Diuretic Treatment in Heart Failure: A Practical Guide for Clinicians
Abstract
:1. Introduction
2. Loop Diuretics—Pharmacology
3. Loop Diuretic Pharmacokinetics
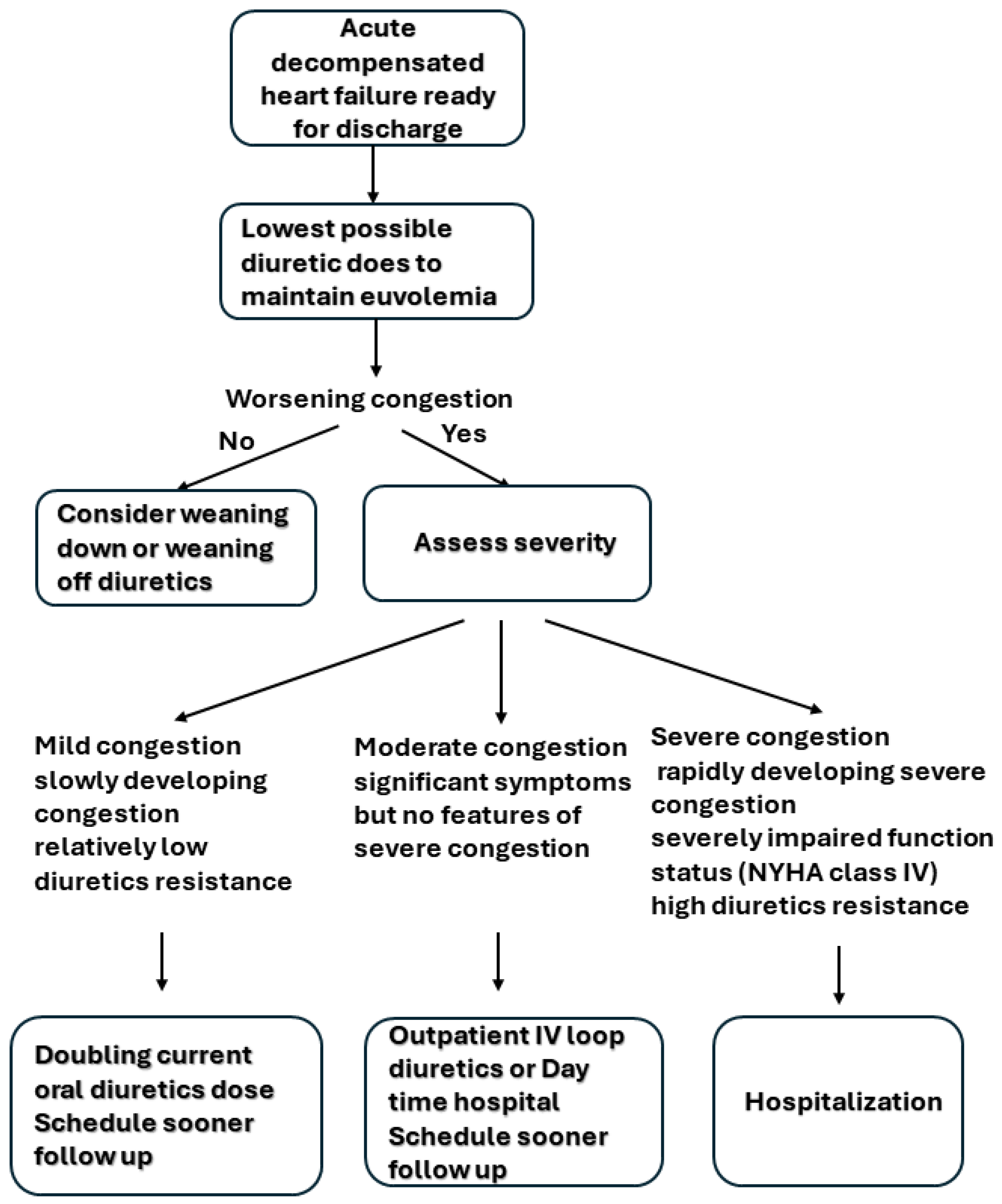
4. Loop Diuretic Use in Acute Decompensated Heart Failure
5. Loop Diuretic Use in Chronic Congestive Heart Failure
6. Adjuncts to Diuretic Treatment
6.1. Thiazides and Thiazides-like Diuretics
6.2. Carbonic Anhydrase Inhibitors
6.3. Mineralocorticoid Receptor Antagonist
6.4. Vasopressin Antagonists
6.5. SGLT-2 Inhibitor
6.6. Inotropes
7. Monitoring and Adverse Effects of Diuretics
7.1. Hypokalemia and Hypomagnesemia
7.2. Hyperkalemia
7.3. Hyponatremia
7.4. Worsening Renal Function
7.5. Hyperuricemia and Gout Attack
7.6. Hypersensitivity
7.7. Ototoxicity
8. Diuretics in Certain Phenotypes
8.1. Diuretics in Chronic Kidney Disease/End-Stage Renal Disease
8.2. Diuretics in Pregnancy
8.3. Diuretics in Elderly
8.4. Diuretics in Heart Failure with Preserved Ejection Fraction (HFpEF)
8.5. Diuretics in Right Ventricular Failure
8.6. Diuretics in Heart Transplant
9. Current Challenges
9.1. Diuretic Resistance
9.2. Incomplete Decongestion
10. Future Direction
Author Contributions
Funding
Conflicts of Interest
References
- Boorsma, E.M.; Ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in heart failure: A contemporary look at physiology, diagnosis and treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef]
- Bart, B.A.; Goldsmith, S.R.; Lee, K.L.; Givertz, M.M.; O’Connor, C.M.; Bull, D.A.; Redfield, M.M.; Deswal, A.; Rouleau, J.L.; LeWinter, M.M.; et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N. Engl. J. Med. 2012, 367, 2296–2304. [Google Scholar] [CrossRef]
- Neuberg, G.W.; Miller, A.B.; O’Connor, C.M.; Belkin, R.N.; Carson, P.E.; Cropp, A.B.; Frid, D.J.; Nye, R.G.; Pressler, M.L.; Wertheimer, J.H.; et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am. Heart J. 2002, 144, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Palmer, L.G.; Schnermann, J. Integrated control of Na transport along the nephron. Clin. J. Am. Soc. Nephrol. 2015, 10, 676–687. [Google Scholar] [CrossRef]
- Oppermann, M.; Hansen, P.B.; Castrop, H.; Schnermann, J. Vasodilatation of afferent arterioles and paradoxical increase of renal vascular resistance by furosemide in mice. Am. J. Physiol. Renal. Physiol. 2007, 293, F279–F287. [Google Scholar] [CrossRef]
- Lapointe, J.Y.; Bell, P.D.; Cardinal, J. Direct evidence for apical Na+:2Cl-:K+ cotransport in macula densa cells. Am. J. Physiol. 1990, 258 Pt 2, F1466–F1469. [Google Scholar] [CrossRef]
- Persson, A.E.; Ollerstam, A.; Liu, R.; Brown, R. Mechanisms for macula densa cell release of renin. Acta Physiol. Scand. 2004, 181, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Mentz, R.J.; Stevens, S.R.; DeVore, A.D.; Lala, A.; Vader, J.M.; AbouEzzeddine, O.F.; Khazanie, P.; Redfield, M.M.; Stevenson, L.W.; O’Connor, C.M.; et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail. 2015, 3, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Planavsky, N.; Hanberg, J.S.; Ahmad, T.; Brisco-Bacik, M.A.; Wilson, F.P.; Jacoby, D.; Chen, M.; Tang, W.H.W.; Cherney, D.Z.I.; et al. Compensatory Distal Reabsorption Drives Diuretic Resistance in Human Heart Failure. J. Am. Soc. Nephrol. 2017, 28, 3414–3424. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.A. Pharmacotherapy in congestive heart failure: Drug absorption in the management of congestive heart failure: Loop diuretics. Congest. Heart Fail. 2003, 9, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Ishii, S.; Maemura, K.; Oki, T.; Yazaki, M.; Fujita, T.; Nabeta, T.; Maekawa, E.; Koitabashi, T.; Ako, J. Association between intestinal oedema and oral loop diuretic resistance in hospitalized patients with acute heart failure. ESC Heart Fail. 2021, 8, 4067–4076. [Google Scholar] [CrossRef]
- Knauf, H.; Mutschler, E. Clinical pharmacokinetics and pharmacodynamics of torasemide. Clin. Pharmacokinet. 1998, 34, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Han, S.Y. Loop Diuretics in Clinical Practice. Electrolytes Blood Press. 2015, 13, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.H. Clinical Pharmacology in Diuretic Use. Clin. J. Am. Soc. Nephrol. 2019, 14, 1248–1257. [Google Scholar] [CrossRef]
- Felker, G.M.; Ellison, D.H.; Mullens, W.; Cox, Z.L.; Testani, J.M. Diuretic Therapy for Patients with Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1178–1195. [Google Scholar] [CrossRef]
- Voelker, J.R.; Cartwright-Brown, D.; Anderson, S.; Leinfelder, J.; Sica, D.A.; Kokko, J.P.; Brater, D.C. Comparison of loop diuretics in patients with chronic renal insufficiency. Kidney Int. 1987, 32, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; O’Connor, C.M.; Braunwald, E.; Heart Failure Clinical Research Network, I. Loop diuretics in acute decompensated heart failure: Necessary? Evil? A necessary evil? Circ. Heart Fail. 2009, 2, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Lee, K.L.; Bull, D.A.; Redfield, M.M.; Stevenson, L.W.; Goldsmith, S.R.; LeWinter, M.M.; Deswal, A.; Rouleau, J.L.; Ofili, E.O.; et al. Diuretic strategies in patients with acute decompensated heart failure. N. Engl. J. Med. 2011, 364, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, S.E.; Osmanska, J.; Petrie, M.C.; Brooksbank, K.J.M.; Clark, A.L.; Docherty, K.F.; Foley, P.W.X.; Guha, K.; Halliday, C.A.; Jhund, P.S.; et al. Dapagliflozin vs. metolazone in heart failure resistant to loop diuretics. Eur. Heart J. 2023, 44, 2966–2977. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Brisco, M.A.; Turner, J.M.; Spatz, E.S.; Bellumkonda, L.; Parikh, C.R.; Tang, W.H. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ. Heart Fail. 2014, 7, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.H.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Grodin, J.L.; Stevens, S.R.; de Las Fuentes, L.; Kiernan, M.; Birati, E.Y.; Gupta, D.; Bart, B.A.; Felker, G.M.; Chen, H.H.; Butler, J.; et al. Intensification of Medication Therapy for Cardiorenal Syndrome in Acute Decompensated Heart Failure. J. Card. Fail. 2016, 22, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Hanberg, J.S.; Cheng, S.; Rao, V.; Onyebeke, C.; Laur, O.; Kula, A.; Chen, M.; Wilson, F.P.; Darlington, A.; et al. Rapid and Highly Accurate Prediction of Poor Loop Diuretic Natriuretic Response in Patients with Heart Failure. Circ. Heart Fail. 2016, 9, e002370. [Google Scholar] [CrossRef]
