Current Concepts in the Treatment of Early Onset Scoliosis
Abstract
:1. Background
2. Non-Operative Treatment
Casting Technique
3. Bracing
4. Preoperative Evaluation
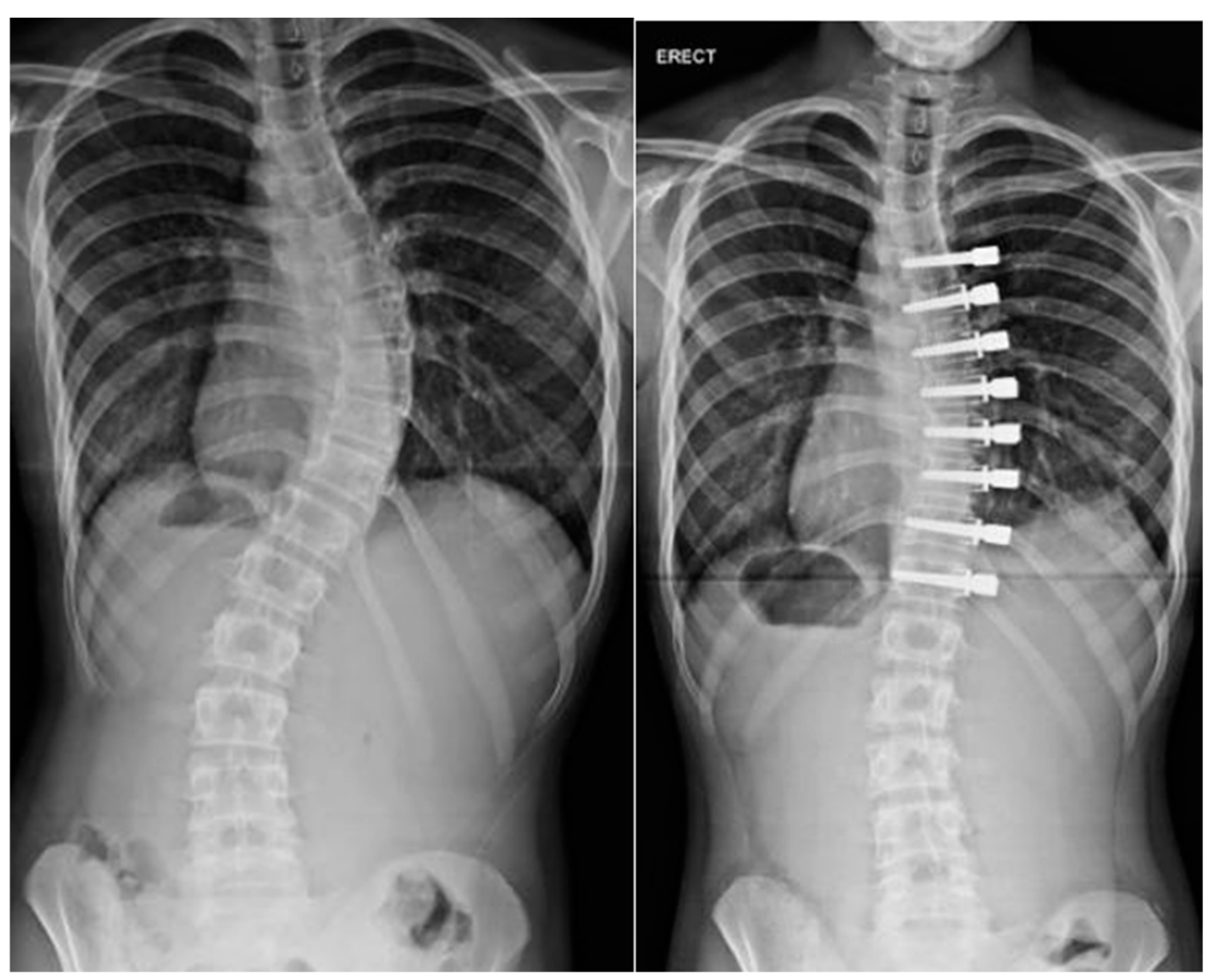
5. Operative Treatment
6. Distraction-Based Methods
7. Magnetically Controlled Growing Rods
8. VEPTR
9. Spring Distraction
10. Growth Modulation Methods
11. Definitive Fusion
12. Conclusions
- The C-EOS is a useful tool in categorizing and understanding risk in patients with early onset scoliosis.
