Failure of Surgical Aortic Valve Prostheses: An Analysis of Heart Team Decisions and Postoperative Outcomes
Abstract
:1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and Co-Morbidities
3.2. Heart Team Decision
3.3. Echocardiographic Data
3.4. Surgical Data
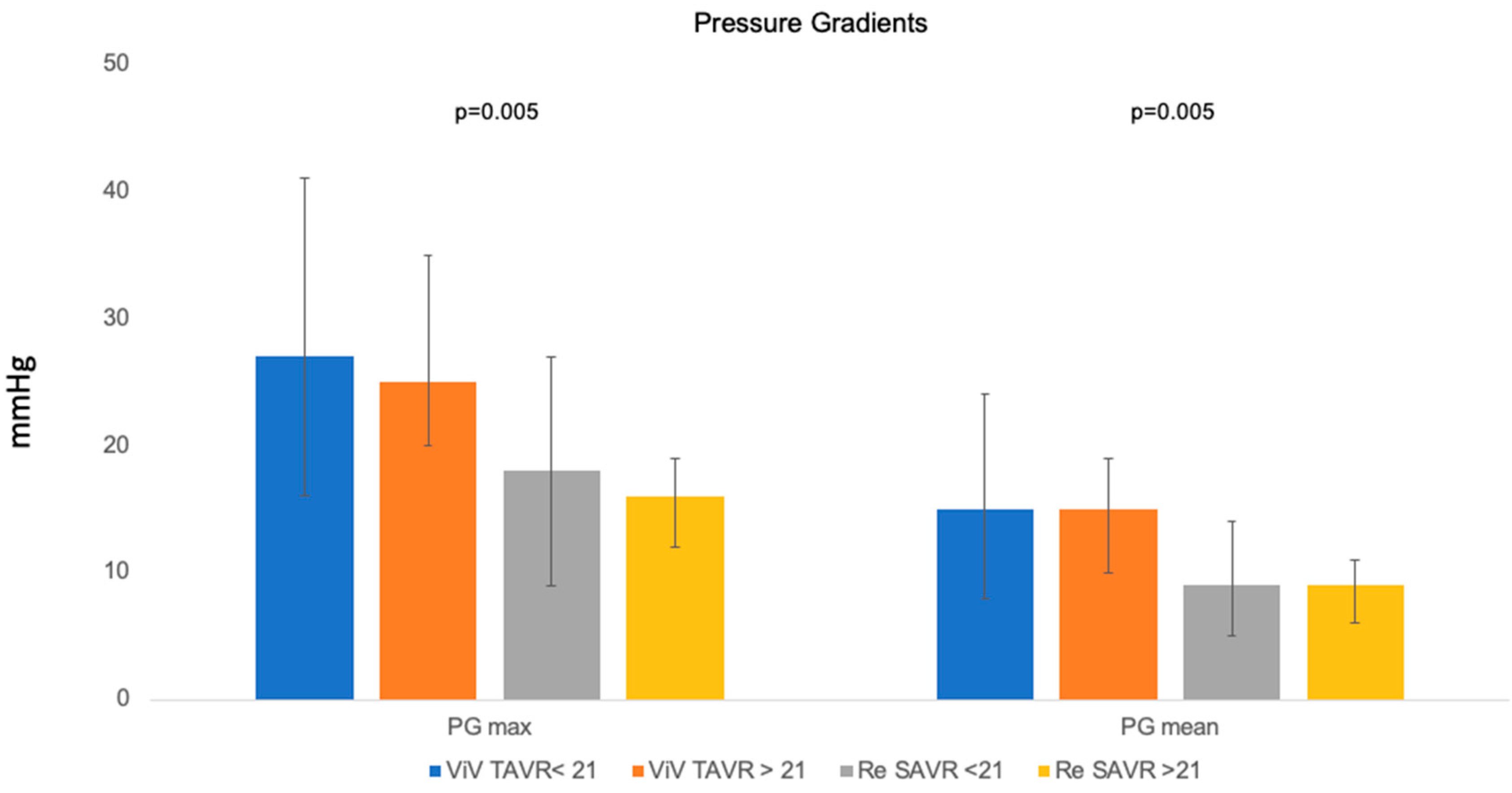
3.5. Valve Type-Based Echocardiographic Data
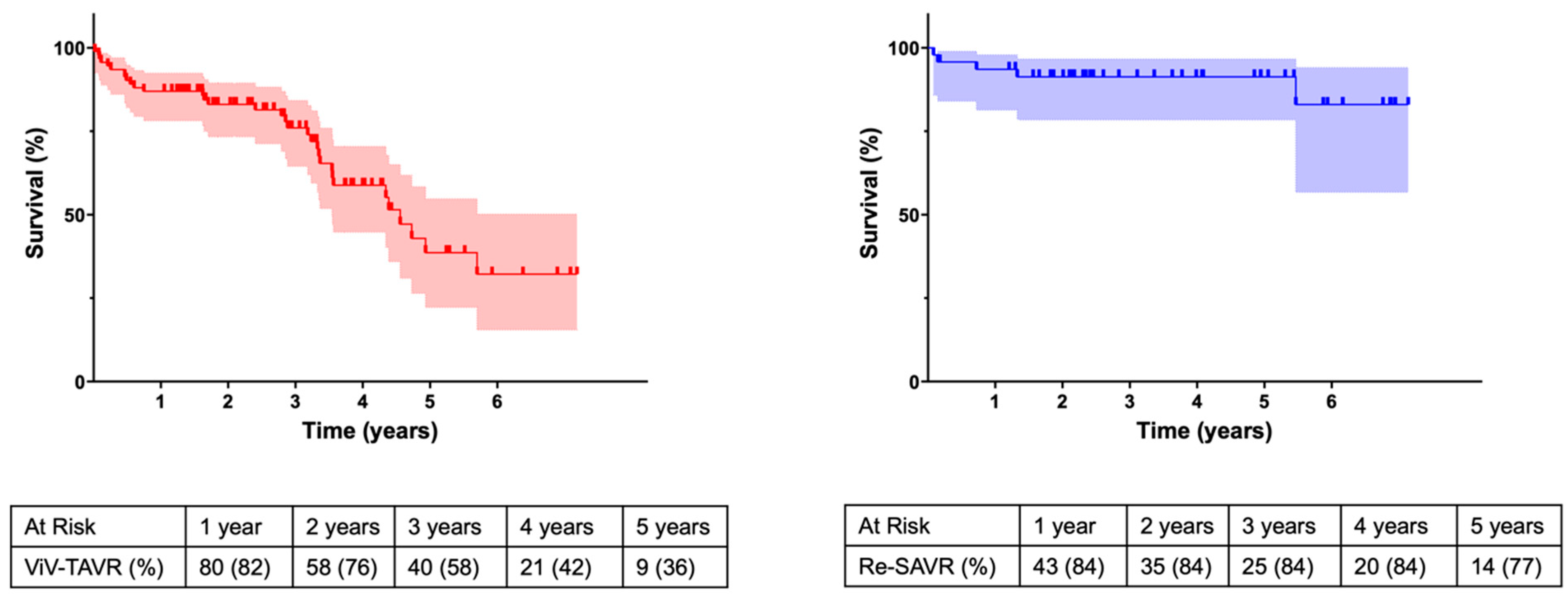
3.6. Postprocedural Morbidities and Outcomes
4. Discussion
- Redo-SAVR, being the more invasive therapy option, enables the surgical treatment of concomitant cardiac pathologies, and allows anticipation for later VIV-TAVR by implanting the largest possible valve prostheses or implantation of mechanical prostheses if mandatory.
- ViV-TAVR offers a treatment for elderly or intermediate-risk profile patients with comparable short-term mortality. However, this therapy is associated with increased pressure gradients and a high prevalence of paravalvular leakage.
