The Role of Systemic Inflammation in the Pathogenesis of Spontaneous Intracranial Hemorrhage in the Presence or Absence of Effective Cerebral Blood Flow
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Measurement of Biomarkers
2.3. Methods of SHI Verification and Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- Severe ICH (coma, MOD, ALV) both without and with IECBF are extremely critical conditions with a high percentage of 28-day mortality at 80.8% (without IECBF) and 96.2% (with IECBF).
- Verification of systemic hyperinflammation using integral criteria in the ranges of days 1–3/5–8 from the onset of critical condition show that in Group 1 (without IECBF), the presence of SHI is detected in 60.9/78.8%, while in Group 2 (with IECBF) in just 0.0/8.7%.
- The low probability of SHI development in the group with IECBF is pathogenetically associated with low blood concentrations of NSE, a marker of blood–brain barrier permeability for brain tissue damage products and other neuroinflammatory factors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziai, W.C.; Carhuapoma, J.R. Intracerebral Hemorrhage. Continuum 2018, 24, 1603–1622. [Google Scholar] [CrossRef] [PubMed]
- Ekkert, A.; Šliachtenko, A.; Utkus, A.; Jatužis, D. Intracerebral Hemorrhage Genetics. Genes 2022, 13, 1250. [Google Scholar] [CrossRef]
- Fonville, A.F.; Al-Shahi Salman, R. Long-term prognosis after intracerebral haemorrhage: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2014, 85, 660–667. [Google Scholar] [CrossRef]
- de Oliveira Manoel, A.L.; Goffi, A.; Zampieri, F.G.; Turkel-Parrella, D.; Duggal, A.; Marotta, T.R.; Macdonald, R.L.; Abrahamson, S. The critical care management of spontaneous intracranial hemorrhage: A contemporary review. Crit. Care 2016, 20, 272. [Google Scholar] [CrossRef] [PubMed]
- Montaño, A.; Hanley, D.F.; Hemphill, J.C., 3rd. Hemorrhagic stroke. Handb. Clin. Neurol. 2021, 176, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Magid-Bernstein, J.; Girard, R.; Polster, S.; Srinath, A.; Romanos, S.; Awad, I.A.; Sansing, L.H. Cerebral Hemorrhage: Pathophysiology, Treatment, and Future Directions. Circ. Res. 2022, 130, 1204–1229. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, K.; Zhao, Y.; Zhou, L.; Liu, Y.; Zhao, J. Role of Ferroptosis in Stroke. Cell Mol. Neurobiol. 2023, 43, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C., 3rd; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef] [PubMed]
