Abstract
Objectives: The objective of this study was to evaluate the real-world drug survival, adherence, and discontinuation risk of biologics disease-modifying anti-rheumatic drugs (bDMARDs) among patients with ankylosing spondylitis (AS). Methods: This was a retrospective study using a computerized database. Biologic-naïve and biologic-experienced AS patients who initiated treatment with bDMARDs (tumor necrosis factor alpha inhibitors {TNF-αis} or interleukin-17 inhibitor {IL-17i}) during 2015–2018 were included. Adherence was assessed using the proportion of days covered (PDC) method. Drug survival was analyzed using Kaplan–Meier estimates. Risk of discontinuation was estimated by the Cox proportional hazard model. Results: We identified 343 eligible patients utilizing 481 lines of therapy. The mean age was 44.6 years (SD ± 13.4), 57.7% were males, and 69.7% were biologic-naïve at baseline. The proportion of highly adherent patients (PDC ≥ 0.8) in the biologic-naïve group was 63.5% for golimumab, 69.2% for etanercept, and 71.6% for adalimumab (p > 0.9). Among the biologic-experienced group, secukinumab had the highest proportion of adherent patients (75.7%) and etanercept the lowest (50.0%) reaching statistical difference (p < 0.001). The Kaplan–Meier analysis did not show a significant difference in drug survival in either the biologic-naïve or the biologic-experienced groups (p = 0.85). Multivariable analysis demonstrated a similar risk for discontinuation for etanercept, golimumab, and secukinumab compared with adalimumab, regardless of biologic-experience status. Conclusions: Adherence, drug survival, and risk for discontinuation were similar for all TNF-αis and the IL-17i SEC, regardless of biologic-experience status. As drug survival is an indirect measure of drug efficacy, n, in real-world settings, we believe caregivers can integrate these results into treatment considerations.
1. Introduction
Ankylosing spondylitis (AS) is a chronic, multi-systemic inflammatory disorder which is a member of the spondyloarthropathy group [1]. It primarily affects the sacroiliac joints and the axial skeleton, manifesting clinically as inflammatory back pain and stiffness [2]. Peripheral arthritis, along with enthesitis and dactylitis, is found in about 30% of patients [3,4]. In addition, extra-articular manifestations, most commonly acute anterior uveitis, psoriasis, and inflammatory bowel disease, [5] can occur, embroiling the patient’s condition and treatment decisions [6]. In some cases, chronic axial inflammation can result in progressive structural damage, leading to impaired spinal mobility and postural abnormalities [7,8]. Such irreversible damage is associated with impaired quality of life [7] and social and economic burden [8]. Hence, treatment goals need to be integrative, abrogating inflammation, reducing overall disease activity, and halting structural damage in order to preserve normal function and social participation [9].
Currently, following NSAID treatment failure, the pharmacological armamentarium includes biological disease-modifying anti-rheumatic drugs (bDMARDs), tumor necrosis factor alpha (TNF-α) inhibitors, anti-interleukin (IL)-17A antibodies, and the latest addition in the form of targeted synthetic (ts) DMARDs, Janus kinase (JAK) inhibitors [10]. These therapies have revolutionized [11] care and prognosis for AS patients [12], bringing significant clinical relief and halting disease progression and spinal damage [13].
By and large, the efficacy and safety of the different treatment options seem to be similar [14]. However, data regarding these agents are obtained from placebo-controlled trails, significantly lacking comparative head-to-head studies [9].
Several studies examined biologic drug survival among AS patients [15,16,17,18,19], yet these studies are heterogeneous and differ by follow-up period, the variety of drugs included, patients’ characteristics, and local healthcare systems’ regulations and financing. Therefore, we aimed to compare the real-world adherence and drug survival of different bDMARDs in adult AS patients.