- Brinkley, D.M., Jr.; Burpee, L.J.; Chaudhry, S.P.; Smallwood, J.A.; Lindenfeld, J.; Lakdawala, N.K.; Desai, A.S.; Stevenson, L.W. Spot Urine Sodium as Triage for Effective Diuretic Infusion in an Ambulatory Heart Failure Unit. J. Card. Fail. 2018, 24, 349–354. [Google Scholar] [CrossRef]
- Singh, D.; Shrestha, K.; Testani, J.M.; Verbrugge, F.H.; Dupont, M.; Mullens, W.; Tang, W.H. Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. J. Card. Fail. 2014, 20, 392–399. [Google Scholar] [CrossRef]
- Dauw, J.; Charaya, K.; Lelonek, M.; Zegri-Reiriz, I.; Nasr, S.; Paredes-Paucar, C.P.; Borbely, A.; Erdal, F.; Benkouar, R.; Cobo-Marcos, M.; et al. Protocolized Natriuresis-Guided Decongestion Improves Diuretic Response: The Multicenter ENACT-HF Study. Circ. Heart Fail. 2024, 17, e011105. [Google Scholar] [CrossRef] [PubMed]
- ter Maaten, J.M.; Valente, M.A.; Damman, K.; Hillege, H.L.; Navis, G.; Voors, A.A. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nat. Rev. Cardiol. 2015, 12, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, A.E.; Kelley, A.M.; Hempel, C.A.; Loeffler, W.J.; Echtenkamp, C.A.; Hassan, A.K. Discharge Diuretic Dose and 30-Day Readmission Rate in Acute Decompensated Heart Failure. Ann. Pharmacother. 2016, 50, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Schale, S.; Brambatti, M.; You, H.; Hansen, P.; McCain, J.; Lin, J.; Greenberg, B. Impact of transitioning patients to oral diuretics 24 hours before discharge from heart failure hospitalization on 30 day outcomes. Int. J. Cardiol. 2022, 364, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Ivey-Miranda, J.B.; Rao, V.S.; Cox, Z.L.; Moreno-Villagomez, J.; Mahoney, D.; Maulion, C.; Bellumkonda, L.; Turner, J.M.; Collins, S.; Wilson, F.P.; et al. In-Hospital Observation on Oral Diuretics after Treatment for Acute Decompensated Heart Failure: Evaluating the Utility. Circ. Heart Fail. 2023, 16, e010206. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Strait, K.M.; Dharmarajan, K.; Partovian, C.; Coca, S.G.; Kim, N.; Li, S.X.; Testani, J.M.; Khan, U.; Krumholz, H.M. Dominance of furosemide for loop diuretic therapy in heart failure: Time to revisit the alternatives? J. Am. Coll. Cardiol. 2013, 61, 1549–1550. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Gamba, G.; Jaquet, F.; Hess, B. Torasemide vs. furosemide in primary care patients with chronic heart failure NYHA II to IV—Efficacy and quality of life. Eur. J. Heart Fail. 2003, 5, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Cosin, J.; Diez, J.; TORIC investigators. Torasemide in chronic heart failure: Results of the TORIC study. Eur. J. Heart Fail. 2002, 4, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Mentz, R.J.; Anstrom, K.J.; Eisenstein, E.L.; Sapp, S.; Greene, S.J.; Morgan, S.; Testani, J.M.; Harrington, A.H.; Sachdev, V.; Ketema, F.; et al. Effect of Torsemide vs Furosemide after Discharge on All-Cause Mortality in Patients Hospitalized with Heart Failure: The TRANSFORM-HF Randomized Clinical Trial. JAMA 2023, 329, 214–223. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef]
- Pellicori, P.; Cleland, J.G.; Zhang, J.; Kallvikbacka-Bennett, A.; Urbinati, A.; Shah, P.; Kazmi, S.; Clark, A.L. Cardiac Dysfunction, Congestion and Loop Diuretics: Their Relationship to Prognosis in Heart Failure. Cardiovasc. Drugs Ther. 2016, 30, 599–609. [Google Scholar] [CrossRef]
- Rohde, L.E.; Rover, M.M.; Figueiredo Neto, J.A.; Danzmann, L.C.; Bertoldi, E.G.; Simoes, M.V.; Silvestre, O.M.; Ribeiro, A.L.P.; Moura, L.Z.; Beck-da-Silva, L.; et al. Short-term diuretic withdrawal in stable outpatients with mild heart failure and no fluid retention receiving optimal therapy: A double-blind, multicentre, randomized trial. Eur. Heart J. 2019, 40, 3605–3612. [Google Scholar] [CrossRef]
- Croset, F.; Llacer, P.; Nunez, J.; Campos, J.; Garcia, M.; Perez, A.; Fernandez, C.; Fabregate, M.; Lopez, G.; Tello, S.; et al. Loop diuretic down-titration at discharge in patients hospitalized for acute heart failure. ESC Heart Fail. 2024, 11, 1739–1747. [Google Scholar] [CrossRef]
- Girerd, N.; Mewton, N.; Tartiere, J.M.; Guijarro, D.; Jourdain, P.; Damy, T.; Lamblin, N.; Bayes-Genis, A.; Pellicori, P.; Januzzi, J.L.; et al. Practical outpatient management of worsening chronic heart failure. Eur. J. Heart Fail. 2022, 24, 750–761. [Google Scholar] [CrossRef]
- Buckley, L.F.; Stevenson, L.W.; Cooper, I.M.; Knowles, D.M.; Matta, L.; Molway, D.W.; Navarro-Velez, K.; Rhoten, M.N.; Shea, E.L.; Stern, G.M.; et al. Ambulatory Treatment of Worsening Heart Failure with Intravenous Loop Diuretics: A Four-Year Experience. J. Card. Fail. 2020, 26, 798–799. [Google Scholar] [CrossRef]