4.1. Surgery or Intervention—The Heart Team Approach
4.2. Hemodynamic Outcomes
4.3. Outcomes
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deharo, P.; Bisson, A.; Herbert, J.; Lacour, T.; Saint Etienne, C.; Jaussaud, N.; Theron, A.; Collart, F.; Bourguignon, T.; Cuisset, T.; et al. Valve-in-valve transcatheter aortic valve implantation after failed surgically implanted aortic bioprosthesis versus native transcatheter aortic valve implantation for aortic stenosis: Data from a nationwide analysis. Arch. Cardiovasc. Dis. 2021, 114, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, N.; Atti, V.; Munir, A.B.; Karam, B.; Patel, N.J.; Kumar, V.; Vemula, P.; Edla, S.; Asti, D.; Paturu, A.; et al. Valve in valve transcatheter aortic valve implantation (ViV-TAVI) versus redo-Surgical aortic valve replacement (redo-SAVR): A systematic review and meta-analysis. J. Interv. Cardiol. 2018, 31, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.H.; Yandrapalli, S.; Zaid, S.; Shetty, S.S.; Aronow, W.S.; Ahmad, H.; Tang, G.H.L. Valve-in-Valve Transcatheter Implantation Versus Redo Surgical Aortic Valve Replacement. Am. J. Cardiol. 2020, 125, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Singh, H.; Lämmer, J.; Othman, H.; Yamasaki, H.; Rosman, H.S.; Bossone, E.; Mehta, R.H.; Eggebrecht, H. Meta-Analysis of Transcatheter Valve-in-Valve Implantation Versus Redo Aortic Valve Surgery for Bioprosthetic Aortic Valve Dysfunction. Am. J. Cardiol. 2018, 121, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Kherallah, R.Y.; Koneru, S.; Krajcer, Z.; Preventza, O.; Dougherty, K.G.; Mccormack, M.L.; Costello, B.T.; Coulter, S.; Strickman, N.E.; Carlos, J.; et al. Hemodynamic outcomes after valve-in-valve transcatheter aortic valve replacement: A single-center experience. Ann. Cardiothorac. Surg. 2021, 10, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Lee, C.; Tully, A.; Fang, J.C.; Sugeng, L.; Elmariah, S.; Grubb, K.J.; Young, M.N. Building and Optimizing the Interdisciplinary Heart Team. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101067. [Google Scholar] [CrossRef]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; Lancellotti, P.; Sondergaard, L.; Ludman, P.F.; Tamburino, C.; et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interve. Eur. Heart J. 2017, 38, 3382–3390. [Google Scholar] [CrossRef]
- Antonides, C.F.J.; Mack, M.J.; Kappetein, A.P. Approaches to the Role of The Heart Team in Therapeutic Decision Making for Heart Valve Disease. Struct. Heart 2017, 1, 249–255. [Google Scholar] [CrossRef]
- Davierwala, P.M.; Marin-Cuartas, M.; Misfeld, M.; Borger, M.A. The value of an “Endocarditis Team”. Ann. Cardiothorac. Surg. 2019, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Varghese, S.; Al Ahmad, A.; Jebran, A.F.; Waezi, N.; Niehaus, H.; Baraki, H.; Kutschka, I. Complex Valve Surgery in Elderly Patients: Increasingly Necessary and Surprisingly Feasible. Thorac. Cardiovasc. Surg. 2020, 68, 107–113. [Google Scholar] [CrossRef]
- Peterss, S.; Fortmann, C.; Pichlmaier, M.; Bagaev, E.; Shrestha, M.L.; Hagl, C.; Haverich, A.; Khaladj, N. Advanced age: A contraindication for triple-valve surgery? J. Heart Valve Dis. 2012, 21, 641–649. [Google Scholar] [PubMed]