2. Methods
2.1. Study Design and Data Source
This retrospective cohort study was conducted using the computerized databases of Maccabi Healthcare Services (MHS). MHS is the second largest state-mandated health provider operating in Israel, providing healthcare services for over 2.6 million members (25% of the Israeli population). The MHS databases integrate data from the MHS central laboratory, medication prescriptions, and purchases throughout the MHS pharmacy network, primary care, expert consultations, hospitalizations, procedures, and socio-demographic data. Physician diagnoses are coded using the International Classification of Disease, 9th Edition (ICD-9-CM), codes, as well as internal MHS codes for sub-classification.
This study was conducted in accordance with the protocol, applicable regulations, and guidelines governing clinical study conduct and the ethical principles that have their origin in the Declaration of Helsinki. The MHS Institutional Review Board (IRB) approved the study protocol and related documents. MHS’s IRB waived the requirement to obtain any informed consent for this secondary analysis of existing data (approval number 0108-18-BBL, 18 December 2018).
2.2. Study Population and Follow-Up
According to the Israeli regulatory guidelines, AS patients are eligible for bDMARDs or tsDMARDs if they fulfill prerequisite requirements. Prior to procurement, patients are required to receive an authorization from the MHS drugs authorization center, confirming they comply with the guidelines.
The study population included patients who first purchased ≥ 1 of the following drugs between 1 January 2015 and 31 December 2017 for the indication of AS: TNF-α inhibitors (adalimumab {ADA}, infliximab {IFX}, golimumab {GLM}, etanercept {ETN}, certolizumab pegol {CTZ}); IL-17 inhibitor (secukinumab {SEC}). The additional IL-17 inhibitor was not available for MHS patients during the study follow-up period; neither were the JAK inhibitors tofatacitinib and upadacitinib. All patients were prescribed with these drugs for the indication of AS only, according to the MHS drug authorization center. The first purchase was defined as the index date for the study. Included patients were adults (age ≥ 18 years) and MHS members for ≥12 months before and after the index date. During the study follow-up period, all the aforementioned drugs were available as first-line treatment after utilizing ≥ 2 different non-steroidal anti-inflammatory drugs (NSAIDs).
Patients were followed until the earliest of the following dates: death, leaving the MHS, or the end of the follow-up period (31 December 2018).
2.3. Study Variables
We included biologic-naïve patients (those with no previous purchase of any of the drugs included in the study before 1 January 2015), as well as biologic-experienced patients (those treated with any of the drugs included in the study before 1 January 2015, or who switched and started treatment with any of the other drugs included in the study, between 1 January 2015 and 31 December 2017).
Initiation of a new line of therapy was defined by the first purchase of the drug. For all patients, we followed all lines of therapy used during the study follow-up period and numbered them. For those defined as biologic-experienced when entering the study, we numbered the lines of therapy used during the study follow-up period, using data on bDMARDs dispensed up to 10 years before entering the study.
Adherence in the first 12 months was evaluated by using the proportion of days covered (PDC) method. PDC reflects the number of days covered by the dispensed drug divided by the total follow-up time for a specific line of therapy. PDC was categorized as follows: non-adherent (PDC < 40%), moderately adherent (40% ≤ PDC < 80%), or highly adherent (PDC ≥ 80%) [20,21,22,23]. For the sake of adherence in the first 12 months, patients were followed from the treatment initiation until the earliest of the following: switching, end of study follow-up, and 12 months after treatment initiation. Only lines of therapy in which patients had at least three months’ follow-up were included.
Drug survival was measured from the initiation of treatment (i.e., the index date) until treatment discontinuation, defined as the first gap of 120 days or more after the last supply date. Patients who discontinued their current drug were further classified as follows: switching (starting a new treatment, with any of the drugs included in the study) or stopping (≥120 days treatment gap, without switching).
Additional data retrieved from the database included socio-demographic factors (age, sex, residential area, socioeconomic status {SES}, and smoking status) and baseline comorbidities according to MHS registries (cardiovascular disease, diabetes, hypertension, obesity, and osteoporosis). Depression and anxiety were defined according to antidepressants and benzodiazepines dispensed 180 days before the index date. The Charlson’s comorbidity index (CCI) was calculated at baseline. Additional data at baseline included years since AS diagnosis, visits to a primary care physician 180 days before the index date, and being hospitalized at least once 180 days before the index date.