- Recent expert consensus was reached for conservative management in all patients aged 3 or younger with preference to casting and has reinforced avoidance of surgery whenever possible.
- Medical optimization of children with EOS is of utmost importance prior to any surgical management with particular attention to nutrition and pulmonary strategies.
- Consensus for surgical intervention was reached for EOS patients older in age (6- or 9-year-olds) with increased (30°) curve progression, greater curve degree, and those with rigid curves. Distraction-based systems remain highly favored in young patients with less enthusiasm in older children [70].
Author Contributions
Funding
Conflicts of Interest
References
- Williams, B.A.; Matsumoto, H.; McCalla, D.J.; Akbarnia, B.A.; Blakemore, L.C.; Betz, R.R.; Flynn, J.M.; Johnston, C.E.; McCarthy, R.E.; Roye, D.P., Jr.; et al. Development and initial validation of the Classification of Early-Onset Scoliosis (C-EOS). J. Bone Jt. Surg. Am. 2014, 96, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Cyr, M.; Hilaire, T.S.; Pan, Z.; Thompson, G.H.; Vitale, M.G.; Garg, S. Classification of early onset scoliosis has excellent interobserver and intraobserver reliability. J. Pediatr. Orthop. 2017, 37, e1–e3. [Google Scholar] [CrossRef]
- Mehta, M.H. Growth as a corrective force in the early treatment of progressive infantile scoliosis. J. Bone Jt. Surg. Br. 2005, 87, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, G.T.; D’Astous, J.L.; Nielson, A.N.; MacWilliams, B.A.; Heflin, J.A. Minimum 5-Year Follow-up of Mehta Casting to Treat Idiopathic Early-Onset Scoliosis. J. Bone Jt. Surg. Am. 2019, 101, 1530–1538. [Google Scholar] [CrossRef]
- Fedorak, G.T.; MacWilliams, B.A.; Stasikelis, P.; Szczodry, M.; Lerman, J.; Pahys, J.M.; D’Astous, J. Age-Stratified Outcomes of Mehta Casting in Idiopathic Early-Onset Scoliosis: A Multicenter Review. J. Bone Jt. Surg. Am. 2022, 104, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Welborn, M.C.; D’Astous, J.; Bratton, S.; Heflin, J. Infantile Idiopathic Scoliosis: Factors Affecting EDF Casting Success. Spine Deform. 2018, 6, 614–620. [Google Scholar] [CrossRef]
- Demirkiran, H.G.; Bekmez, S.; Celilov, R.; Ayvaz, M.; Dede, O.; Yazici, M. Serial derotational casting in congenital scoliosis as a time-buying strategy. J. Pediatr. Orthop. 2015, 35, 43–49. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, X.-J.; Sun, N.; Sun, L.; Guo, D.; Qi, X.-Y.; Bai, Y.-S.; Sun, B.-S. The therapeutic characteristics of serial casting on congenital scoliosis: A comparison with non-congenital cases from a single-center experience. J. Orthop. Surg. Res. 2017, 12, 56. [Google Scholar] [CrossRef]
- LaValva, S.A.A.; MacAlpine, E.; Gupta, P.; Hammerberg, K.; Thompson, G.H.; Sturm, P.; Garg, S.; Anari, J.; Sponseller, P.; Flynn, J.; et al. Serial Casting in Neuromuscular and Syndromic Early-onset Scoliosis (EOS) Can Delay Surgery Over 2 Years. J. Pediatr. Orthop. 2020, 40, e772–e779. [Google Scholar] [CrossRef]
- Fletcher, N.D.; McClung, A.; Rathjen, K.E.; Denning, J.R.; Browne, R.; Johnston, C.E., 3rd. Serial casting as a delay tactic in the treatment of moderate-to-severe early-onset scoliosis. J. Pediatr. Orthop. 2012, 32, 664–671. [Google Scholar] [CrossRef]