- Sedeek, A.F.; Greason, K.L.; Sandhu, G.S.; Dearani, J.A.; Holmes, D.R.J.; Schaff, H.V. Transcatheter Valve-in-Valve Vs Surgical Replacement of Failing Stented Aortic Biological Valves. Ann. Thorac. Surg. 2019, 108, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Woitek, F.J.; Stachel, G.; Kiefer, P.; Haussig, S.; Leontyev, S.; Schlotter, F.; Mende, M.; Hommel, J.; Crusius, L.; Spindler, A.; et al. Treatment of failed aortic bioprostheses: An evaluation of conventional redo surgery and transfemoral transcatheter aortic valve-in-valve implantation. Int. J. Cardiol. 2020, 300, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Tang, G.H.L.; Sangiorgi, G.; Pedicino, D.; Enriquez-Sarano, M.; Maisano, F.; Taramasso, M. Lifetime Management of Aortic Stenosis: Transcatheter Versus Surgical Treatment for Young and Low-Risk Patients. Circ. Cardiovasc. Interv. 2022, 15, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Edelman, J.J.; Khan, J.M.; Rogers, T.; Shults, C.; Satler, L.F.; Ben-Dor, I.I.; Waksman, R.; Thourani, V.H. Valve-in-Valve TAVR: State-of-the-Art Review. Innovations 2019, 14, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.Y.; Rocha, R.V.; Wijeysundera, H.C.; Austin, P.C.; Dvir, D.; Fremes, S.E. Surgical valve selection in the era of transcatheter aortic valve replacement in the Society of Thoracic Surgeons Database. J. Thorac. Cardiovasc. Surg. 2020, 159, 416–427.e8. [Google Scholar] [CrossRef]
- Salaun, E.; Clavel, M.-A.; Rodés-Cabau, J.; Pibarot, P. Bioprosthetic aortic valve durability in the era of transcatheter aortic valve implantation. Heart 2018, 104, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Bilkhu, R.; Jahangiri, M.; Otto, C.M. Patient-prosthesis mismatch following aortic valve replacement. Heart 2019, 105, s28–s33. [Google Scholar] [CrossRef]
- Patel, P.M.; Chiou, E.; Cao, Y.; Binongo, J.; Guyton, R.A.; Leshnower, B.; Grubb, K.J.; Chen, E.P. Isolated Redo Aortic Valve Replacement Versus Valve-in-Valve Transcatheter Valve Replacement. Ann. Thorac. Surg. 2021, 112, 539–545. [Google Scholar] [CrossRef]
- Bleiziffer, S.; Simonato, M.; Webb, J.G.; Rodés-Cabau, J.; Pibarot, P.; Kornowski, R.; Windecker, S.; Erlebach, M.; Duncan, A.; Seiffert, M.; et al. Long-term outcomes after transcatheter aortic valve implantation in failed bioprosthetic valves. Eur. Heart J. 2020, 41, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Dahlbacka, S.; Laakso, T.; Kinnunen, E.-M.; Moriyama, N.; Laine, M.; Virtanen, M.; Maaranen, P.; Ahvenvaara, T.; Tauriainen, T.; Husso, A.; et al. Patient-Prosthesis Mismatch Worsens Long-Term Survival: Insights From the FinnValve Registry. Ann. Thorac. Surg. 2021, 111, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.C.; Daneshvar, S.A.; Fonarow, G.C.; Stebbins, A.; Vemulapalli, S.; Desai, N.D.; Malenka, D.J.; Thourani, V.H.; Rymer, J.; Kosinski, A.S. Prosthesis-Patient Mismatch in Patients Undergoing Transcatheter Aortic Valve Replacement: From the STS/ACC TVT Registry. J. Am. Coll. Cardiol. 2018, 72, 2701–2711. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Webb, J.G.; Bleiziffer, S.; Pasic, M.; Waksman, R.; Kodali, S.; Barbanti, M.; Latib, A.; Schaefer, U.; Rodés-Cabau, J.; et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014, 312, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention 2022, 17, e1126–e1196. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, F.L.M.; Dvir, D.; Rodes-Cabau, J.; Ribeiro, H.B. Valve-in-Valve Challenges: How to Avoid Coronary Obstruction. Front. Cardiovasc. Med. 2019, 6, 120. [Google Scholar] [CrossRef]
| RS (n = 53) | ViV (n = 103) | p-Value | |
|---|---|---|---|
| Age (years) | 68 (59–77) | 79 (75–83) | <0.001 |
| Female | 23 (43.4) | 44 (42.7) | 1.000 |
| Body Mass Index (kg/m2) | 25.3 (22.9–28.9) | 25.3 (23.3–28.5) | 0.855 |
| EuroSCORE II | 5.7 (3.5–8.5) | 9.2 (5.4–13.6) | <0.001 |
| STS—PROM | 2.0 (1.3–2.8) | 4.1 (2.6–6.8) | <0.001 |
| Index surgery (%) | |||
| Biological prosthesis | 43 (81.1) | 103 (100) | - |
| Mechanical prosthesis | 10 (18.8) | - | - |
| Concomitant procedure | 18 (33.9) | 50 (48.5) | |
| Time to Redo-surgery (years) | 9 (5–14.5) | 10 (7–12) | 0.978 |
| Indication for Redo-surgery (%) | 0.688 | ||
| AS | 23 (43.4) | 38 (36.9) | - |
| AR | 7 (13.2) | 5 (4.9) | - |
| MAVD—predominant AS | 10 (18.9) | 43 (41.7) | - |
| MAVD—predominant AR | 9 (17.0) | 17 (16.5) | - |
| PVL | 4 (7.5) | 0 (0.0) | - |
| Co-morbidities | |||
| Arterial hypertension (%) | 43 (81.1) | 99 (96.1) | 0.005 |
| IDDM (%) | 3 (5.7) | 22 (21.4) | 0.011 |
| Hyperlipoproteinemia (%) | 38 (71.7) | 85 (82.5) | 0.182 |
| Hyperuricemia (%) | 5 (9.4) | 26 (25.2) | 0.020 |
| Atrial fibrillation (%) | 20 (37.7) | 42 (40.8) | 0.733 |
| CAD (%) | 17 (32.1) | 71 (68.9) | <0.001 |
| Previous PTCA/Stenting (%) | 6 (11.3) | 28 (27.2) | 0.025 |
| Previous MI (<90 days) (%) | 1 (1.8) | 5 (4.8) | 0.665 |
| Previous pacemaker implantation (%) | 5 (9.4) | 17 (16.5) | 0.431 |
| COPD (%) | 5 (9.4) | 10 (9.7) | 1.000 |
| PAD (%) | 2 (3.8) | 9 (8.7) | 0.335 |
| CVD (%) | 9 (17.0) | 29 (28.2) | 0.168 |
| Previous stroke (%) | 2 (3.8) | 14 (13.6) | 0.091 |
| CKD (%) | 11 (20.8) | 56 (54.4) | <0.001 |
| Hemodialysis (%) | 0 (0.0) | 6 (5.8) | 0.096 |
| ECMO preoperative (%) | 0 (0.0) | 1 (1.0) | 1.000 |
| Anticoagulation | |||
| VKA (%) | 17 (32.1) | 10 (9.7) | 0.001 |
| NOAC (%) | 5 (9.4) | 31 (30.1) | 0.004 |
| SAPT (%) | 28 (52.8) | 56 (53.3) | 0.867 |
| DAPT (%) | 1 (1.9) | 3 (2.9) | 1.000 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Age (years) | 1.061 | 1.020–1.104 | 0.004 |
| IDDM | 1.853 | 0.453–7.584 | 0.391 |
| CAD | 2.648 | 1.160–6.048 | 0.021 |
| CNI | 2.711 | 1.079–6.811 | 0.034 |
| VKA | 0.311 | 0.107–0.906 | 0.032 |
| NOAC | 1.599 | 0.462–1.599 | 0.459 |
| RS (n = 53) | ViV (n = 103) | p-Value | |
|---|---|---|---|
| Before secondary intervention | |||
| LVEF (%) | 0.994 | ||
| ≥50 | 41 (1.2) | 77 (74.7) | |
| 31–49% | 10 (18.8) | 21 (20.3) | |
| ≤30 | 1 (1.8) | 5 (4.8) | |
| Aortic stenosis (%) | 0.010 | ||
| low to mild | 14 (26.4) | 6 (5.8) | |
| moderate to severe | 38 (71.6) | 90 (87.3) | |
| Aortic regurgitation | 0.078 | ||
| low to mild (%) | 25 (47.1) | 64 (62.1) | |