2.4. Statistical Analyses
Patients’ disposition, baseline characteristics, adherence rates, discontinuation rates, type, and time to discontinuation are presented using descriptive statistics (n, %, mean ± SD or median, IQR, as appropriate). Adherence was assessed by treatment status and individual drug. Differences in adherence rates were assessed using Chi-square tests.
Discontinuation rate, type, and time to discontinuation in the first 12 months were assessed among patients with ≥12 months follow-up and presented by treatment experience status and individual drug. Time to treatment discontinuation by treatment experience status was also analyzed and plotted using Kaplan–Meier estimates. The log-rank test was used to evaluate statistical significance differences between individual drugs.
The risk of treatment discontinuation of each drug was estimated separately for the biologic-naïve and the biologic-experienced groups using a multivariable Cox proportional hazards regression model. The model was adjusted for age, sex, SES, time since diagnosis, and CCI.
Two-sided p < 0.05 was considered statistically significant. All statistical analyses were performed with IBM-SPSS V.25.0 standard statistical software for Windows and R V.3.5.
3. Results
Between January 2015 and December 2017, 343 eligible AS patients were identified (Table 1). The mean age was 44.6 years (SD ± 13.4) with a slight male predominance (57.7%) and for 257 patients (74.9%) the time since diagnosis at baseline was under two years. The most prevalent comorbidities were obesity (23.6%), depression or anxiety (22.7%), and hypertension (21.0%). Most patients (69.7%) were biologic-naive, and 482 lines of therapy were used during follow-up. Because only one patient used Tofacitinib, it was excluded from all analyses. Therefore, a total of 481 lines of treatment were included.

Table 1.
Baseline characteristics of AS patients (n = 343).
3.1. Patients’ Disposition
Among the 239 patients using their first line of therapy, the most common drug used was ADA (42.7%), followed by ETN (32.6%) and GOL (21.8%). The remaining 2.9% were treated with IFX (n = 6) and SEC (n = 1). Among the 143 patients on their second line of therapy (including biologic-naive patients who switched during follow-up), ADA (36.4%), ETN (25.2%), and GOL (28.0%) remained the most prevalent drugs. Figure 1 depicts the disposition of patients according to their treatment episode. The proportion of patients utilizing CTZ and IFX increased to 10.5%. Among the patients utilizing a third line of therapy (n = 99), we noticed a decrease in the proportion of patients prescribed ADA, ETN, and GOL (10.1%, 12.1%, and 33.3%, respectively), while the proportion of patients prescribed with SEC increased to 28.3%, along with an increment in the proportion of patients using IFX (9.1%) and CTZ (7.1%).
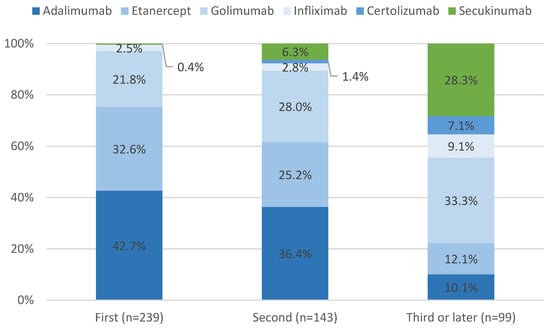
Figure 1.
Patient disposition by treatment lines.
3.2. Adherence to Treatment
Among biologic-naïve patients (Table 2), the proportion of highly adherent patients (PDC ≥ 0.8) was similar (p > 0.9) and highest for ADA (71.6%), followed by ETN (69.2%) and GOL (63.5%). Among the biologic-experienced group, the highest adherence was recorded for SEC (75.7%), followed by ADA (69.4%), GOL (60.3%), and ETN (50.0%) (p < 0.001).

Table 2.
Adherence in the first 12 months by biologic-experience status.