- Gomez, J.A.; Grzywna, A.; Miller, P.E.; Karlin, L.I.; Garg, S.; Sanders, J.O.; Sturm, P.F.; Sponseller, P.D.; D’Astous, J.L.; Glotzbecker, M.P. Initial Cast Correction as a Predictor of Treatment Outcome Success for Infantile Idiopathic Scoliosis. J. Pediatr. Orthop. 2017, 37, e625–e630. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, G.T.; Stasikelis, P.J.; Carpenter, A.M.; Nielson, A.N.; D’Astous, J.L. Optimization of Casting in Early-onset Scoliosis. J. Pediatr. Orthop. 2019, 39, e303–e307. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, B.K.; Tileston, K.; Kaur, J.; Kym, D.; Segovia, N.A.; Imrie, M.; Policy, J.; Rinsky, L.; Vorhies, J. Innovative technique for early-onset scoliosis casting using Jackson table. Spine Deform. 2022, 10, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- LaValva, S.M.; Pediatric Spine Study Group; MacAlpine, E.M.; Kawakami, N.; Gandhi, J.S.; Morishita, K.; Sturm, P.F.; Garg, S.; Glotzbecker, M.P.; Anari, J.B.; et al. Awake serial body casting for the management of infantile idiopathic scoliosis: Is general anesthesia necessary? Spine Deform. 2020, 8, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Thometz, J.; Liu, X.; Rizza, R.; English, I.; Tarima, S. Effect of an elongation bending derotation brace on the infantile or juvenile scoliosis. Scoliosis Spinal Disord. 2018, 13, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thometz, J.; Liu, X.C. Serial CAD/CAM Bracing: An Alternative to Serial Casting for Early Onset Scoliosis. J. Pediatr. Orthop. 2019, 39, e185–e189. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Donzelli, S.; Jurenaite, G.; Negrini, F.; Zaina, F. Efficacy of bracing in early infantile scoliosis: A 5-year prospective cohort shows that idiopathic respond better than secondary-2021 SOSORT award winner. Eur. Spine J. 2021, 30, 3498–3508. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.L. Casting vs. Bracing for Idiopathic Early-Onset Scoliosis (CVBT); ClinicalTrials.gov Identifier: NCT04500041; University of Iowa: Iowa City, IA, USA, 2022. [Google Scholar]
- Minsk, M.K.; Venuti, K.D.; Daumit, G.L.; Sponseller, P.D. Effectiveness of the Rigo Chêneau versus Boston-style orthoses for adolescent idiopathic scoliosis: A retrospective study. Scoliosis Spinal Disord. 2017, 12, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, B.A.; The Pediatric Spine Study Group; McClung, A.; Blakemore, L.C.; Shah, S.A.; Pawelek, J.B.; Sponseller, P.D.; Parent, S.; Emans, J.B.; Sturm, P.F.; et al. MRI utilization and rates of abnormal pretreatment MRI findings in early-onset scoliosis: Review of a global cohort. Spine Deform. 2020, 8, 1099–1107. [Google Scholar] [CrossRef]
- Tong, Y.; Udupa, J.K.; McDonough, J.M.; Wileyto, E.P.; Capraro, A.; Wu, C.; Ho, S.; Galagedera, N.; Talwar, D.; Mayer, O.H.; et al. Quantitative Dynamic Thoracic MRI: Application to Thoracic Insufficiency Syndrome in Pediatric Patients. Radiology 2019, 292, 206–213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Udupa, J.K.; Tong, Y.; Capraro, A.; McDonough, J.M.; Mayer, O.H.; Ho, S.; Wileyto, P.; Torigian, D.A.; Campbell, R.M., Jr. Understanding Respiratory Restrictions as a Function of the Scoliotic Spinal Curve in Thoracic Insufficiency Syndrome: A 4D Dynamic MR Imaging Study. J. Pediatr. Orthop. 2020, 40, 183–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohanty, S.P.; Kanhangad, M.P.; Kurup, J.K.N.; Saiffudeen, S. Vertebral, intraspinal and other organ anomalies in congenital scoliosis. Eur. Spine J. 2020, 29, 2449–2456. [Google Scholar] [CrossRef]