- Budinčević, H.; Meštrović, A.; Demarin, V. Stroke Scales as Assessment Tools in Emergency Settings: A Narrative Review. Medicina 2022, 58, 1541. [Google Scholar] [CrossRef]
- Li, X.; Chen, G. CNS-peripheral immune interactions in hemorrhagic stroke. J. Cereb. Blood Flow. Metab. 2023, 43, 185–197. [Google Scholar] [CrossRef]
- Saand, A.R.; Yu, F.; Chen, J.; Chou, S.H. Systemic inflammation in hemorrhagic strokes—A novel neurological sign and therapeutic target? J. Cereb. Blood Flow. Metab. 2019, 39, 959–988. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, S.N.; DeLong, J.H.; Kozberg, M.G.; Mazur-Hart, D.J.; van Veluw, S.J.; Alkayed, N.J.; Sansing, L.H. Role of Inflammatory Processes in Hemorrhagic Stroke. Stroke 2023, 54, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ma, Q.; Li, L.; Zhang, L.; Yang, Z.; Huang, Y.; Han, Z.; Wang, R.; Tao, Z.; Zheng, Y.; et al. Alterations of inflammatory cytokines in super-acute stroke patients and the potential pathogenesis. J. Clin. Neurosci. 2022, 99, 35–43. [Google Scholar] [CrossRef]
- Yang, M.; He, Y.; Deng, S.; Xiao, L.; Tian, M.; Xin, Y.; Lu, C.; Zhao, F.; Gong, Y. Mitochondrial Quality Control: A Pathophysiological Mechanism and Therapeutic Target for Stroke. Front. Mol. Neurosci. 2022, 14, 786099. [Google Scholar] [CrossRef]
- Okada, T.; Suzuki, H.; Travis, Z.D.; Zhang, J.H. The Stroke-Induced Blood-Brain Barrier Disruption: Current Progress of Inspection Technique, Mechanism, and Therapeutic Target. Curr. Neuropharmacol. 2020, 18, 1187–1212. [Google Scholar] [CrossRef]
- Kang, C.; You, Y.; Ahn, H.J.; Park, J.S.; Jeong, W.; Min, J.H.; In, Y.N.; Yoo, I.; Cho, Y.; Ryu, S.; et al. Blood-brain barrier disruption as a cause of various serum neuron-specific enolase cut-off values for neurological prognosis in cardiac arrest patients. Sci. Rep. 2022, 12, 2186. [Google Scholar] [CrossRef]
- Lu, W.; Jiang, C.; Wang, Z.; Chen, Y.; Bai, R.; Yan, G.; Wang, G.; Ren, H. Lactic acid, neuron-specific enolase, and blood-brain barrier index after a severe traumatic brain injury: A prospective study. Br. J. Neurosurg. 2024, 38, 220–224. [Google Scholar] [CrossRef]
- Dagonnier, M.; Donnan, G.A.; Davis, S.M.; Dewey, H.M.; Howells, D.W. Acute Stroke Biomarkers: Are We There Yet? Front. Neurol. 2021, 12, 619721. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xie, J.; Zhang, H.; Zheng, H.; Zheng, W.; Pang, C.; Ca, Y.; Deng, B. Neuron-specific enolase in hypertension patients with acute ischemic stroke and its value forecasting long-term functional outcomes. BMC Geriatr. 2023, 23, 294. [Google Scholar] [CrossRef]
