The Outcome after Endovascular and Open Repair of Abdominal Aortic Aneurysms—A Binational Study Conducted between 1998 and 2017
Abstract
:1. Introduction
2. Methods
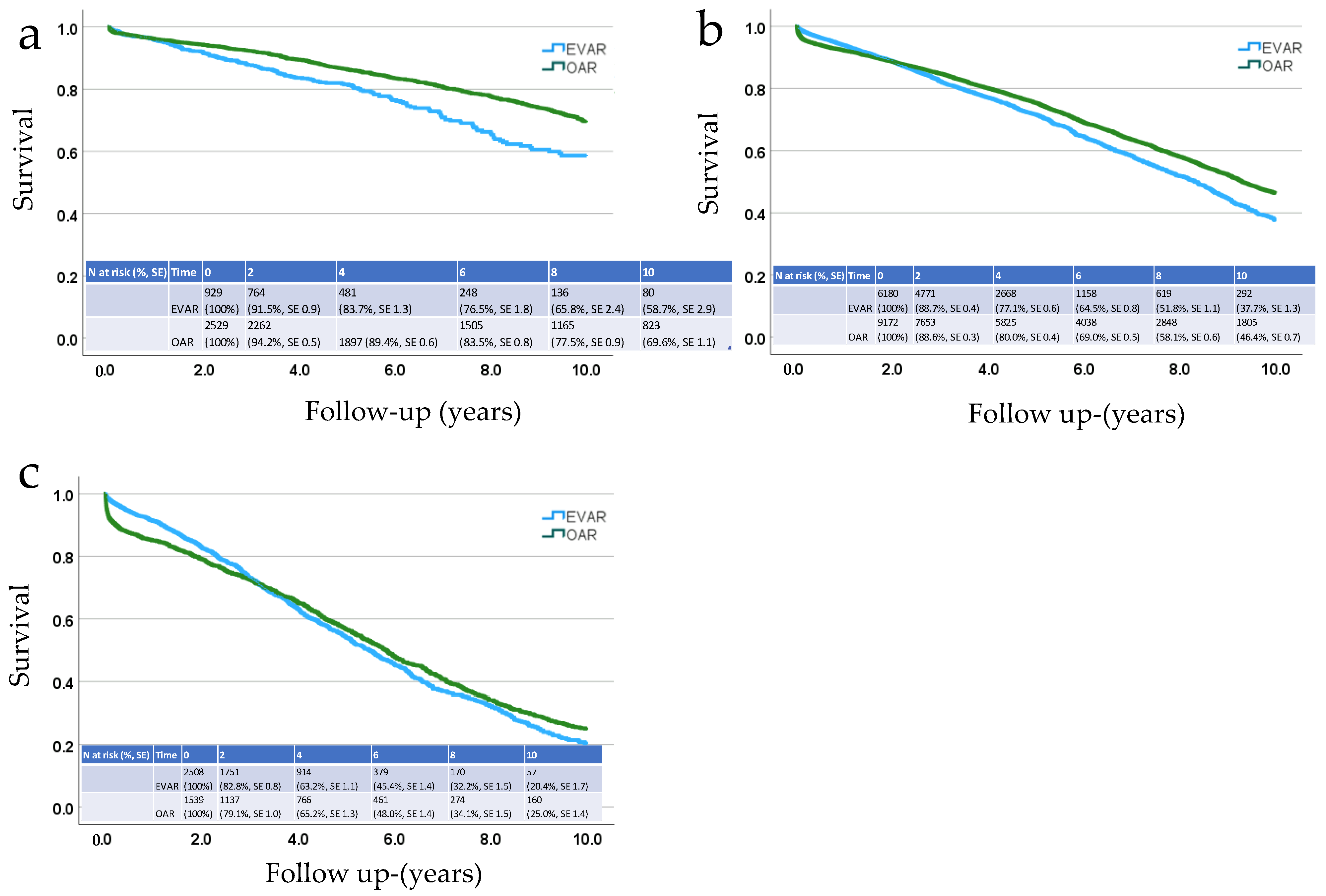
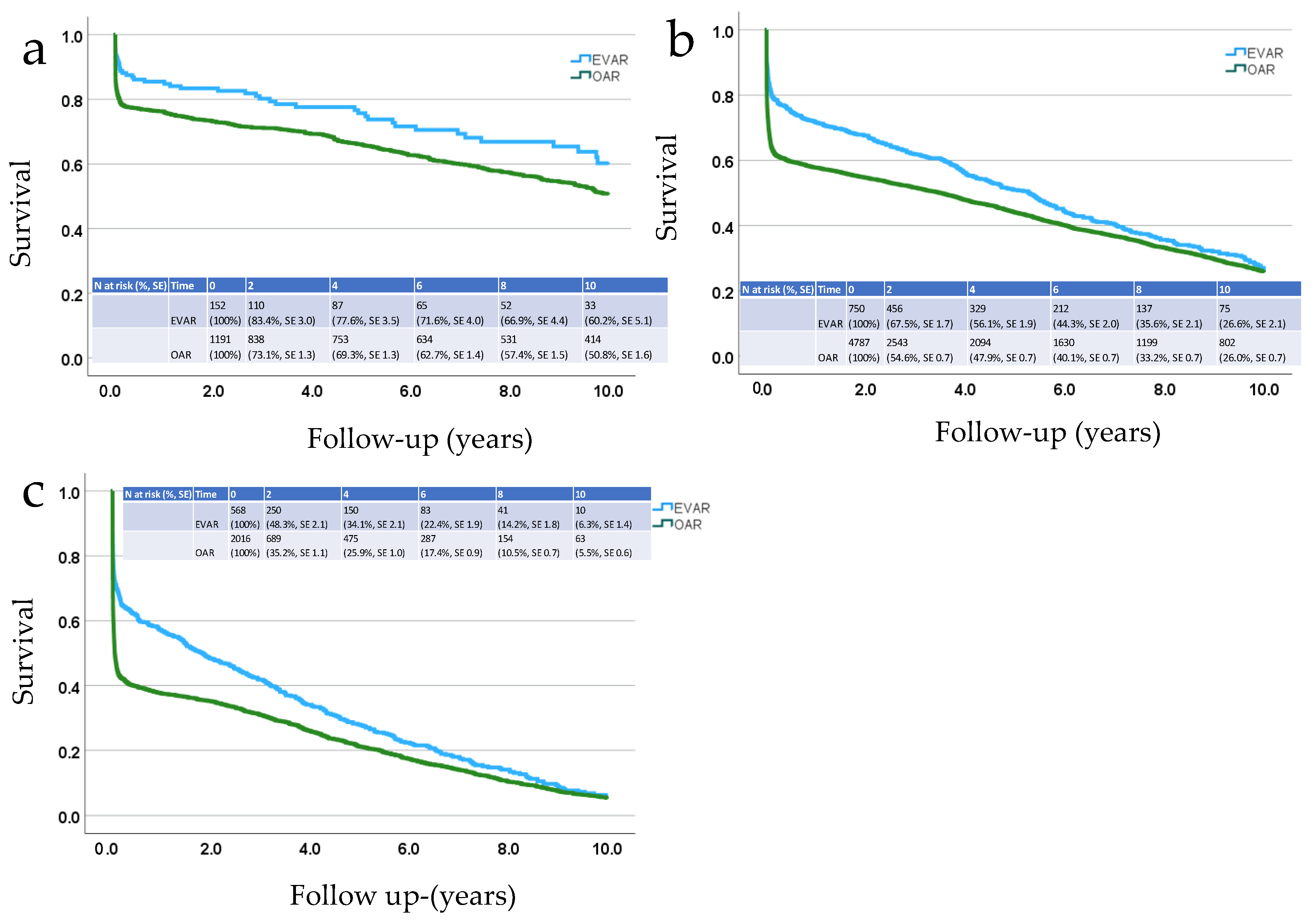
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parodi, J.C.; Palmaz, J.C.; Barone, H.D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann. Vasc. Surg. 1991, 5, 491–499. [Google Scholar] [CrossRef] [PubMed]
- United Kingdom EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm. N. Engl. J. Med. 2010, 362, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Freischlag, J.A.; Kyriakides, T.C.; Padberg, F.T.; Matsumura, J.S.; Kohler, T.R.; Lin, P.H.; Jean-Claude, J.M.; Cikrit, D.F.; Swanson, K.M.; et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: A randomized trial. JAMA 2009, 302, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, J.L.; Baas, A.F.; Buth, J.; Prinssen, M.; Verhoeven, E.L.; Cuypers, P.W.; van Sambeek, M.R.; Balm, R.; Grobbee, D.E.; Blankensteijn, J.D. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N. Engl. J. Med. 2010, 362, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.T.; Laukontaus, S.J.; Sund, R.; Aho, P.S.; Kantonen, I.; Albäck, A.; Venermo, M. A population-based study of abdominal aortic aneurysm treatment in Finland 2000 to 2014. Circulation 2017, 136, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Powell, J.; Thompson, S.; Epstein, D.; Sculpher, M.; Greenhalgh, R. The UK endovascular aneurysm repair (EVAR) trials: Randomised trials of EVAR versus standard therapy. Health Technol. Assess. 2012, 16, 1–218. [Google Scholar] [CrossRef] [PubMed]