- Wu, N.; Liu, L.; Zhang, Y.; Wang, L.; Wang, S.; Zhao, S.; Li, G.; Yang, Y.; Lin, G.; Shen, J.; et al. Retrospective Analysis of Associated Anomalies in 636 Patients with Operatively Treated Congenital Scoliosis. J. Bone Jt. Surg Am. 2023, 105, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O.H.; Redding, G. Early changes in pulmonary function after vertical expandable prosthetic titanium rib insertion in children with thoracic insufficiency syndrome. J. Pediatr. Orthop. 2009, 29, 35–38. [Google Scholar] [CrossRef]
- Redding, G.J.; Mayer, O.H. Structure-respiration function relationships before and after surgical treatment of early-onset scoliosis. Clin. Orthop. Relat. Res. 2011, 469, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Kawakami, N.M.; Matsumoto, H.; Redding, G.J. Preoperative 6-Minute Walk Performance in Children With Congenital Scoliosis. J. Pediatr. Orthop. 2020, 40, e818–e821. [Google Scholar] [CrossRef] [PubMed]
- Striegl, A.; Chen, M.L.; Kifle, Y.; Song, K.; Redding, G. Sleep-disordered breathing in children with thoracic insufficiency syndrome. Pediatr. Pulmonol. 2010, 45, 469–474. [Google Scholar] [CrossRef]
- Skaggs, D.L.; Akbarnia, B.A.; Flynn, J.M.; Myung, K.S.; Sponseller, P.D.; Vitale, M.G.; Chest Wall and Spine Deformity Study Group; Growing Spine Study Group; Pediatric Orthopaedic Society of North America; Scoliosis Research Society Growing Spine Study Committee. A classification of growth friendly spine implants. J. Pediatr. Orthop. 2014, 34, 260–274. [Google Scholar] [CrossRef]
- Welborn, M.C.; Krajbich, J.I.; D’Amato, C. Use of Magnetic Spinal Growth Rods (MCGR) With and Without Preoperative Halo-gravity Traction (HGT) for the Treatment of Severe Early-onset Scoliosis (EOS). J. Pediatr. Orthop. 2019, 39, e293–e297. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Duah, H.O.; Wulff, I.; Osei Tutu, H.; Mahmud, R.; Yankey, K.P.; Akoto, H.; Boachie-Adjei, O.; FOCOS Spine Research Group. The Use of Halo Gravity Traction in the Treatment of Severe Early Onset Spinal Deformity. Spine 2019, 44, E841–E845. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; McElroy, M.J.; Akbarnia, B.A.; Salari, P.; Oliveira, D.; Thompson, G.H.; Emans, J.B.; Yazici, M.; Skaggs, D.L.; Shah, S.A.; et al. Growing rods for spinal deformity: Characterizing consensus and variation in current use. J. Pediatr. Orthop. 2010, 30, 264.e70. [Google Scholar] [CrossRef] [PubMed]
- Bess, S.; Akbarnia, B.A.; Thompson, G.H.; Sponseller, P.D.; Shah, S.A.; El Sebaie, H.; Boachie-Adjei, O.; Karlin, L.I.; Canale, S.; Poe-Kochert, C.; et al. Complications of growing-rod treatment for early-onset scoliosis: Analysis of one hundred and forty patients. J. Bone Joint Surg. Am. 2010, 92, 2533.e43. [Google Scholar] [CrossRef] [PubMed]
- Sewell, M.D.; Malagelada, F.; Wallace, C.; Gibson, A.; Noordeen, H.; Tucker, S.; Molloy, S.; Lehovsky, J. A Preliminary Study to Assess Whether Spinal Fusion for Scoliosis Improves Carer-assessed Quality of Life for Children With GMFCS Level IV or V Cerebral Palsy. J. Pediatr. Orthop. 2016, 36, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Akbarnia, B.A.; Breakwell, L.M.; Marks, D.S.; McCarthy, R.E.; Thompson, A.G.; Canale, S.K.; Kostial, P.N.; Tambe, A.; Asher, M.A.; Growing Spine Study Group. Dual growing rod technique followed for three to eleven years until final fusion: The effect of frequency of lengthening. Spine 2008, 33, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.H.; Akbarnia, B.A.; Kostial, P.; Poe-Kochert, C.; Armstrong, D.G.; Roh, J.; Lowe, R.; Asher, M.A.; Marks, D.S. Comparison of single and dual growing rod techniques followed through definitive surgery: A preliminary study. Spine 2005, 30, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Varley, E.S.; Pediatric Spine Study Group; Pawelek, J.B.; Mundis, G.M., Jr.; Oetgen, M.E.; Sturm, P.F.; Akbarnia, B.A.; Yaszay, B. The role of traditional growing rods in the era of magnetically controlled growing rods for the treatment of early-onset scoliosis. Spine Deform. 