- Buckley, L.F.; Carter, D.M.; Matta, L.; Cheng, J.W.; Stevens, C.; Belenkiy, R.M.; Burpee, L.J.; Young, M.A.; Weiffenbach, C.S.; Smallwood, J.A.; et al. Intravenous Diuretic Therapy for the Management of Heart Failure and Volume Overload in a Multidisciplinary Outpatient Unit. JACC Heart Fail. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Wierda, E.; Dickhoff, C.; Handoko, M.L.; Oosterom, L.; Kok, W.E.; de Rover, Y.; de Mol, B.; van Heerebeek, L.; Schroeder-Tanka, J.M. Outpatient treatment of worsening heart failure with intravenous and subcutaneous diuretics: A systematic review of the literature. ESC Heart Fail. 2020, 7, 892–902. [Google Scholar] [CrossRef]
- Welling, P.G. Pharmacokinetics of the thiazide diuretics. Biopharm. Drug Dispos. 1986, 7, 501–535. [Google Scholar] [CrossRef]
- Jentzer, J.C.; DeWald, T.A.; Hernandez, A.F. Combination of loop diuretics with thiazide-type diuretics in heart failure. J. Am. Coll. Cardiol. 2010, 56, 1527–1534. [Google Scholar] [CrossRef]
- Cox, Z.L.; Hung, R.; Lenihan, D.J.; Testani, J.M. Diuretic Strategies for Loop Diuretic Resistance in Acute Heart Failure: The 3T Trial. JACC Heart Fail. 2020, 8, 157–168. [Google Scholar] [CrossRef]
- Trullas, J.C.; Morales-Rull, J.L.; Casado, J.; Carrera-Izquierdo, M.; Sanchez-Marteles, M.; Conde-Martel, A.; Davila-Ramos, M.F.; Llacer, P.; Salamanca-Bautista, P.; Perez-Silvestre, J.; et al. Combining loop with thiazide diuretics for decompensated heart failure: The CLOROTIC trial. Eur. Heart J. 2023, 44, 411–421. [Google Scholar] [CrossRef]
- Brisco-Bacik, M.A.; Ter Maaten, J.M.; Houser, S.R.; Vedage, N.A.; Rao, V.; Ahmad, T.; Wilson, F.P.; Testani, J.M. Outcomes Associated with a Strategy of Adjuvant Metolazone or High-Dose Loop Diuretics in Acute Decompensated Heart Failure: A Propensity Analysis. J. Am. Heart Assoc. 2018, 7, e009149. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin. Ther. Pat. 2018, 28, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S.; Testani, J.M.; Pitt, B. Pathophysiology of Diuretic Resistance and Its Implications for the Management of Chronic Heart Failure. Hypertension 2020, 76, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Meekers, E.; Dauw, J.; Martens, P.; Dhont, S.; Verbrugge, F.H.; Nijst, P.; Ter Maaten, J.M.; Damman, K.; Mebazaa, A.; Filippatos, G.; et al. Renal function and decongestion with acetazolamide in acute decompensated heart failure: The ADVOR trial. Eur. Heart J. 2023, 44, 3672–3682. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Verbrugge, F.H.; Dauw, J.; Nijst, P.; Meekers, E.; Augusto, S.N.; Ter Maaten, J.M.; Heylen, L.; Damman, K.; Mebazaa, A.; et al. Pre-treatment bicarbonate levels and decongestion by acetazolamide: The ADVOR trial. Eur. Heart J. 2023, 44, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am. J. Cardiol. 1996, 78, 902–907. [CrossRef]
- Bansal, S.; Lindenfeld, J.; Schrier, R.W. Sodium retention in heart failure and cirrhosis: Potential role of natriuretic doses of mineralocorticoid antagonist? Circ. Heart Fail. 2009, 2, 370–376. [Google Scholar] [CrossRef]
- Butler, J.; Anstrom, K.J.; Felker, G.M.; Givertz, M.M.; Kalogeropoulos, A.P.; Konstam, M.A.; Mann, D.L.; Margulies, K.B.; McNulty, S.E.; Mentz, R.J.; et al. Efficacy and Safety of Spironolactone in Acute Heart Failure: The ATHENA-HF Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.; Xue, Y.; van Veldhuisen, D.J.; Voors, A.A.; Jaarsma, T.; Pang, P.S.; Butler, J.; Pitt, B.; Clopton, P.; de Boer, R.A. Effect of spironolactone on 30-day death and heart failure rehospitalization (from the COACH Study). Am. J. Cardiol. 2014, 114, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Stockand, J.D. Vasopressin regulation of renal sodium excretion. Kidney Int. 2010, 78, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am. J. Cardiol. 2005, 95, 8B–13B. [Google Scholar] [CrossRef]
- Konstam, M.A.; Gheorghiade, M.; Burnett, J.C., Jr.; Grinfeld, L.; Maggioni, A.P.; Swedberg, K.; Udelson, J.E.; Zannad, F.; Cook, T.; Ouyang, J.; et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA 2007, 297, 1319–1331. [Google Scholar] [CrossRef]
- Jujo, K.; Saito, K.; Ishida, I.; Furuki, Y.; Kim, A.; Suzuki, Y.; Sekiguchi, H.; Yamaguchi, J.; Ogawa, H.; Hagiwara, N. Randomized pilot trial comparing tolvaptan with furosemide on renal and neurohumoral effects in acute heart failure. ESC Heart Fail. 2016, 3, 177–188. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S.; et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef]
- Mondritzki, T.; Mai, T.A.; Vogel, J.; Pook, E.; Wasnaire, P.; Schmeck, C.; Huser, J.; Dinh, W.; Truebel, H.; Kolkhof, P. Cardiac output improvement by pecavaptan: A novel dual-acting vasopressin V1a/V2 receptor antagonist in experimental heart failure. Eur. J. Heart Fail. 2021, 23, 743–750. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Norton, L.; DeFronzo, R.A. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am. J. Physiol. Renal. Physiol. 2015, 309, F889–F900. [Google Scholar] [CrossRef]
- Marton, A.; Saffari, S.E.; Rauh, M.; Sun, R.N.; Nagel, A.M.; Linz, P.; Lim, T.T.; Takase-Minegishi, K.; Pajarillaga, A.; Saw, S.; et al. Water Conservation Overrides Osmotic Diuresis During SGLT2 Inhibition in Patients with Heart Failure. J. Am. Coll. Cardiol. 2024, 83, 1386–1398. [Google Scholar] [CrossRef]
- Borges-Junior, F.A.; Silva Dos Santos, D.; Benetti, A.; Polidoro, J.Z.; Wisnivesky, A.C.T.; Crajoinas, R.O.; Antonio, E.L.; Jensen, L.; Caramelli, B.; Malnic, G.; et al. Empagliflozin Inhibits Proximal Tubule NHE3 Activity, Preserves GFR, and Restores Euvolemia in Nondiabetic Rats with Induced Heart Failure. J. Am. Soc. Nephrol. 2021, 32, 1616–1629. [Google Scholar] [CrossRef]