- Blankensteijn, J.D.; de Jong, S.E.; Prinssen, M.; van der Ham, A.C.; Buth, J.; van Sterkenburg, S.M.; Verhagen, H.J.; Buskens, E.; Grobbee, D.E. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N. Engl. J. Med. 2005, 352, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Becquemin, J.-P.; Pillet, J.-C.; Lescalie, F.; Sapoval, M.; Goueffic, Y.; Lermusiaux, P.; Steinmetz, E.; Marzelle, J. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low-to moderate-risk patients. J. Vasc. Surg. 2011, 53, 1167–1173. [Google Scholar] [CrossRef]
- Lederle, F.A.; Freischlag, J.A.; Kyriakides, T.C.; Matsumura, J.S.; Padberg, F.T.J.; Kohler, T.R.; Kougias, P.; Jean-Claude, J.M.; Cikrit, D.F.; Swanson, K.M. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N. Engl. J. Med. 2012, 367, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.T.; Sweeting, M.J.; Ulug, P.; Blankensteijn, J.D.; A Lederle, F.; Becquemin, J.-P.; Greenhalgh, R.M.; Beard, J.D.; Buxton, M.J.; Brown, L.C.; et al. Meta-analysis of individual-patient data from EVAR-1, DREAM, OVER and ACE trials comparing outcomes of endovascular or open repair for abdominal aortic aneurysm over 5 years. Br. J. Surg. 2017, 104, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.K.; Day, J.; Horrocks, M. Editor’s choice—volume–outcome relationships in elective abdominal aortic aneurysm surgery: Analysis of the UK hospital episodes statistics database for the Getting It Right First Time (GIRFT) programme. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Trenner, M.; Kuehnl, A.; Salvermoser, M.; Reutersberg, B.; Geisbuesch, S.; Schmid, V.; Eckstein, H.-H. Editor’s choice—High annual hospital volume is associated with decreased in hospital mortality and complication rates following treatment of abdominal aortic aneurysms: Secondary data analysis of the nationwide German DRG statistics from 2005 to 2013. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Sawang, M.; Paravastu, S.C.; Liu, Z.; Thomas, S.D.; Beiles, C.B.; Mwipatayi, B.P.; Verhagen, H.J.; Verhoeven, E.L.; Varcoe, R.L. The relationship between operative volume and peri-operative mortality after non-elective aortic aneurysm repair in Australia. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Verzini, F.; Isernia, G.; De Rango, P.; Simonte, G.; Parlani, G.; Loschi, D.; Cao, P. Abdominal aortic endografting beyond the trials: A 15-year single-center experience comparing newer to older generation stent-grafts. J. Endovasc. Ther. 2014, 21, 439–447. [Google Scholar] [CrossRef] [PubMed]
- NOMESCO Classification of Surgical Procedures. 2011. Available online: https://norden.diva-portal.org/smash/get/diva2:968721/FULLTEXT01.pdf (accessed on 7 January 2024).
- Statistics Sweden Statistical Database. Available online: https://www.statistikdatabasen.scb.se/pxweb/en/ssd/ (accessed on 2 September 2023).
- Statistics Finland Statistical Database. Available online: https://stat.fi/tup/tilastotietokannat/index_en.html (accessed on 2 September 2023).