2021, 9, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Takaso, M.; Moriya, H.; Kitahara, H.; Minami, S.; Takahashi, K.; Isobe, K.; Yamagata, M.; Otsuka, Y.; Nakata, Y.; Inoue, M. New remote-controlled growing-rod spinal instrumentation possibly applicable for scoliosis in young children. J. Orthop. Sci. 1998, 3, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Akbarnia, B.A.; Pawelek, J.B.; Cheung, K.M.C.; Demirkiran, G.; Elsebaie, H.; Emans, J.B.; Johnston, C.E.; Mundis, G.M.; Noordeen, H.; Skaggs, D.L.; et al. Traditional growing rods versus magnetically controlled growing rods for the surgical treatment of early-onset scoliosis: A case-matched 2-year study. Spine Deform. 2014, 2, 2493–2497. [Google Scholar] [CrossRef] [PubMed]
- Lebel, D.E.; Rocos, B.; Helenius, I.; Sigal, A.; Struder, D.; Yazici, M.; Bekmez, S.; Hasler, C.C.; Pesenti, S.; Jouve, J.L. Magnetically controlled growing rods graduation: Deformity control with high complication rate. Spine 2021, 46, E1105–E1112. [Google Scholar] [CrossRef]
- Thakar, C.; Kieser, D.C.; Mardare, M.; Haleem, S.; Fairbank, J.; Nnadi, C. Systematic review of the complications associated with magnetically controlled growing rods for the treatment of early onset scoliosis. Eur. Spine J. 2018, 27, 2062–2071. [Google Scholar] [CrossRef]
- Zhang, T.; Sze, K.Y.; Peng, Z.W.; Cheung, K.M.; Lui, Y.F.; Wong, Y.W.; Kwan, K.Y.; Cheung, J.P. Systematic investigation of metallosis associated with magnetically controlled growing rod implantation for early-onset scoliosis. Bone Jt J. 2020, 102-B, 1375–1383. [Google Scholar]
- McIntosh, A.L.; Booth, A.; Oetgen, M.E. Unplanned return to the operating room (UPROR) occurs in 40% of MCGR patients at an average of 2 years after initial implantation. Spine Deform 2024. Epub ahead of print. [Google Scholar] [PubMed]
- Matsumoto, H.; Skaggs, D.L.; Akbarnia, B.A.; Pawelek, J.B.; Hilaire, T.S.; Levine, S.; Sturm, P.; Perez-Grueso, F.J.S.; Luhmann, S.J.; Sponseller, P.D.; et al. Comparing health-related quality of life and burden of care between early-onset scoliosis patients treated with magnetically controlled growing rods and traditional growing rods: A multicenter study. Spine Deform. 2021, 9, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Sankar, W.N.; Skaggs, D.L.; Yazici, M.; Johnston, C.E.; Shah, S.A.; Javidan, P.; Kadakia, R.V.; Day, T.F.; Akbarnia, B.A. Lengthening of dual growing rods and the Law of Diminishing Returns. Spine 2010, 36, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Heyer, J.H.; Anari, J.B.; Baldwin, K.D.; Mitchell, S.L.; Luhmann, S.J.; Sturm, P.F.; Flynn, J.M.; Cahill, P.J.; on behalf of the Pediatric Spine Study Group. Lengthening Behavior of Magnetically Controlled Growing Rods in Early-Onset Scoliosis: A Multicenter Study. J. Bone Jt. Surg. 2022, 104, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Doany, M.E.; Olgun, Z.D.; Kinikli, G.I.; Bekmez, S.; Kocyigit, A.; Demirkiran, G.; Karaagaoglu, A.E.; Yazici, M. Health-Related Quality of Life in Early-Onset Scoliosis Patients Treated Surgically: EOSQ Scores in Traditional Growing Rod Versus Magnetically Controlled Growing Rods. Spine 2018, 43, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Klyce, W.; Mitchell, S.L.; Pawelek, J.; Skaggs, D.L.; Sanders, J.O.; Shah, S.A.; McCarthy, R.E.; Luhmann, S.J.; Sturm, P.F.; Flynn, J.M.; et al. Characterizing use of growth-friendly implants for early-onset scoliosis: A 10-year update. J. Pediatr. Orthop. 