- Schulze, P.C.; Bogoviku, J.; Westphal, J.; Aftanski, P.; Haertel, F.; Grund, S.; von Haehling, S.; Schumacher, U.; Mobius-Winkler, S.; Busch, M. Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients with Acute Decompensated Heart Failure (EMPAG-HF). Circulation 2022, 146, 289–298. [Google Scholar] [CrossRef]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Cox, Z.L.; Collins, S.P.; Hernandez, G.A.; McRae, A.T., 3rd; Davidson, B.T.; Adams, K.; Aaron, M.; Cunningham, L.; Jenkins, C.A.; Lindsell, C.J.; et al. Efficacy and Safety of Dapagliflozin in Patients with Acute Heart Failure. J. Am. Coll. Cardiol. 2024, 83, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Manzi, G.; Pierucci, N.; Laviola, D.; Piro, A.; D’Amato, A.; Filomena, D.; Matteucci, A.; Severino, P.; Miraldi, F.; et al. SGLT2i effect on atrial fibrillation: A network meta-analysis of randomized controlled trials. J. Cardiovasc. Electrophysiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022, 102, S1–S127. [Google Scholar] [CrossRef]
- Califf, R.M.; Adams, K.F.; McKenna, W.J.; Gheorghiade, M.; Uretsky, B.F.; McNulty, S.E.; Darius, H.; Schulman, K.; Zannad, F.; Handberg-Thurmond, E.; et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST). Am. Heart J. 1997, 134, 44–54. [Google Scholar] [CrossRef]
- Packer, M.; Carver, J.R.; Rodeheffer, R.J.; Ivanhoe, R.J.; DiBianco, R.; Zeldis, S.M.; Hendrix, G.H.; Bommer, W.J.; Elkayam, U.; Kukin, M.L.; et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N. Engl. J. Med. 1991, 325, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Ichai, C.; Soubielle, J.; Carles, M.; Giunti, C.; Grimaud, D. Comparison of the renal effects of low to high doses of dopamine and dobutamine in critically ill patients: A single-blind randomized study. Crit. Care Med. 2000, 28, 921–928. [Google Scholar] [CrossRef]
- Chen, H.H.; Anstrom, K.J.; Givertz, M.M.; Stevenson, L.W.; Semigran, M.J.; Goldsmith, S.R.; Bart, B.A.; Bull, D.A.; Stehlik, J.; LeWinter, M.M.; et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA 2013, 310, 2533–2543. [Google Scholar] [CrossRef]
- Wan, S.H.; Stevens, S.R.; Borlaug, B.A.; Anstrom, K.J.; Deswal, A.; Felker, G.M.; Givertz, M.M.; Bart, B.A.; Tang, W.H.; Redfield, M.M.; et al. Differential Response to Low-Dose Dopamine or Low-Dose Nesiritide in Acute Heart Failure with Reduced or Preserved Ejection Fraction: Results From the ROSE AHF Trial (Renal Optimization Strategies Evaluation in Acute Heart Failure). Circ. Heart Fail. 2016, 9, e002593. [Google Scholar] [CrossRef]
- Cuffe, M.S.; Califf, R.M.; Adams, K.F., Jr.; Benza, R.; Bourge, R.; Colucci, W.S.; Massie, B.M.; O’Connor, C.M.; Pina, I.; Quigg, R.; et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA 2002, 287, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, F.; Damman, K.; Nalbantgil, S.; Van Laake, L.W.; Tops, L.F.; Thum, T.; Adamopoulos, S.; Bonios, M.; Coats, A.J.; Crespo-Leiro, M.G.; et al. Inotropic therapy in patients with advanced heart failure. A clinical consensus statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2023, 25, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N. Engl. J. Med. 1997, 336, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, Z.D.; Goldberger, A.L. Therapeutic ranges of serum digoxin concentrations in patients with heart failure. Am. J. Cardiol. 2012, 109, 1818–1821. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.; Su, C.Y.; Chan, A.L.; Lian, P.W.; Leu, H.B.; Hsu, Y.J. Risk of digoxin intoxication in heart failure patients exposed to digoxin-diuretic interactions: A population-based study. Br. J. Clin. Pharmacol. 2010, 70, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, J.R.; Diaz, R.; Felker, G.M.; McMurray, J.J.V.; Metra, M.; Solomon, S.D.; Adams, K.F.; Anand, I.; Arias-Mendoza, A.; Biering-Sorensen, T.; et al. Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. N. Engl. J. Med. 2021, 384, 105–116. [Google Scholar] [CrossRef]
- Abraham, W.T.; Kuck, K.H.; Goldsmith, R.L.; Lindenfeld, J.; Reddy, V.Y.; Carson, P.E.; Mann, D.L.; Saville, B.; Parise, H.; Chan, R.; et al. A Randomized Controlled Trial to Evaluate the Safety and Efficacy of Cardiac Contractility Modulation. JACC Heart Fail. 2018, 6, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F. Regulation of Potassium Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Quamme, G.A. Control of magnesium transport in the thick ascending limb. Am. J. Physiol. 1989, 256 Pt 2, F197–F210. [Google Scholar] [CrossRef]
- Milionis, H.J.; Alexandrides, G.E.; Liberopoulos, E.N.; Bairaktari, E.T.; Goudevenos, J.; Elisaf, M.S. Hypomagnesemia and concurrent acid-base and electrolyte abnormalities in patients with congestive heart failure. Eur. J. Heart Fail. 2002, 4, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.; Epstein, F.H. Signs and symptoms of electrolyte disorders. Yale J. Biol. Med. 1970, 43, 76–109. [Google Scholar] [PubMed]
- Ferreira, J.P.; Butler, J.; Rossignol, P.; Pitt, B.; Anker, S.D.; Kosiborod, M.; Lund, L.H.; Bakris, G.L.; Weir, M.R.; Zannad, F. Abnormalities of Potassium in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2836–2850. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, P.; Dobre, D.; McMurray, J.J.; Swedberg, K.; Krum, H.; van Veldhuisen, D.J.; Shi, H.; Messig, M.; Vincent, J.; Girerd, N.; et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: Results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ. Heart Fail. 2014, 7, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Anker, S.D.; Lund, L.H.; Coats, A.J.S.; Filippatos, G.; Siddiqi, T.J.; Friede, T.; Fabien, V.; Kosiborod, M.; Metra, M.; et al. Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: The DIAMOND trial. Eur. Heart J. 2022, 43, 4362–4373. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Rossi, J.S.; Cotts, W.; Shin, D.D.; Hellkamp, A.S.; Pina, I.L.; Fonarow, G.C.; DeMarco, T.; Pauly, D.F.; Rogers, J.; et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch. Intern. Med. 2007, 167, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Steels, P.; Grieten, L.; Nijst, P.; Tang, W.H.; Mullens, W. Hyponatremia in acute decompensated heart failure: Depletion versus dilution. J. Am. Coll. Cardiol. 2015, 65, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Leviel, F.; Hubner, C.A.; Houillier, P.; Morla, L.; El Moghrabi, S.; Brideau, G.; Hassan, H.; Parker, M.D.; Kurth, I.; Kougioumtzes, A.; et al. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J. Clin. Investig. 2010, 120, 1627–1635. [Google Scholar] [CrossRef]