- Ulug, P.; Hinchliffe, R.J.; Sweeting, M.J.; Gomes, M.; Thompson, M.T.; Thompson, S.G.; Grieve, R.J.; Ashleigh, R.; Greenhalgh, R.M.; Powell, J.T. Strategy of endovascular versus open repair for patients with clinical diagnosis of ruptured abdominal aortic aneurysm: The IMPROVE RCT. Health Technol. Assess. 2018, 22, 1–122. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, K.; Wanhainen, A.; Björck, M.; Djavani-Gidlund, K.; Mani, K. Nationwide study of ruptured abdominal aortic aneurysms during twenty years (1994–2013). Ann. Surg. 2021, 274, e160–e166. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Behrendt, C.-A.; Falster, M.O.; Varcoe, R.L.M.; Zheng, X.; Peters, F.; Beiles, B.M.; Schermerhorn, M.L.; Jorm, L.; Beck, A.W.; et al. Long-term mortality and reintervention after endovascular and open abdominal aortic aneurysm repairs in Australia, Germany, and the United States. Ann. Surg. 2022, 278, e626–e633. [Google Scholar] [CrossRef] [PubMed]
- Sund, R.; Gissler, M. Use of Health Registers. In Handbook of Epidemiology; Ahrens, W., Pigeot, I., Eds.; Springer: New York, NY, USA, 2022; pp. 1–27. [Google Scholar] [CrossRef]
- Wanhainen, A.; Van Herzeele, I.; Goncalves, F.B.; Montoya, S.B.; Berard, X.; Boyle, J.R.; D’oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef] [PubMed]
| EVAR | OAR | All | ||
|---|---|---|---|---|
| Intact AAA | ||||
| Number of operations | 9619 | 13,241 | 22,860 | |
| SWE 7073 | SWE 8854 | SWE 15,927 | ||
| FIN 2549 | FIN 4387 | FIN 6933 | ||
| % women | 14.5% | 16.0% | 15.4% | p = 0.002 |
| SWE 15.4% | SWE 17.8% | SWE 16.7% | p < 0.001 | |
| FIN 12.0% | FIN 12.4% | FIN 12.2% | p = 0.621 | |
| Mean age (all) | 74.3 (SD 7.4) | 70.8 (SD 7.5) | 72.3 (SD 7.7) | p < 0.001 |
| SWE 73.8 (SD 7.3) | SWE 71.1 (SD 7.2) | SWE 72.3 (SD 7.4) | p < 0.001 | |
| FIN 75.5 (SD 7.7) | FIN 70.3 (SD 8.1) | FIN 72.2 (SD 8.3) | p < 0.001 | |
| Mean age (men) | 74.0 (SD 7.4) | 70.5 (SD 7.5) | 72.0 (SD 7.6) | p < 0.001 |
| SWE 73.6 (SD 7.3) | SWE 70.8 (SD 7.2) | SWE 72.1 (SD 7.4) | p < 0.001 | |
| FIN 75.1 (SD 7.7) | FIN 69.8 (SD 7.9) | FIN 71.8 (SD 8.2) | p < 0.001 | |
| Mean age (women) | 75.9 (SD 7.3) | 72.7 (SD 7.2) | 74.0 (SD 7.6) | p < 0.001 |
| SWE 75.0 (SD 7.2) | SWE 72.3 (SD 7.0) | SWE 73.4 (SD 7.2) | p < 0.001 | |
| FIN 78.9 (SD 6.7) | FIN 73.8 (SD 8.5) | FIN 75.6 (SD 8.3) | p < 0.001 | |
| Ruptured AAA | ||||
| Number of operations | 1470 | 7994 | 9464 | |
| SWE 1318 | SWE 5949 | SWE 7267 | ||
| FIN 152 | FIN 2045 | FIN 2197 | ||
| % women | 18.8% | 16.4% | 16.8% | p = 0.027 |
| SWE 18.9% | SWE 18.0% | SWE 18.2% | p = 0.449 | |
| FIN 17.8% | FIN 11.8% | FIN 12.2% | p = 0.034 | |