- Roth, C.; Ferbert, A.; Matthaei, J.; Kaestner, S.; Engel, H.; Gehling, M. Progress of intracranial pressure and cerebral perfusion pressure in patients during the development of brain death. J. Neurol. Sci. 2019, 398, 171–175. [Google Scholar] [CrossRef]
- Salih, F.; Holtkamp, M.; Brandt, S.A.; Hoffmann, O.; Masuhr, F.; Schreiber, S.; Weissinger, F.; Vajkoczy, P.; Wolf, S. Intracranial pressure and cerebral perfusion pressure in patients developing brain death. J. Crit. Care 2016, 34, 1–6. [Google Scholar] [CrossRef]
- Corrêa, D.G.; de Souza, S.R.; Nunes, P.G.C.; Coutinho, A.C., Jr.; da Cruz, L.C.H., Jr. The role of neuroimaging in the determination of brain death. Radiol. Bras. 2022, 55, 365–372. [Google Scholar] [CrossRef]
- Slessarev, M.; Mahmoud, O.; McIntyre, C.W.; Ellis, C.G. Cerebral Blood Flow Deviations in Critically Ill Patients: Potential Insult Contributing to Ischemic and Hyperemic Injury. Front. Med. 2021, 7, 615318. [Google Scholar] [CrossRef] [PubMed]
- Rózsa, L.; Szabó, S.; Gombi, R.; Mikó, L.; Balázs, E. Koponyaüri nyomásfokozódást és agyhalált kísérö agyi véráramlás változások vizsgálata transcranialis Doppler szonográfiával [Intracranial pressure increase and changes in cerebrovascular circulation, associated with brain death, studied by transcranial Doppler sonography]. Orv. Hetil. 1991, 132, 2785–2788. [Google Scholar] [PubMed]
- Machado, C.; Pérez-Nellar, J.; Estevez, M.; Gonzalez, E. Evidence-based guideline update: Determining brain death in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2011, 76, 307; author reply 308–309. [Google Scholar] [CrossRef]
- Zotova, N.V.; Chereshnev, V.A.; Gusev, E.Y. Systemic inflammation: Methodological approaches to Iidentification of the common pathological process. PLoS ONE 2016, 11, e0155138. [Google Scholar] [CrossRef]
- Zotova, N.; Zhuravleva, Y.; Chereshnev, V.; Gusev, E. Acute and Chronic Systemic Inflammation: Features and Differences in the Pathogenesis, and Integral Criteria for Verification and Differentiation. Int. J. Mol. Sci. 2023, 24, 1144. [Google Scholar] [CrossRef] [PubMed]
- Brazhnikov, A.; Zotova, N.; Solomatina, L.; Sarapultsev, A.; Spirin, A.; Gusev, E. Shock-Associated Systemic Inflammation in Amniotic Fluid Embolism, Complicated by Clinical Death. Pathophysiology 2023, 30, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Zotova, N.V.; Zhuravleva, Y.A.; Zubova, T.E.; Gusev, E.Y. Integral estimation of systemic inflammatory response under sepsis. Gen. Physiol. Biophys. 2020, 39, 13–26. [Google Scholar] [CrossRef]