- Laureno, R. Central pontine myelinolysis following rapid correction of hyponatremia. Ann. Neurol. 1983, 13, 232–242. [Google Scholar] [CrossRef]
- Shirakabe, A.; Hata, N.; Kobayashi, N.; Okazaki, H.; Matsushita, M.; Shibata, Y.; Nishigoori, S.; Uchiyama, S.; Asai, K.; Shimizu, W. Worsening renal function definition is insufficient for evaluating acute renal failure in acute heart failure. ESC Heart Fail. 2018, 5, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Navis, G.; Voors, A.A.; Asselbergs, F.W.; Smilde, T.D.; Cleland, J.G.; van Veldhuisen, D.J.; Hillege, H.L. Worsening renal function and prognosis in heart failure: Systematic review and meta-analysis. J. Card. Fail. 2007, 13, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S.S.; Abraham, W.; Butler, J.; Forman, D.E.; Loh, E.; Massie, B.M.; O’Connor, C.M.; Rich, M.W.; Stevenson, L.W.; Young, J.; et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J. Card. Fail. 2002, 8, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Valente, M.A.; Voors, A.A.; O’Connor, C.M.; van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Loungani, R.; Doerfler, S.M.; Coles, A.; Greene, S.J.; Cooper, L.B.; Fiuzat, M.; O’Connor, C.M.; Rogers, J.G.; Mentz, R.J. Worsening renal function during decongestion among patients hospitalized for heart failure: Findings from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial. Am. Heart J. 2018, 204, 163–173. [Google Scholar] [CrossRef]
- Brisco, M.A.; Zile, M.R.; Hanberg, J.S.; Wilson, F.P.; Parikh, C.R.; Coca, S.G.; Tang, W.H.; Testani, J.M. Relevance of Changes in Serum Creatinine During a Heart Failure Trial of Decongestive Strategies: Insights From the DOSE Trial. J. Card. Fail. 2016, 22, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, H.; Dresser, T.; Aggarwal, K. Acute effect of furosemide on glomerular filtration rate in diastolic dysfunction. Ren. Fail. 2007, 29, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Jackson, K.; Rao, V.S.; Tang, W.H.W.; Brisco-Bacik, M.A.; Chen, H.H.; Felker, G.M.; Hernandez, A.F.; O’Connor, C.M.; Sabbisetti, V.S.; et al. Worsening Renal Function in Patients with Acute Heart Failure Undergoing Aggressive Diuresis Is Not Associated with Tubular Injury. Circulation 2018, 137, 2016–2028. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Skouri, H.; Verbrugge, F.H.; Fudim, M.; Iacoviello, M.; Franke, J.; Flammer, A.J.; et al. Renal effects of guideline-directed medical therapies in heart failure: A consensus document from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Mathew, J.; Lerman, L.O.; Lieske, J.C.; Larson, J.J.; Alexander, M.P.; Poggio, E.; Glassock, R.J.; Rule, A.D. Single-Nephron Glomerular Filtration Rate in Healthy Adults. N. Engl. J. Med. 2017, 376, 2349–2357. [Google Scholar] [CrossRef]
- Shirakabe, A.; Hata, N.; Kobayashi, N.; Okazaki, H.; Matsushita, M.; Shibata, Y.; Uchiyama, S.; Sawatani, T.; Asai, K.; Shimizu, W. Worsening renal failure in patients with acute heart failure: The importance of cardiac biomarkers. ESC Heart Fail. 2019, 6, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Spieker, L.E.; Ruschitzka, F.T.; Luscher, T.F.; Noll, G. The management of hyperuricemia and gout in patients with heart failure. Eur. J. Heart Fail. 2002, 4, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Bruderer, S.; Bodmer, M.; Jick, S.S.; Meier, C.R. Use of diuretics and risk of incident gout: A population-based case-control study. Arthritis Rheumatol. 2014, 66, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Ritter, F.; Franzeck, F.; Geisshardt, J.; Walker, U.A.; Osthoff, M. Gout Arthritis During Admission for Decompensated Heart Failure-A Descriptive Analysis of Risk Factors, Treatment and Prognosis. Front. Med. 2022, 9, 789414. [Google Scholar] [CrossRef] [PubMed]
- Drug and Therapeutics Bulletin. Latest guidance on the management of gout. BMJ 2018, 362, k2893. [Google Scholar] [CrossRef]
- Khan, D.A.; Knowles, S.R.; Shear, N.H. Sulfonamide Hypersensitivity: Fact and Fiction. J. Allergy Clin. Immunol. Pract. 2019, 7, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Onesti, G.; Moyer, J.H.; Swartz, C. Ethacrynic acid and furosemide. Diuretic and hemodynamic effects and clinical uses. Am. J. Cardiol. 1971, 27, 407–415. [Google Scholar] [CrossRef]
- Strom, B.L.; Schinnar, R.; Apter, A.J.; Margolis, D.J.; Lautenbach, E.; Hennessy, S.; Bilker, W.B.; Pettitt, D. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N. Engl. J. Med. 2003, 349, 1628–1635. [Google Scholar] [CrossRef]
- Ding, D.; Liu, H.; Qi, W.; Jiang, H.; Li, Y.; Wu, X.; Sun, H.; Gross, K.; Salvi, R. Ototoxic effects and mechanisms of loop diuretics. J. Otol. 2016, 11, 145–156. [Google Scholar] [CrossRef]
- Brown, C.B.; Ogg, C.S.; Cameron, J.S. High dose frusemide in acute renal failure: A controlled trial. Clin. Nephrol. 1981, 15, 90–96. [Google Scholar]
- Gallagher, K.L.; Jones, J.K. Furosemide-induced ototoxicity. Ann. Intern. Med. 1979, 91, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Salvador, D.R.; Rey, N.R.; Ramos, G.C.; Punzalan, F.E. Continuous infusion versus bolus injection of loop diuretics in congestive heart failure. Cochrane Database Syst. Rev. 2005, 2005, CD003178. [Google Scholar] [CrossRef] [PubMed]