| moderate to severe (%) | 28 (52.8) | 32 (31.0) | |
| Mitral insufficiency | 0.595 | ||
| low to mild (%) | 37 (69.8) | 76 (73.7) | |
| moderate to severe (%) | 15 (28.3) | 20 (19.4) | |
| Tricuspid insufficiency (%) | 0.115 | ||
| moderate to severe (%) | 3 (5.6) | 19 (18.4) | |
| Pulmonary hypertension (%) | 12 (22.6) | 13 (12.6) | 0.113 |
| AV prosthesis PG max (mmHg) | 73 (57–96) | 61 (45–73) | 0.694 |
| AV prosthesis PG mean (mmHg) | 45 (33–67) | 37 (26–45) | 0.391 |
| AV prosthesis PVL (%) | 7 (13.2) | 9 (8.7) | 0.364 |
| EOA (cm2) | 0.7 (0.5–0.8) | 0.7 (0.6–0.9) | 0.747 |
| After secondary intervention | |||
| PG max (mmHg) | 18 (10–30) | 26 (19–38) | <0.001 |
| PG mean (mmHg) | 9 (6–15) | 15 (9–21) | <0.001 |
| PG mean ≥ 20 mmHg (%) | 1 (1.8) | 25 (24.2) | <0.001 |
| PVL (%) | 1 (1.9) | 23 (25.3) | 0.013 |
| RS (n = 53) | ViV (n = 103) | |
|---|---|---|
| Bypass time (min) | 156 (119–211) | - |
| Cross-clamp time (min) | 103 (82–141) | - |
| Concomitant procedures (%) | 33 (62.2) | 16 (15.5) |
| Aortic root enlargement (%) | 12 (22.6) | - |
| Aortic root replacement (%) | 3 (5.7) | - |
| CABG surgery (%) | 7 (13.2) | - |
| Mitral valve reconstruction (%) | 1 (1.9) | - |
| Mitral valve replacement (%) | 13 (24.5) | - |
| Tricuspid valve replacement (%) | 2 (3.8) | - |
| PTCA/Stenting (%) | - | 16 (15.5) |
| Valve prosthesis | ||
| Biological (%) | 40 (75.4) | 103 (100) |
| Self-expandable TAVR | 71 (68.9) | |
| Balloon-expandable TAVR | 32 (31.1) | |
| Mechanical (%) | 13 (24.5) | - |
| (a) | |||
| Bioprosthetic (n = 40) | Mechanical (n = 13) | p-Value | |
| PG max (mmHg) | 18 (11–20) | 21 (13–30) | 0.629 |
| PG mean (mmHg) | 9 (6–11) | 13 (8–17) | 0.577 |
| PVL (%) | 1 (3.8) | 0 (0.0) | 1.000 |
| (b) | |||
| SE (n = 71) | BE (n = 32) | p-Value | |
| PG max (mmHg) | 22 (17–28) | 28 (24–25) | 0.073 |
| PG mean (mmHg) | 13 (9–21) | 16 (13–21) | 0.153 |
| PVL (%) | 20 (32.8) | 3 (10.0) | 0.021 |
| RS (n = 53) | ViV (n = 103) | p-Value | |
|---|---|---|---|
| Cardiovascular morbidities | |||
| CPR (%) | 0 (0.0) | 6 (5.8) | 0.096 |
| Coronary obstruction (%) | 0 (0.0) | 3 (2.9) | 0.551 |
| Myocardial infarction (%) | 0 (0.0) | 2 (1.9) | 0.548 |
| Atrial fibrillation (%) | 8 (15.1) | 5 (4.9) | 0.036 |
| Arrythmia | |||
| AV—block (%) | 4 (7.5) | 8 (2.6) | 1.000 |
| Bundle branch block (%) | 5 (9.4) | 21 (20.4) | 0.112 |
| Pacemaker implantation (%) | 3 (5.7) | 6 (5.8) | 1.000 |
| Other | |||
| Bleeding (%) | 5 (9.4) | 9 (8.7) | 1.000 |
| Blood transfusion (%) | 38 (71.6) | 5 (4.8) | <0.001 |
| Re-explorative surgery (%) | 6 (11.3) | 0 (0.0) | 0.001 |
| Adverse cerebrovascular events (%) | 0 (0.0) | 5 (4.9) | 0.167 |
| Sepsis (%) | 5 (9.4) | 3 (2.9) | 0.122 |
| Pneumonia (%) | 7 (13.2) | 5 (4.9) | 0.108 |
| Surgical site infection (%) | 5 (9.4) | 1 (1.0) | 0.018 |
| Groin complication (%) | 1 (1.9) | 8 (7.8) | 0.168 |
| Organ support | |||
| Hemodialysis | 7 (13.2) | 2 (1.9) | 0.008 |
| IABP | 1 (1.9) | 0 (0.0) | 0.340 |
| ECMO | 2 (3.8) | 3 (2.9) | 1.000 |
| RS (n = 53) | ViV (n = 103) | p-Value | |
|---|---|---|---|
| Length of ICU stay (days) | 3 (3–5) | 1 (1–3) | <0.001 |