3.3. Discontinuation and Drug Survival
Table 3 displays the discontinuation rate in the first 12 months by biologic-experience status. Among the biologic-naïve group, the discontinuation rate was highest for GOL (34.6%), followed by ADA (30.4%) and ETN (28.2%) (p = 0.7). Among the biologic-experienced group, the discontinuation rate was highest for ETN (47.4%) and lowest for SEC (30.8%) (p = 0.3).

Table 3.
Discontinuation rate and type in the first 12 months among patients with ≥12 months follow-up, by biologic-experience status.
The Kaplan–Meier analysis (Figure 2) shows similar survival rates for ADA, GOL, and ETN among the biologic-naïve group (p = 0.85). Among the biologic-experienced group, after 12 months, GOL and SEC displayed similar drug survival rates, followed by ADA and ETN; however, the results did not reach statistical significance (p = 0.94).
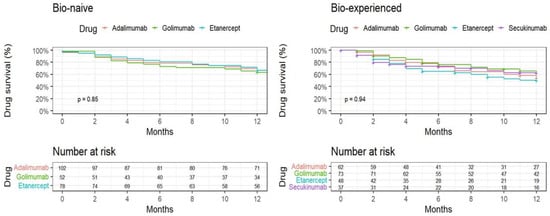
Figure 2.
One year Kaplan–Meier plot time to discontinuation, by treatment experience status and individual drug.
The multivariable models (Table 4) showed that in the biologic-naïve group, females had an increased risk for treatment discontinuation (HR 2.03, 95% CI 1.26–3.28). A similar risk for discontinuation was observed for GOL and ETN compared to ADA (HR 1.23, 95% CI 0.68–2.23; HR 0.89, 95% CI 0.51–1.55, respectively). Among the treatment-experienced group, longer disease duration was associated with reduced risk for discontinuation (HR 0.90, 95% CI 0.82–1.00). In the biologic-naïve group, GOL, ETN, and SEC had similar risk for discontinuation compared with ADA (HR 0.99, 95% CI 0.53–1.84; HR 1.38, 95% CI 0.73–2.61; HR 1.09, 95% CI 0.67–1.05, respectively).

Table 4.
HRs and 95% CIs for treatment discontinuation in the first 12 months among AS patients with ≥12 months follow-up, by biologic-experience status.
4. Discussion
Our study aimed to evaluate adherence, drug survival, and risk for discontinuation of biologic-naïve and -experienced AS patients in a real-world setting.
Adherence to biologics in the first 12 months in the biologic-naïve group was similar (p = 0.497) across all medications of interest, with 63.5–71.6% of the patients being highly adherent. In the biologic-experienced group, the highest rate of highly adherent patients was recorded for SEC (75.7%) and the lowest for ETN (50.0%), reflecting significant differences between the different drugs (p < 0.001).
The relatively high adherence rate in both treatment groups is worth noting. We previously reported high adherence rates to ADA among patients with AS compared to other inflammatory diseases [24], postulating that the relative paucity at the time of biologics for AS (compared to rheumatoid arthritis or psoriatic arthritis) is a plausible explanation.
Interestingly, we have found that the highest adherence, together with the lowest discontinuation rate (30.8%), was among patients prescribed with SEC in comparison to TNF-αis. In addition, the proportion of patients treated with SEC increased gradually between lines of therapy, reaching 28% when prescribed as a third line of therapy or above. Taken together, we believe these properties reinforce the aforementioned tendency to maintain treatment when other options are becoming less available. At the same time, drug survival is an important proxy measure for the effectiveness of treatments for inflammatory diseases [15], and the adherence rate may also reflect the drug’s efficacy [25]. Therefore, the high proportion of highly adherent patients treated with SEC in our study and its relatively high level of drug survival in our study and in other studies [26,27] might indicate that SEC can be an effective treatment option for patients with AS.
As seen in previous studies [16,28], drug survival decreased with time. In our study, none of the drugs utilized by either biologic-naïve or biologic-experienced patients showed superiority in terms of drug survival or discontinuation risk.