- Gusev, E.Y.; Zotova, N.V. Cellular stress and general pathological processes. Curr. Pharm. Des. 2019, 25, 251–297. [Google Scholar] [CrossRef]
- Gusev, E.; Zhuravleva, Y. Inflammation: A New Look at an Old Problem. Int. J. Mol. Sci. 2022, 23, 4596. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Dhapola, R.; Sharma, P.; Nagar, P.; Medhi, B.; HariKrishnaReddy, D. The impact of cytokines in neuroinflammation-mediated stroke. Cytokine Growth Factor. Rev. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, Z.; Yu, J.; Yang, X.; He, F.; Liu, Z.; Che, F.; Chen, X.; Ren, H.; Hong, M.; et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog. Neurobiol. 2019, 178, 101610. [Google Scholar] [CrossRef] [PubMed]
- Maida, C.D.; Norrito, R.L.; Rizzica, S.; Mazzola, M.; Scarantino, E.R.; Tuttolomondo, A. Molecular Pathogenesis of Ischemic and Hemorrhagic Strokes: Background and Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 6297. [Google Scholar] [CrossRef] [PubMed]
- Fringuello, A.; Tatman, P.D.; Wroblewski, T.; Thompson, J.A.; Yu, X.; Lillehei, K.O.; Kowalski, R.G.; Graner, M.W. Cytokine-Laden Extracellular Vesicles Predict Patient Prognosis after Cerebrovascular Accident. Int. J. Mol. Sci. 2021, 22, 7847. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Gabazza, E.C.; Asakura, H.; Koike, K.; Okamoto, K.; Maruyama, I.; Shiku, H.; Nobori, T. Comparison of diagnostic criteria for disseminated intravascular coagulation (DIC): Diagnostic criteria of the International Society of Thrombosis and Hemostasis and of the Japanese Ministry of Health and Welfare for overt DIC. Am. J. Hematol. 2003, 74, 17–22. [Google Scholar] [CrossRef]
- Papageorgiou, C.; Jourdi, G.; Adjambri, E.; Walborn, A.; Patel, P.; Fareed, J.; Elalamy, I.; Hoppensteadt, D.; Gerotziafas, G.T. Disseminated Intravascular Coagulation: An Update on Pathogenesis, Diagnosis, and Therapeutic Strategies. Clin. Appl. Thromb. Hemost. 2018, 24 (Suppl. 9), 8S–28S. [Google Scholar] [CrossRef]

| Evaluation Parameters | Group 1 (without IECBF) | Group 2 (with IECBF) |
|---|---|---|
| n, 1–3/5–8 days | 52/49 | 26/23 |
| Age, years, Me ± SD (Group 1A and 2A) | 54.0 ± 11.5 | 50.5 ± 10.8 |
| Paul f/m, %, (Group 1A and 2A) | 50/50 | 36/64 |
| Intracerebral hemorrhage intraventricular, % | 21.1 * | 42.3 * |
| Multiple organ failure (MOF), % | 100 | 100 |
| Artificial lung ventilation (ALV), % | 100 | 100 |