2020, 40, e740–e746. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.M., Jr.; Smith, M.D.; Mayes, T.C.; Mangos, J.A.; Willey-Courand, D.B.; Kose, N.; Pinero, R.F.; Alder, M.E.; Duong, H.L.; Surber, J.L. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J. Bone Jt. Surg. Am. 2004, 86-A, 1659–1674. [Google Scholar]
- El-Hawary, R.; Morash, K.; Kadhim, M.; Vitale, M.; Smith, J.; Samdani, A.; Flynn, J. VEPTR Treatment of Early Onset Scoliosis in Children Without Rib Abnormalities: Long-term Results of a Prospective, Multicenter Study. J. Pediatr. Orthop. 2020, 40, e406–e412. [Google Scholar] [CrossRef] [PubMed]
- Waldhausen, J.H.; Redding, G.; White, K.; Song, K. Complications in using the vertical expandable prosthetic titanium rib (VEPTR) in children. J. Pediatr. Surg. 2016, 51, 1747–1750. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, S.P.J.; Tromp, I.N.; Yazici, M.; Kempen, D.H.R.; Castelein, R.M.; Kruyt, M.C. A comparison of growth among growth-friendly systems for scoliosis: A systematic review. Spine J. 2019, 19, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Tabeling, C.S.; Lemans, J.V.C.; Top, A.; Scholten, E.P.; Stempels, H.W.; Schlösser, T.P.C.; Ito, K.; Castelein, R.M.; Kruyt, M.C. The Spring Distraction System for Growth-Friendly Surgical Treatment of Early Onset Scoliosis: A Preliminary Report on Clinical Results and Safety after Design Iterations in a Prospective Clinical Trial. J. Clin. Med. 2022, 11, 3747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luqué, E.R.; Cardoso, A. Treatment of scoliosis without arthrodesis or external support, preliminary report. Orthop. Trans. 1977, 1, 37–38. [Google Scholar]
- McCarthy, R.E.; Luhmann, S.; Lenke, L.; McCullough, F.L.R. The Shilla Growth Guidance Technique for Early-Onset Spinal Deformities at 2-Year Follow-Up: A Preliminary Report. J. Pediatr. Orthop. 2014, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Betz, R.R.; Kim, J.; D’Andrea, L.P.; Mulcahey, M.J.; Balsara, R.K.; Clements, D.H. An innovative technique of vertebral body stapling for the treatment of patients with adolescent idiopathic scoliosis: A feasibility, safety, and utility study. Spine 2003, 28 (Suppl. 20), S255–S265. [Google Scholar] [CrossRef] [PubMed]
- Betz, R.R.; Ranade, A.; Samdani, A.F.; Chafetz, R.; D’andrea, L.P.; Gaughan, J.P.; Asghar, J.; Grewal, H.; Mulcahey, M.J. Vertebral body stapling: A fusionless treatment option for a growing child with moderate idiopathic scoliosis. Spine 2010, 35, 169–176. [Google Scholar] [CrossRef]
- Trobisch, P.D.; Samdani, A.; Cahill, P.; Betz, R.R. Vertebral body stapling as an alternative in the treatment of idiopathic scoliosis. Oper. Orthop. Traumatol. 2011, 23, 227–231. [Google Scholar] [CrossRef]
- Cuddihy, L.; Danielsson, A.J.; Cahill, P.J.; Samdani, A.F.; Grewal, H.; Richmond, J.M.; Mulcahey, M.J.; Gaughan, J.P.; Antonacci, M.D.; Betz, R.R. Vertebral body stapling versus bracing for patients with high-risk moderate idiopathic scoliosis. Biomed Res. Int. 2015, 2015, 438452. [Google Scholar] [CrossRef]