Real-world data relating to SEC drug properties in patients with AS are currently scarce; however, our study sheds some light on the topic, showing similar drug survival between SEC and TNF-αis. Our findings are in line with a meta-analysis by Yu et al. [29], which reported comparable drug survival for all biologics for the treatment of AS. Another study retrospectively exploring the drug survival of SEC, either as the first treatment line or following TNF-αi exposure, was conducted by Diaconu et al. [30]. The authors found that drug survival was 59.7% at 12 months and 31.3% at 24 months, without significant differences in the median drug survival between biologic-naïve versus biologic-experienced subgroups.
In our study, the drug survival was similar for TNF-αis and SEC when given to biologic-experienced patients. These results are supported by other studies, showing that patients switching from either one TNF-αi to another [30,31,32] or to SEC [33] displayed fair survival rates.
In contrast to our findings, former studies found poor drug survival for biologic-experienced compared to biologic-naïve patients. According to a single-center observational study by Gyulas et al. [28], the overall survival time of biologic-naïve AS patients prescribed with TNF-αis was better in comparisons with those on their second treatment line [62.88 months (95% CI: 56.67–69.09) and 39.29 months (95% CI: 31.29–47.03), p = 0.05, respectively], yet the authors state that switching between TNF-αis is still a good therapeutic option for biologic-experienced patients. A real-life multicenter study by Krajewski et al. [34] reported similar results showing that the drug survival of all TNF-αis was shorter for the second than the first TNF-αi (mean difference 6.3 months, p < 0.001).
According to our findings, female biologic-naïve patients displayed an increased risk for treatment discontinuation. However, gender seems to be an equivocal factor in treatment discontinuation. According to Aturi et al. [35], the lack of adherence to treatment was not associated with sex, while in another study, male sex was associated with a better retention rate [36].
There are some limitations in our study. Due to the retrospective design, which is based on administrative data, some data could not be retrieved, including disease activity, the presence of extra-articular symptoms, and concomitant conventional synthetic DMARDs, which influence drug retention [37]. Also, the reasons for drug discontinuation by each subject and whether it was related to loss of efficacy, adverse events, or other factors could not be retrieved from the data. The study did not include all bDMARDs and tsDMARDS currently available for patients with AS. In addition, we did not distinguish AS from non-radiographic axial spondylarthritis, which shows conflicting results regarding treatment adherence [38,39]. The current study also has several strengths. We were able to follow patients for a long period of time and include almost all bDMARDs available for AS, including the IL-17i SEC. We used real-world data without any exclusion or randomization. Additionally, we were able to assess adherence, drug survival, and the risk for treatment discontinuation in treatment-naïve and treatment-experienced patients.
In conclusion, drug adherence, survival, and risk for discontinuation were similar for both TNF-αis and the IL-17i SEC, regardless of biologic-experience status. Etanercept was the TNF-αi with the lowest rate of adherent patients and the highest rate of discontinuation among biologic-experienced patients. The drug survival rate among biologic-experienced patients was similar to that of biologic-naïve patients, indicating that patients who discontinued their first biologic may benefit from switching to another. As these features are indirect methods to appraise drug efficacy and safety [40] in real-world settings, we believe caregivers can integrate these results into treatment considerations.
Author Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by V.R., H.A., G.C. and O.G. The first draft of the manuscript was written by V.R. and O.G.; F.F., A.W. and D.M. provided critical review of the manuscript. All authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
AbbVie Inc. (North Chicago, IL, USA) funded the research. AbbVie and Maccabi participated in the study design, research, analysis, data collection, interpretation of the data, review, and approval of the manuscript and publication.
Institutional Review Board Statement
The study was conducted in accordance with the protocol, applicable regulations, and guidelines governing clinical study conduct and the ethical principles originating in the Declaration of Helsinki. The independent ethics committee and Institutional Review Board, MHS Institutional Review Board, approved the study protocol and related documents (approval number 0108-18-BBL, 18 December 2018). MHS’s IRB waived the requirement to obtain any informed consent for this secondary analysis of existing data.
Informed Consent Statement
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published. No honoraria or payments were made for authorship.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available because the data that support the findings of this study originate from Maccabi Healthcare Services, and restrictions apply to the availability of these data. Due to restrictions, these data can be accessed only by request to the authors and/or Maccabi Healthcare Services.