| Critical coma (GSC = 3 scores), % | 82.7 | 76.9 |
| 28-day mortality, % | 84.6 | 96.2 |
| Criteria DIC syndrome, % | 17.3 | 0 |
| Treatment with vasopressors, % | 23.1 * | 80.8 * |
| NSE > 17.6 ng/mL 1, % | 0 | 21.8 * |
| 28 daily brain death 2 (group 2B, n-23), % | 0 | 87.0 * |
| Organ donors 3 (group 2B, n-23), % | 0 | 21.8 * |
| Factors | Norm Values | Values of Individual Reactivity Levels | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| IL-6, pg/mL | ≤5 | ≤5 | ≤10 | ≤40 | ≤200 | ≤1000 | >1000 | no |
| IL-10, pg/mL | ≤10 | ≤10 | no | ≤20 | ≤50 | ≤200 | ≤1000 | >1000 |
| IL-8, pg/mL | ≤10 | ≤10 | ≤25 | ≤100 | ≤500 | ≤2500 | >250 | no |
| TNF-α, pg/mL | ≤8 | ≤8 | ≤16 | ≤40 | ≤160 | ≤800 | >800 | no |
| PCT, ng/mL | ≤0.1 | ≤0.1 | ≤0.25 | ≤1 | ≤10 | ≤100 | >100 | no |
| Phenomenon of SHI | Criterion | Points | Note |
|---|---|---|---|
| Systemic inflammatory reaction | RL scale (0–5 points) | 2–5 | RL 0–1 rules out the presence of an acute |
| Disseminated intravascular coagulation | D-dimer > 500 ng/mL | 1 | or the presence of criteria for DIC syndrome |
| Distress of the hypothalamic–pituitary–adrenal axis | Cortisol 1 >1380 or <100 nmol/L | 1 | In the absence of a criterion, but with glucocorticoid treatment, 1 point is added |
| Systemic tissue alteration | Troponin I ≥ 0.2 ng/mL and/or myoglobin ≥ 200 ng/mL | 1 | Troponin I is not taken into account in myocardial infarction |
| Multiple organ failure | Scale SOFA | 1 | or other criteria for multiple organ dysfunction syndrome |
| Indicators | 1A | 1B | 2A | 2B |
|---|---|---|---|---|
| Heart rate (N 60–90) | 85.0 ± 21.4 | 89.0 ± 17.1 | 93.0 ± 35.7 | 100.0 ± 31.0 |
| Average blood pressure | 101.3 ± 23.2 * | 99.7 ± 20.8 * | 76.7 ± 16.6 | 73.7 ± 11.5 |
| Central venous pressure (N 8–12 mmHg) | 12.0 ± 2.1 | 12.0 ± 2.9 | 12.0 ± 3.7 | 12.0 ± 3.4 |
| Bilirubin (N < 20 μmol/L) | 33.0 ± 11.7 | 33.5 ± 11.9 | 36.0 ± 11.3 | 36.0 ± 12.7 |
| Creatinine (N < 110 mmol/L) | 93.0 ± 41.2 * | 151.5 ± 63.3 * | 67.0 ± 39.7 | 67.0 ± 33.9 |
| Urea (N 1.7–8.3 mmol/L) | 7.7 ± 4.6 | 11.3 ± 4.8 * | 7.8 ± 4.3 | 7.6 ± 4.0 |
| Platelets (N 180–320 × 103/mm3) | 201.0 ± 95.1 | 208.5 ± 94.9 | 213.0 ± 140.1 | 134.0 ± 94.5 |
| Glucose mmol/L 1 | 10.5 ± 4.4 | 12.6 ± 3.6 | 11.6 ± 4.5 | 11.7 ± 4.8 |
| ALT (N < 40 units) | 49.0 ± 31.9 * | 92.5 ± 75.5 * | 31.0 ± 42.7 | 25.0 ± 47.7 |
| AST (N < 40 units) | 65.0 ± 56.1 * | 88.0 ± 66.2 * | 27.0 ± 26.4 | 26.0 ± 25.6 |