- McAlister, F.A.; Ezekowitz, J.; Tarantini, L.; Squire, I.; Komajda, M.; Bayes-Genis, A.; Gotsman, I.; Whalley, G.; Earle, N.; Poppe, K.K.; et al. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: Impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula. Circ. Heart Fail. 2012, 5, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.A. Diuretic use in renal disease. Nat. Rev. Nephrol. 2011, 8, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Kurella Tamura, M.; Feldman, H.I. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Bovee, D.M.; Cuevas, C.A.; Zietse, R.; Danser, A.H.J.; Mirabito Colafella, K.M.; Hoorn, E.J. Salt-sensitive hypertension in chronic kidney disease: Distal tubular mechanisms. Am. J. Physiol. Renal. Physiol. 2020, 319, F729–F745. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Sinha, A.D.; Cramer, A.E.; Balmes-Fenwick, M.; Dickinson, J.H.; Ouyang, F.; Tu, W. Chlorthalidone for Hypertension in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2021, 385, 2507–2519. [Google Scholar] [CrossRef] [PubMed]
- Obi, Y.; Streja, E.; Rhee, C.M.; Ravel, V.; Amin, A.N.; Cupisti, A.; Chen, J.; Mathew, A.T.; Kovesdy, C.P.; Mehrotra, R.; et al. Incremental Hemodialysis, Residual Kidney Function, and Mortality Risk in Incident Dialysis Patients: A Cohort Study. Am. J. Kidney Dis. 2016, 68, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Bargman, J.M.; Thorpe, K.E.; Churchill, D.N. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J. Am. Soc. Nephrol. 2001, 12, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Medcalf, J.F.; Harris, K.P.; Walls, J. Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 2001, 59, 1128–1133. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Ruocco, G.; Severino, P.; Gennari, L.; Pirrotta, F.; Stefanini, A.; Tramonte, F.; Feola, M.; Mancone, M.; Fedele, F. Effects of Metolazone Administration on Congestion, Diuretic Response and Renal Function in Patients with Advanced Heart Failure. J. Clin. Med. 2021, 10, 4207. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.; Sliwa, K. Decompensated Heart Failure in Pregnancy. Card. Fail. Rev. 2016, 2, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bertozzi-Villa, A.; Coggeshall, M.S.; Shackelford, K.A.; Steiner, C.; Heuton, K.R.; Gonzalez-Medina, D.; Barber, R.; Huynh, C.; Dicker, D.; et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 980–1004. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomstrom-Lundqvist, C.; Cifkova, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, U.; Goland, S.; Pieper, P.G.; Silverside, C.K. High-Risk Cardiac Disease in Pregnancy: Part I. J. Am. Coll. Cardiol. 2016, 68, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Halpern, D.G.; Weinberg, C.R.; Pinnelas, R.; Mehta-Lee, S.; Economy, K.E.; Valente, A.M. Use of Medication for Cardiovascular Disease During Pregnancy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Yusuf, S.; Peto, R. Overview of randomised trials of diuretics in pregnancy. Br. Med. J. (Clin. Res. Ed.) 1985, 290, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Turnheim, K. When drug therapy gets old: Pharmacokinetics and pharmacodynamics in the elderly. Exp. Gerontol. 2003, 38, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes with the Aging Kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.W.; Shock, N.W.; DeFronzo, R.A. The influence of age on the renal response to water deprivation in man. Nephron 1976, 17, 270–278. [Google Scholar] [CrossRef]
- Phillips, P.A.; Rolls, B.J.; Ledingham, J.G.; Forsling, M.L.; Morton, J.J.; Crowe, M.J.; Wollner, L. Reduced thirst after water deprivation in healthy elderly men. N. Engl. J. Med. 1984, 311, 753–759. [Google Scholar] [CrossRef]
- Mets, T.F. Drug-induced orthostatic hypotension in older patients. Drugs Aging 1995, 6, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Han, B.; Zhang, M.; Liu, J.; Cui, Y.; Jiang, H. The association between diuretics and falls in older adults: A systematic review and meta-analysis. Geriatr. Nurs. 2023, 52, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Heerdink, E.R.; Leufkens, H.G.; Herings, R.M.; Ottervanger, J.P.; Stricker, B.H.; Bakker, A. NSAIDs associated with increased risk of congestive heart failure in elderly patients taking diuretics. Arch. Intern. Med. 1998, 158, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.M.; Panjrath, G.S.; Amancherla, K.; Davis, L.L.; Deswal, A.; Dixon, D.L.; Januzzi, J.L., Jr.; Yancy, C.W. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure with Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2023, 81, 1835–1878. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Dong, B.; Xue, R.; Zhao, J.; Wu, Z.; Wu, Y.; Zhou, Y.; Wu, D.; Dong, Y.; He, J.; et al. Effect of aggressive diuresis in acute heart failure with reduced and preserved ejection fraction. ESC Heart Fail. 2021, 8, 3248–3256. [Google Scholar] [CrossRef]
- Abramov, D.; Cohen, R.S.; Katz, S.D.; Mancini, D.; Maurer, M.S. Comparison of blood volume characteristics in anemic patients with low versus preserved left ventricular ejection fractions. Am. J. Cardiol. 2008, 102, 1069–1072. [Google Scholar] [CrossRef]
- Iglesias-Garriz, I.; Olalla-Gomez, C.; Garrote, C.; Lopez-Benito, M.; Martin, J.; Alonso, D.; Rodriguez, M.A. Contribution of right ventricular dysfunction to heart failure mortality: A meta-analysis. Rev. Cardiovasc. Med. 2012, 13, e62–e69. [Google Scholar] [CrossRef] [PubMed]