- Trupia, E.; Hsu, A.C.; Mueller, J.D.; Matsumoto, H.; Bodenstein, L.; Vitale, M. Treatment of idiopathic scoliosis with vertebral body stapling. Spine Deform. 2019, 7, 720–728. [Google Scholar] [CrossRef]
- Newton, P.O. Spinal growth tethering: Indications and limits. Ann. Transl. Med. 2020, 8, 27. [Google Scholar] [CrossRef]
- Baroncini, A.; Courvoisier, A. The different applications of Vertebral Body Tethering—Narrative review and clinical experience. J. Orthop. 2023, 37, 86–92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mackey, C.; Hanstein, R.; Lo, Y.; Vaughan, M.; St Hilaire, T.; Luhmann, S.J.; Vitale, M.G.; Glotzbecker, M.P.; Samdani, A.; Parent, S.; et al. Magnetically Controlled Growing Rods (MCGR) Versus Single Posterior Spinal Fusion (PSF) Versus Vertebral Body Tether (VBT) in Older Early Onset Scoliosis (EOS) Patients: How Do Early Outcomes Compare? Spine 2022, 47, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Cahill, P.J.; Miyanji, F.; Lullo, B.R.; Samdani, A.F.; Lonner, B.S.; Pahys, J.M.; Hwang, S.W.; Haber, L.L.; Alanay, A.; Shah, S.A.; et al. Incidence of Tether Breakage in Anterior Vertebral Body Tethering. J. Pediatr. Orthop. 2024, 44, e323–e328. [Google Scholar] [CrossRef] [PubMed]
- Hedequist, D.J. Surgical treatment of congenital scoliosis. Orthop. Clin. North Am. 2007, 38, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.B.; Moe, J.H. The results of spinal arthrodesis for congenital spinal deformity in patients younger than five years old. J. Bone Jt. Surg. Am. 1982, 64, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, C.J.; Moore, D.P.; Fogarty, E.E.; Dowling, F.E. Long-term results from in situ fusion for congenital vertebral deformity. Spine 2002, 27, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Karol, L.A.; Johnston, C.; Mladenov, K.; Schochet, P.; Walters, P.; Browne, R.H. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J. Bone Jt. Surg. Am. 2008, 90, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.E.; Karol, L.A.; Thornberg, D.; Jo, C.; Eamara, P. The 18-cm Thoracic-Height Threshold and Pulmonary Function in Non-Neuromuscular Early-Onset Scoliosis: A Reassessment. JB JS Open Access. 2021, 6, e21.00093. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Fano, A.N.; Quan, T.; Akbarnia, B.A.; Blakemore, L.C.; Flynn, J.M.; Skaggs, D.L.; Smith, J.T.; Snyder, B.D.; Sponseller, P.D.; et al. Re-evaluating consensus and uncertainty among treatment options for early onset scoliosis: A 10-year update. Spine Deform. 2023, 11, 11–25. [Google Scholar] [CrossRef] [PubMed]

| Idiopathic | Syndromic | Neuromuscular | Congenital |
|---|---|---|---|
| Infantile Juvenile | Jarcho–Levine Marfan’s VACTER Goldenhar Spinal Muscular Atrophy Prader–Willi Neurofibromatosis Skeletal Dysplasia | Cerebral Palsy Spina Bifida Muscular Dystrophy Spinal Cord Injury Chiari Malformation Syringomyelia | Failure of Formation Failure of Segmentation Mixed Failure of Segmentation/Formation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, A.N.; Lark, R.K. Current Concepts in the Treatment of Early Onset Scoliosis. J. Clin. Med. 2024, 13, 4472. https://doi.org/10.3390/jcm13154472
Johnson AN, Lark RK. Current Concepts in the Treatment of Early Onset Scoliosis. Journal of Clinical Medicine. 2024; 13(15):4472. https://doi.org/10.3390/jcm13154472
Chicago/Turabian StyleJohnson, Alexandra N., and Robert K. Lark. 2024. "Current Concepts in the Treatment of Early Onset Scoliosis" Journal of Clinical Medicine 13, no. 15: 4472. https://doi.org/10.3390/jcm13154472