| Na+ (N 136–145 mmol/L) | 143.0 ± 10.5 * | 146.0 ± 11.2 * | 154.0 ± 10.1 | 156.0 ± 9.1 |
| K+ (N 3.5–5.2 mmol/L) | 4.2 ± 0.8 | 3.7 ± 0.9 | 4.6 ± 0.8 | 4.5 ± 1.2 |
| Cl− (N 98—113 mmol/L) | 105.0 ± 3.8 | 105.0 ± 4.3 | 104.0 ± 5.1 | 104.5 ± 4.5 |
| SpO2/FiO2 | 291.3 ± 75.7 | 291.9 ± 62.5 | 310.0 ± 60.7 | 307.5 ± 85.1 |
| Indicators | Control | Group 1A | Group 1B | Group 2A | Group 2B |
|---|---|---|---|---|---|
| SOFA, scores | 0 [1A, 1B, 2A, 2B] | 7.00 (6.00–8.00) [C, 1B, 2A, 2B] | 9.00 (8.00–10.00) [C, 1A, 2A, 2B] | 10.00 (9.00–11.00) [C, 1A, 1B, 2B] | 11.00 (10.00–12.00) [C, 1A, 1B, 2A] |
| Myoglobin, ng/mL | 7.74 (5.61–13.28) [1A, 1B, 2A, 2B] | 79.40 (45.69–173.6) [C, 2A, 2B] | 123.90 (50.58–230.73) [C, 2A, 2B] | 16.15 (13.88–19.15) [C, 1A, 1B] | 18.05 (14.50–26.75) [C, 1A, 1B] |
| Troponin I, ng/mL | 0.00 (0.00–0.00) [1A, 1B] | 0.05 (0.02–0.349) [C, 2B, 2A] | 0.07 (0.03–0.31) [C, 2A, 2B] | 0.00 (0.00–0.00) [1A, 1B] | 0.00 (0.00–0.00) [1A, 1B] |
| D-dimer, ng/mL | 11.5 (4.9–30.3) [1A, 1B, 2A, 2B] | 1228.4 (654.0–3654.0) [C, 1B, 2A, 2B] | 2517.6 (1294.8–4848.0) [C, 1A, 2A, 2B] | 75.0 (42.5–91.6) [C, 1A, 1B] | 105.0 (31.5–216.3) [C, 1A, 1B] |
| IL-6, pg/mL | 0.80 (0.45–1.36) [1A, 1B, 2A, 2B] | 78.35 (42.80–223.10) [C, 1B, 2A, 2B] | 185.32 (77.82–317.70) [C, 1A, 2A, 2B] | 1.40 (1.20–1.50) [C, 2B, 1A, 1B] | 6.25 (2.20–12.28) [C, 1A, 1B, 2A] |
| IL-10, pg/mL | 0.57 (0.00–21.95) [1A, 1B, 2A] | 10.90 (5.37–18.02) [C, 2A, 2B] | 14.46 (4.90–25.10) [C, 2A, 2B] | 1.75 (0.63–4.30) [C, 1A, 1B] | 0.70 (0.23–4.68) [1A, 1B] |
| IL-8, pg/mL | 1.72 (1.35–2.38) [1A, 1B, 2A, 2B] | 16.40 (5.50–48.45) [C, 1B, 2A] | 50.10 (14.70–127.46) [C, 1A, 2A, 2B] | 3.00 (2.90–3.18) [C, 1A, 1B, 2B] | 6.70 (3.53–37.28) [C, 1B, 2A] |
| TNF-α, pg/mL | 0.00 (0.00–0.36) [1A, 1B, 2A, 2B] | 1.36 (0.20–4.00) [C, 1B] | 7.62 (1.05–20.40) [C, 1A, 2A, 2B] | 1.34 (0.93–1.54) [C, 1B, 2B] | 1.54 (1.27–1.87) [C, 1B, 2A] |
| PCT, ng/mL | 0.03 (0.02–0.03) [1A, 1B, 2A, 2B] | 0.89 (0.40–2.96) [C, 2B, 2A] | 1.26 (0.48–3.84) [C, 2A, 2B] | 0.10 (0.10–0.11) [C, 1A, 1B] | 0.11 (0.10–0.16) [C, 1A, 1B] |
| Cortisol, nmol/L | 358.3 (278–450) [1A, 1B, 2A, 2B] | 898.5 (467–1387) [C] | 1006.8 (560.0–1475.0) [C, 2A] | 564.5 (535–575) [C, 1B] | 565.5 (543–871) [C] |
| ACTH, pg/mL | 2.40 (1.36–3.64) [1A, 1B] | 4.00 (2.70–9.20) [C] | No | 6.30 (5.82–6.55) [C] | No |
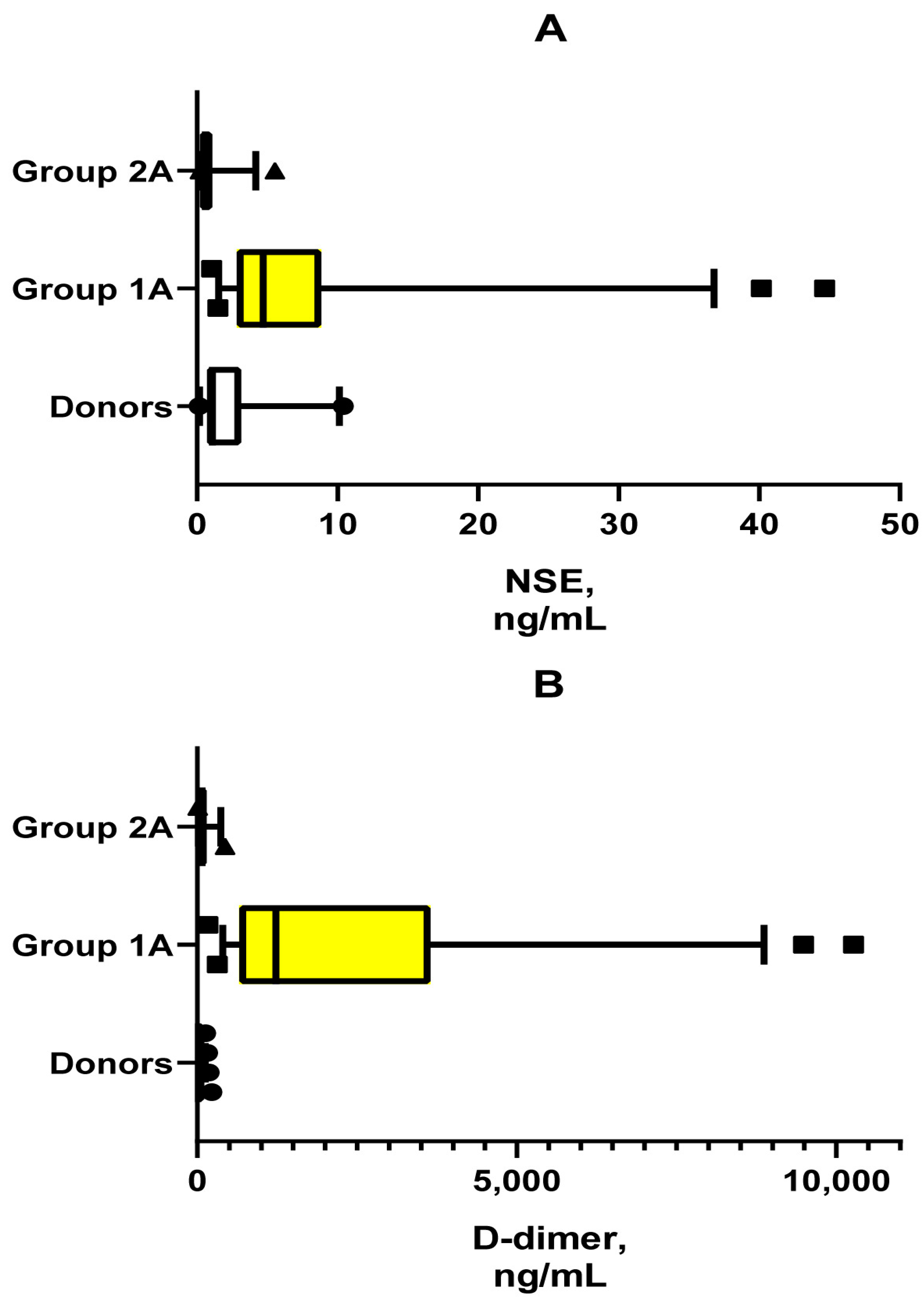
| NSE, ng/mL | 0.99 (0.71–3.83) [1A, 1B] | 4.71 (2.85–8.79) [C, 1B] | No | 0.60 (0.60–0.70) [C, 1A] | No |
| Indicators | Spearman—R | p-Value |
