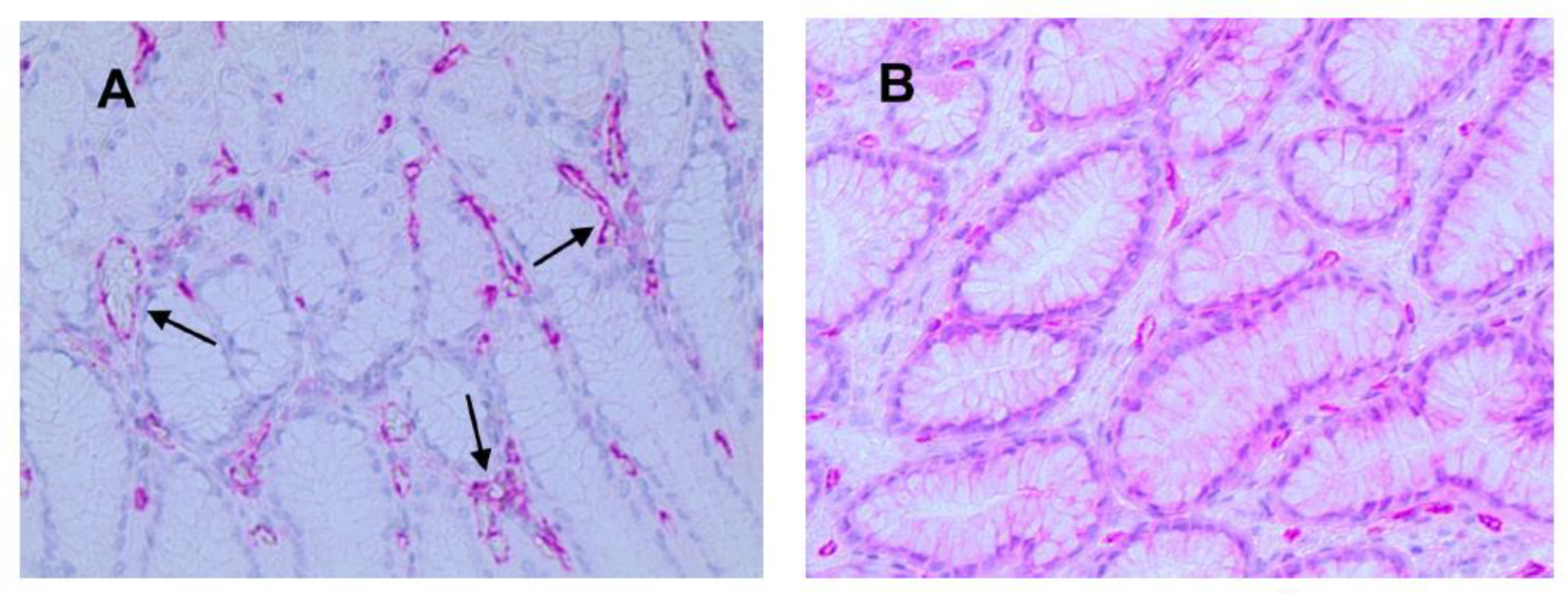
3.1. Immunohistochemical Analysis
3.2. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Milling, S. Adipokines and the control of mast cell functions: From obesity to inflammation? Immunology 2019, 158, 1–2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Kratz, M.; Coats, B.R.; Hisert, K.B.; Hagman, D.; Mutskov, V.; Peris, E.; Schoenfeld, K.Q.; Kuzma, J.N.; Larson, I.; Billing, P.S.; et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014, 20, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Żelechowska, P.; Agier, J.; Kozłowska, E.; Brzezińska-Błaszczyk, E. Mast cells participate in chronic low-grade inflammation within adipose tissue. Obes. Rev. 2018, 19, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Corvera, S.; Solivan-Rivera, J.; Yang Loureiro, Z. Angiogenesis in adipose tissue and obesity. Angiogenesis 2022, 25, 439–453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Gu, Y.; Jin, Y.; Mao, Z. Is Bariatric Surgery Effective for Chinese Patients with Type 2 Diabetes Mellitus and Body Mass Index < 35 kg/m2? A Systematic Review and Meta-analysis. Obes. Surg. 2021, 31, 4083–4092. [Google Scholar]
- Ranieri, G.; Grammatica, L.; Patruno, R.; Zito, A.F.; Valerio, P.; Iacobellis, S.; Gadaleta, C.; Gasparini, G.; Ribatti, D. A possible role of thymidine phosphorylase expression and 5-fluorouracil increased sensitivity in oropharyngeal cancer patients. J. Cell Mol. Med. 2007, 11, 362–368. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Wang, X.; Wang, J.; Yan, Z.; Cheng, J.; Gong, G.; Li, G. Body mass index and risk of gastric cancer: A meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 1395–1408. [Google Scholar] [CrossRef]
- Csendes, A.; Burgos, A.M.; Smok, G.; Beltran, M. Endoscopic and histologic findings of the foregut in 426 patients with morbid obesity. Obes. Surg. 2007, 17, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Arora, M.; Kireet, A.; Bashandy, H.; Gandsas, A. Upper gastrointestinal symptoms and associated disorders in morbidly obese patients: A prospective study. Dig. Dis. Sci. 2009, 54, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Aros, S.; Cremonini, F.; Castillo, J.E.; Chial, H.J.; Burton, D.D.; Ferber, I.; Camilleri, M. Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology 2004, 126, 432–440. [Google Scholar] [CrossRef]
- Delgado-Aros, S.; Camilleri, M.; Castillo, E.J.; Cremonini, F.; Stephens, D.; Ferber, I.; Baxter, K.; Burton, D.; Zinsmeister, A.R. Effect of gastric volume or emptying on meal-related symptoms after liquid nutrients in obesity: A pharmacologic study. Clin. Gastroenterol. Hepatol. 2005, 3, 997–1006. [Google Scholar] [CrossRef]
- Matsuzawa, Y. Adiponectin: A key player in obesity-related disorders. Curr. Pharm. Des. 2010, 16, 1896–1901. [Google Scholar] [CrossRef]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Bulló, M.; Casas-Agustench, P.; Amigó-Correig, P.; Araneta, J.; Salas-Salvadó, J. Inflammation, obesity and comorbidities: The role of diet. Public Health Nutr. 2007, 10, 1164–1172. [Google Scholar] [CrossRef]
- Christiansen, T.; Richelsen, B.; Bruun, J.M. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity, and reduced after weight loss in morbid obese subjects. Int. J. Obes. 2005, 29, 146–150. [Google Scholar] [CrossRef]