- Gorter, T.M.; van Veldhuisen, D.J.; Bauersachs, J.; Borlaug, B.A.; Celutkiene, J.; Coats, A.J.S.; Crespo-Leiro, M.G.; Guazzi, M.; Harjola, V.P.; Heymans, S.; et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: Mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, J.; Akkan, D.; Iversen, K.K.; Kober, L.; Torp-Pedersen, C.; Hassager, C. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur. J. Heart Fail. 2007, 9, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.P.; Mebazaa, A.; Celutkiene, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, M.; Huber, L.C.; Winnik, S.; Mikulicic, F.; Guidetti, F.; Frank, M.; Flammer, A.J.; Ruschitzka, F. Right Ventricular Failure: Pathophysiology, Diagnosis and Treatment. Card. Fail. Rev. 2019, 5, 140–146. [Google Scholar] [CrossRef]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart. Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Baudry, G.; Coutance, G.; Dorent, R.; Bauer, F.; Blanchart, K.; Boignard, A.; Chabanne, C.; Delmas, C.; D’Ostrevy, N.; Epailly, E.; et al. Diuretic dose is a strong prognostic factor in ambulatory patients awaiting heart transplantation. ESC Heart Fail. 2023, 10, 2843–2852. [Google Scholar] [CrossRef] [PubMed]
- Bellet, M.; Cabrol, C.; Sassano, P.; Leger, P.; Corvol, P.; Menard, J. Systemic hypertension after cardiac transplantation: Effect of cyclosporine on the renin-angiotensin-aldosterone system. Am. J. Cardiol. 1985, 56, 927–931. [Google Scholar] [CrossRef]
- Braith, R.W.; Mills, R.M., Jr.; Wilcox, C.S.; Davis, G.L.; Wood, C.E. Breakdown of blood pressure and body fluid homeostasis in heart transplant recipients. J. Am. Coll. Cardiol. 1996, 27, 375–383. [Google Scholar] [CrossRef]
- Niederstadt, C.; Steinhoff, J.; Erbsloh-Moller, B.; Renner, F.; Sack, K.; Rob, P.M. Effect of FK506 on magnesium homeostasis after renal transplantation. Transplant. Proc. 1997, 29, 3161–3162. [Google Scholar] [CrossRef]
- Arthur, J.M.; Shamim, S. Interaction of cyclosporine and FK506 with diuretics in transplant patients. Kidney Int. 2000, 58, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.H. Diuretic therapy and resistance in congestive heart failure. Cardiology 2001, 96, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Trullas, J.C.; Casado, J.; Morales-Rull, J.L.; Formiga, F.; Conde-Martel, A.; Quiros, R.; Epelde, F.; Gonzalez-Franco, A.; Manzano, L.; Montero-Perez-Barquero, M. Prevalence and outcome of diuretic resistance in heart failure. Intern. Emerg. Med. 2019, 14, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.A.; Ferguson, T.W.; Tangri, N. A Machine Learning Model to Predict Diuretic Resistance. Kidney360 2023, 4, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Aznarez, S.; Campos-Saenz de Santamaria, A.; Sanchez-Marteles, M.; Garces-Horna, V.; Josa-Laorden, C.; Gimenez-Lopez, I.; Perez-Calvo, J.I.; Rubio-Gracia, J. The Association Between Intra-abdominal Pressure and Diuretic Response in Heart Failure. Curr. Heart Fail. Rep. 2023, 20, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Kitsios, G.D.; Mascari, P.; Ettunsi, R.; Gray, A.W. Co-administration of furosemide with albumin for overcoming diuretic resistance in patients with hypoalbuminemia: A meta-analysis. J. Crit. Care 2014, 29, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Emmett, M. Metabolic Alkalosis: A Brief Pathophysiologic Review. Clin. J. Am. Soc. Nephrol. 2020, 15, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, J.J.; Bhandari, S.; Clark, A.L. Hypochloraemia in Patients with Heart Failure: Causes and Consequences. Cardiol. Ther. 2020, 9, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Hanberg, J.S.; Rao, V.; Ter Maaten, J.M.; Laur, O.; Brisco, M.A.; Perry Wilson, F.; Grodin, J.L.; Assefa, M.; Samuel Broughton, J.; Planavsky, N.J.; et al. Hypochloremia and Diuretic Resistance in Heart Failure: Mechanistic Insights. Circ. Heart Fail. 2016, 9, e003180. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Lack of durable natriuresis and objective decongestion following SGLT2 inhibition in randomized controlled trials of patients with heart failure. Cardiovasc. Diabetol. 2023, 22, 197. [Google Scholar] [CrossRef]
- Polyzogopoulou, E.; Bezati, S.; Karamasis, G.; Boultadakis, A.; Parissis, J. Early Recognition and Risk Stratification in Cardiogenic Shock: Well Begun Is Half Done. J. Clin. Med. 2023, 12, 2643. [Google Scholar] [CrossRef] [PubMed]
- Girerd, N.; Seronde, M.F.; Coiro, S.; Chouihed, T.; Bilbault, P.; Braun, F.; Kenizou, D.; Maillier, B.; Nazeyrollas, P.; Roul, G.; et al. Integrative Assessment of Congestion in Heart Failure Throughout the Patient Journey. JACC Heart Fail. 2018, 6, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Kociol, R.D.; McNulty, S.E.; Hernandez, A.F.; Lee, K.L.; Redfield, M.M.; Tracy, R.P.; Braunwald, E.; O’Connor, C.M.; Felker, G.M.; NHLBI Heart Failure Network Steering Committee and Investigators. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ. Heart Fail. 2013, 6, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.F., Jr.; Fonarow, G.C.; Emerman, C.L.; LeJemtel, T.H.; Costanzo, M.R.; Abraham, W.T.; Berkowitz, R.L.; Galvao, M.; Horton, D.P.; ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am. Heart J. 2005, 149, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Brisco, M.A.; Chen, J.; McCauley, B.D.; Parikh, C.R.; Tang, W.H. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: Importance of sustained decongestion. J. Am. Coll. Cardiol. 2013, 62, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Chen, J.; McCauley, B.D.; Kimmel, S.E.; Shannon, R.P. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010, 122, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Santaguida, P.L.; Don-Wauchope, A.C.; Oremus, M.; McKelvie, R.; Ali, U.; Hill, S.A.; Balion, C.; Booth, R.A.; Brown, J.A.; Bustamam, A.; et al. BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: A systematic review. Heart Fail. Rev. 2014, 19, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B. Successful Decongestion as a Clinical Target, Performance Indicator, and as a Study Endpoint in Hospitalized Heart Failure Patients. JACC Heart Fail. 2023, 11, 126–129. [Google Scholar] [CrossRef]