| Mean age (all) | 76.2 (SD 8.2) | 73.6 (SD 8.0) | 74.0 (SD 8.1) | p < 0.001 |
| SWE 76.3 (SD 8.1) | SWE 73.9 (SD 7.7) | SWE 74.3 (SD 7.9) | p < 0.001 | |
| FIN 74.8 (SD 9.4) | FIN 72.7 (SD 8.7) | FIN 72.8 (SD 8.8) | p = 0.005 | |
| Mean age (men) | 75.7 (SD 8.3) | 73.0 (SD 8.0) | 73.4 (SD 8.1) | p < 0.001 |
| SWE 75.9 (SD 8.1) | SWE 73.4 (SD 7.8) | SWE 73.9 (SD 7.9) | p < 0.001 | |
| FIN 73.4 (SD 9.2) | FIN 72.0 (SD 8.6) | FIN 72.0 (SD 8.6) | p = 0.070 | |
| Mean age (women) | 78.3 (SD 7.6) | 76.4 (SD 7.4) | 76.7 (SD 7.4) | p < 0.001 |
| SWE 77.9 (SD 7.6) | SWE 76.0 (SD 7.2) | SWE 76.4 (SD 7.3) | p < 0.001 | |
| FIN 81.3 (SD 7.2) | FIN 78.0 (SD 7.7) | FIN 78.3 (SD 7.7) | p = 0.031 |
| HR | 95% CI | p Value | ||
|---|---|---|---|---|
| AAA | Country (Finland vs. Sweden) | 1.22 | 1.16–1.27 | <0.001 |
| Female sex | 0.93 | 0.88–0.99 | 0.020 | |
| Age (10-year increments) | 1.81 | 1.75–1.87 | <0.001 | |
| EVAR vs. OAR | 1.21 | 1.15–1.27 | <0.001 | |
| Year of operation | 0.97 | 0.97–0.98 | <0.001 | |
| rAAA | Country (Finland vs. Sweden) | 1.03 | 0.97–1.10 | 0.310 |
| Female sex | 1.14 | 1.07–1.21 | <0.001 | |
| Age (10-year increments) | 1.81 | 1.75–1.87 | <0.001 | |
| EVAR vs. OAR | 0.81 | 0.75–0.87 | <0.001 | |
| Year of operation | 0.98 | 0.98–0.99 | <0.001 |
| Age | Mean Survival (Years), 95% CI | ||
| AAA | <65 | 13.22 | 12.90–13.53 |
| 65–79 | 9.67 | 9.52–9.81 | |
| ≥80 | 6.84 | 6.57–7.11 | |
| RAAA | <65 | 10.29 | 9.82–10.77 |
| 65–79 | 5.75 | 5.57–5.93 | |
| ≥80 | 2.59 | 2.44–2.74 | |
| If alive after first 90 days | |||
| AAA | <65 | 13.49 | 13.17–13.81 |
| 65–79 | 10.06 | 9.91–10.21 | |
| ≥80 | 7.28 | 7.00–7.57 | |
| RAAA | <65 | 13.07 | 12.59–13.55 |
| 65–79 | 9.07 | 8.85–9.29 | |
| ≥80 | 5.53 | 5.30–5.79 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirinen, R.; Laine, M.T.; Mani, K.; Gunnarsson, K.; Wanhainen, A.; Sund, R.; Venermo, M. The Outcome after Endovascular and Open Repair of Abdominal Aortic Aneurysms—A Binational Study Conducted between 1998 and 2017. J. Clin. Med. 2024, 13, 4449. https://doi.org/10.3390/jcm13154449
Pirinen R, Laine MT, Mani K, Gunnarsson K, Wanhainen A, Sund R, Venermo M. The Outcome after Endovascular and Open Repair of Abdominal Aortic Aneurysms—A Binational Study Conducted between 1998 and 2017. Journal of Clinical Medicine. 2024; 13(15):4449. https://doi.org/10.3390/jcm13154449
Chicago/Turabian StylePirinen, Riku, Matti T. Laine, Kevin Mani, Kim Gunnarsson, Anders Wanhainen, Reijo Sund, and Maarit Venermo. 2024. "The Outcome after Endovascular and Open Repair of Abdominal Aortic Aneurysms—A Binational Study Conducted between 1998 and 2017" Journal of Clinical Medicine 13, no. 15: 4449. https://doi.org/10.3390/jcm13154449