- Lesna, I.K.; Cejkova, S.; Kralova, A.; Fronek, J.; Petras, M.; Sekerkova, A.; Thieme, F.; Janousek, L.; Poledne, R. Human adipose tissue accumulation is associated with proinflammatory changes in subcutaneous rather than visceral adipose tissue. Nutr. Diabetes 2017, 7, e264. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, C.M.; Ackermann, P.J.; Ostermann, A.L.; Adams-Quack, P.; Vogt, M.C.; Tran, M.L.; Nikolajev, A.; Waisman, A.; Garbers, C.; Theurich, S.; et al. Obesity exacerbates colitis-associated cancer via IL-6-regulated macrophage polarisation and CCL-20/CCR-6-mediated lymphocyte recruitment. Nat. Commun. 2018, 9, 1646. [Google Scholar] [CrossRef]
- Devericks, E.N.; Carson, M.S.; McCullough, L.E.; Coleman, M.F.; Hursting, S.D. The obesity-breast cancer link: A multidisciplinary perspective. Cancer Metastasis Rev. 2022, 41, 607–625. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eguchi, K.; Manabe, I.; Oishi-Tanaka, Y.; Ohsugi, M.; Kono, M.; Ogat, F.; Yagi, N.; Ohto, U.; Kimoto, M.; Miyake, K.; et al. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab. 2012, 15, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Fu, W.; Lee, Y.S.; Olefsky, J.M. The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat. Rev. Endocrinol. 2020, 16, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kolb, R.; Phan, L.; Borcherding, N.; Liu, Y.; Yuan, F.; Janowski, A.M.; Xie, Q.; Markan, K.R.; Li, W.; Potthoff, M.J.; et al. Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat. Commun. 2016, 7, 13007. [Google Scholar] [CrossRef]
- Bauernfeind, F.; Ablasser, A.; Bartok, E.; Kim, S.; Schmid-Burgk, J.; Cavlar, T.; Hornung, V. Inflammasomes: Current understanding and open questions. Cell Mol. Life Sci. 2011, 68, 765–783. [Google Scholar] [CrossRef]
- Dyson, J.; Jaques, B.; Chattopadhyay, D.; Lochan, R.; Graham, J.; Das, D.; Aslam, T.; Patanwala, I.; Gaggar, S.; Cole, M.; et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes, and a multidisciplinary team. J. Hepatol. 2014, 60, 110–117. [Google Scholar] [CrossRef]
- Caruso, A.; Gelsomino, L.; Panza, S.; Accattatis, F.M.; Naimo, G.D.; Barone, I.; Giordano, C.; Catalano, S.; Andò, S. Leptin: A Heavyweight Player in Obesity-Related Cancers. Biomolecules 2023, 13, 1084. [Google Scholar] [CrossRef]


| Gender n | Age Range | BMI | DMT2n | OSASn | H. pylori |
|---|---|---|---|---|---|
| F 21 | 28 ys | 44.3 (Mean Value) | 7 | 10 | None |
| M 9 | 24 ys | 42.4 (Mean Value) | 5 | 2 | None |
| Tissue Type | MVD ×20 Magnification (0.19 mm2 Area) | MCPT ×20 Magnification (0.19 mm2 Area) | ATMs ×20 Magnification (0.19 mm2 Area) |
|---|---|---|---|
| NT | 13± 5 a | 6 a ± 4 | 14 ± 5 |
| GTO | 30 ± 7 a | 13 a ± 6 | 38 ± 9 |
| p-value (t-test) | p < 0.05 | p < 0.05 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammendola, M.; Vescio, F.; Rotondo, C.; Arturi, F.; Luposella, M.; Zuccalà, V.; Battaglia, C.; Laganà, D.; Ranieri, G.; Navarra, G.; et al. Macrophages and Mast Cells in the Gastric Mucosa of Patients with Obesity Undergoing Sleeve Gastrectomy. J. Clin. Med. 2024, 13, 4434. https://doi.org/10.3390/jcm13154434
Ammendola M, Vescio F, Rotondo C, Arturi F, Luposella M, Zuccalà V, Battaglia C, Laganà D, Ranieri G, Navarra G, et al. Macrophages and Mast Cells in the Gastric Mucosa of Patients with Obesity Undergoing Sleeve Gastrectomy. Journal of Clinical Medicine. 2024; 13(15):4434. https://doi.org/10.3390/jcm13154434
Chicago/Turabian StyleAmmendola, Michele, Francesca Vescio, Cataldo Rotondo, Franco Arturi, Maria Luposella, Valeria Zuccalà, Caterina Battaglia, Domenico Laganà, Girolamo Ranieri, Giuseppe Navarra, and et al. 2024. "Macrophages and Mast Cells in the Gastric Mucosa of Patients with Obesity Undergoing Sleeve Gastrectomy" Journal of Clinical Medicine 13, no. 15: 4434. https://doi.org/10.3390/jcm13154434