|---|---|---|
| SOFA | −0.705673 | 0.000000 |
| Myoglobin | 0.649737 | 0.000000 |
| D-dimer | 0.774284 | 0.000000 |
| IL-6 | 0.730473 | 0.000000 |
| IL-10 | 0.440968 | 0.000167 |
| IL-8 | 0.591767 | 0.000000 |
| PCT | 0.655436 | 0.000000 |
| TNF-α | 0.099814 | 0.418025 |
| Cortisol | 0.331982 | 0.005679 |
| Troponin I | 0.728685 | 0.000000 |
| RL | 0.767600 | 0.000000 |
| Scale SI | 0.799648 | 0.000000 |
| Indicators | Group 1A | Group 1B | Group 2A | Group 2B |
|---|---|---|---|---|
| RL-0 | 3.9 [2A, 2B] | 4.1 [2A, 2B] | 100 [1A, 1B] | 68.2 [1A, 1B] |
| RL-1 | 15.7 [2A, 1B] | 2 [1A] | 0 [1A] | 9.1 [No] |
| RL-2 | 31.4 [2A] | 22.4 [2A] | 0 [1A, 1B] | 18.2 [No] |
| RL-3 | 41.2 [2A, 2B] | 53.1 [2A, 2B] | 0 [1A, 1B] | 4.5 [1A, 1B] |
| RL-4 | 5.9 [No] | 18.4 [2A, 2B] | 0 [2B] | 0 [2B] |
| RL-5 | 2.0 [No] | 0 [No] | 0 [No] | 0 [No] |
| Troponin I > 0.2 ng/mL | 33.3 [2A, 2B] | 40.8 [2A, 2B] | 0 [1A, 1B] | 4.3 [1A, 1B] |
| Myoglobin > 200 ng/mL | 19.6 [2A, 2B] | 34.7 [2A, 2B] | 0 [1A, 1B] | 0 [1A, 1B] |
| Tissue alteration 1 | 43.1 [2A, 2B] | 59.3 [2A, 2B] | 0 [1A, 1B] | 4.3 [1A, 1B] |
| Cortisol > 1380 or < 100 nmol/L | 25.5 [2A] | 40.1 [2A, 2B] | 0 [1A, 1B] | 8.7 [1B] |
| D-dimer > 500 ng/mL | 92.2 [2A, 2B] | 95.9 [2A, 2B] | 0 [1A, 1B] | 8.7 [1A, 1B] |
| Multiple organ failure (SOFA) | 100 [No] | 100 [No] | 100 [No] | 100 [No] |
| Pre-SHI | 32.7 [2A, 2B, 1B] | 8.2 [1A] | 0 [1A] | 8.7 [1A] |
| Scale SHI | 61.5 [2A, 2B, 1B] | 87.8 [2A, 2B, 1A] | 0 [1A, 1B] | 8.7 [1A, 1B] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusev, E.; Solomatina, L.; Bochkarev, P.; Zudova, A.; Chereshnev, V. The Role of Systemic Inflammation in the Pathogenesis of Spontaneous Intracranial Hemorrhage in the Presence or Absence of Effective Cerebral Blood Flow. J. Clin. Med. 2024, 13, 4454. https://doi.org/10.3390/jcm13154454
Gusev E, Solomatina L, Bochkarev P, Zudova A, Chereshnev V. The Role of Systemic Inflammation in the Pathogenesis of Spontaneous Intracranial Hemorrhage in the Presence or Absence of Effective Cerebral Blood Flow. Journal of Clinical Medicine. 2024; 13(15):4454. https://doi.org/10.3390/jcm13154454
Chicago/Turabian StyleGusev, Evgenii, Liliya Solomatina, Peter Bochkarev, Alevtina Zudova, and Valeriy Chereshnev. 2024. "The Role of Systemic Inflammation in the Pathogenesis of Spontaneous Intracranial Hemorrhage in the Presence or Absence of Effective Cerebral Blood Flow" Journal of Clinical Medicine 13, no. 15: 4454. https://doi.org/10.3390/jcm13154454