- Binanay, C.; Califf, R.M.; Hasselblad, V.; O’Connor, C.M.; Shah, M.R.; Sopko, G.; Stevenson, L.W.; Francis, G.S.; Leier, C.V.; Miller, L.W.; et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA 2005, 294, 1625–1633. [Google Scholar] [CrossRef]
- Lindenfeld, J.; Zile, M.R.; Desai, A.S.; Bhatt, K.; Ducharme, A.; Horstmanshof, D.; Krim, S.R.; Maisel, A.; Mehra, M.R.; Paul, S.; et al. Haemodynamic-guided management of heart failure (GUIDE-HF): A randomised controlled trial. Lancet 2021, 398, 991–1001. [Google Scholar] [CrossRef]

| Trials | Intervention/Medication | Patient Population | Outcome Measured | Conclusions |
|---|---|---|---|---|
| DOES | (2 × 2 factorial design)
|
|
|
|
| CARRESS-HF | Slow continuous ultrafiltration versus stepped pharmacological treatment of loop diuretics. |
|
|
|
| ENACT-HF | Protocolized natriuresis-based diuretics dosing versus standard of care. |
|
|
|
| Transform HF | Torsemide versus furosemide. |
|
|
|
| 3T Trial | Oral metolazone, intravenous chlorothiazide, or tolvaptan therapy in addition to loop diuretics. |
|
|
|
| CLOROTIC | Hydrochlorothiazide versus placebo, in addition to intravenous loop diuretics. |
|
|
|
| EMPAG-HF | Early initiation (day one of hospitalization) of empagliflozin versus placebo in addition to standard medical treatment of acute decompensated heart failure. |
|
|
|
| EMPULSE | Empagliflozin versus placebo, in addition to standard medical treatment of acute decompensated heart failure. |
|
|
|
| DAPA-RESIST | Dapagliflozin versus metolazone for heart failure with diuretic resistance. |
|
|
|
| DICTATE-AHF | Early initiation of dapagliflozin versus placebo in addition to standard medical treatment of acute decompensated heart failure. |
|
|
|
| ADVOR | Intravenous administration of acetazolamide (500 mg daily) versus placebo in addition to standardized intravenous loop diuretics. |
|
|
|
| ATHENA-HF | High-dose spironolactone versus low-dose or placebo for acute decompensated heart failure patient. |
|
|
|
| ROSE HF | Low-dose dopamine (2 μg/kg/min) or low-dose nesiritide (0.005 μg/kg/min without bolus) in addition to standard diuresis. |
|
|
|
| OPTIME-CHF | Short-term use of milrinone versus placebo in addition to standard therapy. |
|
|
|
| CHAMPION | Implantable pulmonary artery pressure monitors to guide the medical management of patients with heart failure. |
|
|
|
| Cause of Diuretic Resistance | Mechanism | Strategy to Overcome Diuretic Resistance |
|---|---|---|
| Prerenal cause | ||
| Venous congestion, intestinal edema | Reduced absorption of loop diuretics from the gastrointestinal tract; Venous congestion causes increases in renal venous pressure, leading to a drop in glomerular filtration rate. | Use IV diuretics or diuretics that have better bioavailability. |
| Increased abdominal pressure | A rise in intrabdominal pressure causes increases in renal venous pressure, leading to a drop in Glomerular filtration rate. | Address constipation, urinary retention, and significant ascites. |
| Low cardiac output | Low cardiac output decreases kidney. | Inotropes, vasodilators, advanced therapy such as left ventricular assist device. |
| Hypoalbuminemia | Loop diuretics are highly protein-bound. Hypoalbuminemia reduces the level of active form of loop diuretics. | Albumin infusion (poor effect) improves nutrition. |
| Intrarenal cause | ||
| Proximal tubular injury | Reduced delivery of loop diuretics to target site. | Treat underlying etiology of acute tubular injury. |
| Neurohormonal activation | Extracellular fluid volume contraction and activation of the renin–angiotensin–aldosterone system promotes the proximal solute reabsorption and decrease the delivery of sodium and chloride to the distal tubule. | Carbonic anhydrase inhibitor. Sodium-glucose cotransporter-2 inhibitors. Mineralocorticoid receptor antagonists and other guideline-directed medical therapy. |
| Compensatory reabsorption in distal convoluted tubule or collecting tubule | Epithelial cell of distal tubule undergoes both hypertrophy and hyperplasia in response to loop diuretics that increases solute delivery to distal segments. | Thiazide diuretics; Mineralocorticoid receptor antagonists |
| Free water excretion impairment | Excessive vasopressin leads to significant water retention and severe hyponatremia. | Vasopressin antagonist. |
| Albuminuria | Furosemide can bind to albumin within the tubular lumen, which reduces the level of active and unbound drug that is capable of binding to the tubular receptor. | Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, mineralocorticoid receptor antagonists. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Rodriguez, M.; El Hachem, K.; Krittanawong, C. Diuretic Treatment in Heart Failure: A Practical Guide for Clinicians. J. Clin. Med. 2024, 13, 4470. https://doi.org/10.3390/jcm13154470
Wu L, Rodriguez M, El Hachem K, Krittanawong C. Diuretic Treatment in Heart Failure: A Practical Guide for Clinicians. Journal of Clinical Medicine. 2024; 13(15):4470. https://doi.org/10.3390/jcm13154470
Chicago/Turabian StyleWu, Lingling, Mario Rodriguez, Karim El Hachem, and Chayakrit Krittanawong. 2024. "Diuretic Treatment in Heart Failure: A Practical Guide for Clinicians" Journal of Clinical Medicine 13, no. 15: 4470. https://doi.org/10.3390/jcm13154470