| Length of hospital stay (days) | 15 (13–20) | 11 (8–14) | <0.001 |
| Discharge | <0.001 | ||
| Cardiac rehabilitation (%) | 43 (81.1) | 41 (39.8) | |
| Inpatient care (%) | 4 (7.54) | 14 (13.5) | |
| Home (%) | 3 (5.66) | 44 (42.7) | |
| VARC-3 Early Safety (%) | 43 (81.1) | 90 (87.3) | 0.343 |
| 30-day mortality (%) | 3 (5.7) | 3 (2.9) | 0.409 |
| Myocardial infarction (%) | 0 (0.0%) | 1 (0.9%) | 1.000 |
| New pacemaker implantation (%) | 3 (5.6%) | 6 (5.8%) | 1.000 |
| Hospital admission for cardiac reasons (%) | 8 (15.1%) | 11 (10.7%) | 0.801 |
| Redo aortic valve surgery (%) | 0 (0.0%) | 1 (0.9%) | 1.000 |
| Major bleeding (%) | 1 (1.9%) | 3 (2.9%) | 1.000 |
| Ischemic stroke (%) | 3 (5.7%) | 2 (1.9%) | 0.370 |
| RSs (n = 29) | ViV (n = 103) | p-Value | |
|---|---|---|---|
| Cardiovascular morbidities | |||
| CPR (%) | 0 (0.0) | 6 (5.8) | 0.338 |
| Coronary obstruction (%) | 0 (0.0) | 3 (2.9) | 0.551 |
| Myocardial infarction (%) | 0 (0.0) | 2 (1.9) | 0.548 |
| Atrial fibrillation (%) | 5 (17.2) | 5 (4.9) | 0.041 |
| Arrythmia | |||
| AV—block (%) | 2 (6.9) | 8 (2.6) | 1.000 |
| Bundle branch block (%) | 3 (10.3) | 21 (20.4) | 0.282 |
| Pacemaker implantation (%) | 1 (3.4) | 6 (5.8) | 1.000 |
| Other | |||
| Bleeding (%) | 1 (3.4) | 9 (8.7) | 0.691 |
| Blood transfusion (%) | 18 (62.1) | 5 (4.8) | <0.001 |
| Re-explorative surgery (%) | 1 (3.4) | 0 (0.0) | 0.220 |
| Adverse cerebrovascular events (%) | 0 (0.0) | 5 (4.9) | 0.585 |
| Sepsis (%) | 2 (6.9) | 3 (2.9) | 0.302 |
| Pneumonia (%) | 1 (3.4) | 5 (4.9) | 1.000 |
| Surgical site infection (%) | 0 (0.0) | 1 (1.0) | 1.000 |
| Groin complication (%) | 0 (0.0) | 8 (7.8) | 0.199 |
| Organ support | |||
| Hemodialysis (%) | 2 (6.9) | 2 (1.9) | 0.121 |
| IABP (%) | 0 (0.0) | 0 (0.0) | - |
| ECMO (%) | 0 (0.0) | 3 (2.9) | 1.000 |
| Outcomes | |||
| Length of ICU stay (days) | 2 (2–3) | 1 (1–3) | 0.011 |
| Length of hospital stay (days) | 15 (12–21) | 11 (8–14) | <0.001 |
| VARC-3 Early Safety (%) | 26 (89.7) | 90 (87.3) | 1.000 |
| 30-day mortality (%) | 1 (3.4) | 3 (2.9) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnackenburg, P.; Saha, S.; Ali, A.; Horke, K.M.; Buech, J.; Mueller, C.S.; Sadoni, S.; Orban, M.; Kaiser, R.; Doldi, P.M.; et al. Failure of Surgical Aortic Valve Prostheses: An Analysis of Heart Team Decisions and Postoperative Outcomes. J. Clin. Med. 2024, 13, 4461. https://doi.org/10.3390/jcm13154461
Schnackenburg P, Saha S, Ali A, Horke KM, Buech J, Mueller CS, Sadoni S, Orban M, Kaiser R, Doldi PM, et al. Failure of Surgical Aortic Valve Prostheses: An Analysis of Heart Team Decisions and Postoperative Outcomes. Journal of Clinical Medicine. 2024; 13(15):4461. https://doi.org/10.3390/jcm13154461
Chicago/Turabian StyleSchnackenburg, Philipp, Shekhar Saha, Ahmad Ali, Konstanze Maria Horke, Joscha Buech, Christoph S. Mueller, Sebastian Sadoni, Martin Orban, Rainer Kaiser, Philipp Maximilian Doldi, and et al. 2024. "Failure of Surgical Aortic Valve Prostheses: An Analysis of Heart Team Decisions and Postoperative Outcomes" Journal of Clinical Medicine 13, no. 15: 4461. https://doi.org/10.3390/jcm13154461