Conflicts of Interest
F Faccin is a full-time AbbVie employee and may own AbbVie stock and/or options. V Rosenberg, G Chodick, and O Gendelman declare that they have no conflicts of interest. H. Amital received consultant fees from AbbVie for this project. After completion of the manuscript, G Chodick changed his affiliation with Maccabi Healthcare Services, and his new affiliation is Maccabi Healthcare Services, Tel Aviv, Israel. His other affiliation, with Tel Aviv University, remains the same. Dennis McGonagle work is supported by the Leeds National Institute of Health Research Biomedical Research Centre.
References
- Ghasemi-Rad, M.; Attaya, H.; Lesha, E.; Vegh, A.; Maleki-Miandoab, T.; Nosair, E.; Sepehrvand, N.; Davarian, A.; Rajebi, H.; Pakniat, A.; et al. Ankylosing spondylitis: A state of the art factual backbone. World J. Radiol. 2015, 7, 236–252. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, Z.; Zhang, Y.; Lin, C.; He, B.; Fang, L.; Tu, L.; Zhao, M.; Wu, X.; Gu, J. Pain- and Fatigue-Related Functional and Structural Changes in Ankylosing Spondylitis: An fRMI Study. Front. Med. 2020, 7, 193. [Google Scholar] [CrossRef]
- López-Medina, C.; Dougados, M.; Ruyssen-Witrand, A.; Moltó, A. Evaluation of concomitant peripheral arthritis in patients with recent onset axial spondyloarthritis: 5-year results from the DESIR cohort. Arthritis Res. Ther. 2019, 21, 139. [Google Scholar] [CrossRef]
- de Winter, J.J.; van Mens, L.J.; van der Heijde, D.; Landewé, R.; Baeten, D.L. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: A meta-analysis. Arthritis Res. Ther. 2016, 18, 196. [Google Scholar] [CrossRef]
- Maghraoui, A.E. Extra-articular manifestations of ankylosing spondylitis: Prevalence, characteristics and therapeutic implications. Eur. J. Intern. Med. 2011, 22, 554–560. [Google Scholar] [CrossRef]
- Elewaut, D.; Matucci-Cerinic, M. Treatment of ankylosing spondylitis and extra-articular manifestations in everyday rheumatology practice. Rheumatology 2009, 48, 1029–1035. [Google Scholar] [CrossRef]
- Braun, J.; Haibel, H.; de Hooge, M.; Landewé, R.; Rudwaleit, M.; Fox, T.; Readie, A.; Richards, H.B.; Porter, B.; Martin, R.; et al. Spinal radiographic progression over 2 years in ankylosing spondylitis patients treated with secukinumab: A historical cohort comparison. Arthritis Res. Ther. 2019, 21, 142. [Google Scholar] [CrossRef]
- Malinowski, K.P.; Kawalec, P. The indirect costs of ankylosing spondylitis: A systematic review and meta-analysis. Expert. Rev. Pharmacoecon. Outcomes Res. 2015, 15, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, S.; Nikiphorou, E.; Sepriano, A.; Ortolan, A.; Webers, C.; Baraliakos, X.; Landewé, R.B.M.; Van den Bosch, F.E.; Boteva, B.; Bremander, A.; et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann. Rheum. Dis. 2023, 82, 19–34. [Google Scholar] [CrossRef]
- Juanola, X.; Ramos, M.J.M.; Belzunegui, J.M.; Fernández-Carballido, C.; Gratacós, J. Treatment Failure in Axial Spondyloarthritis: Insights for a Standardized Definition. Adv. Ther. 2022, 39, 1490–1501. [Google Scholar] [CrossRef]
- Zhu, W.; He, X.; Cheng, K.; Zhang, L.; Chen, D.; Wang, X.; Qiu, G.; Cao, X.; Weng, X. Ankylosing spondylitis: Etiology, pathogenesis, and treatments. Bone Res. 2019, 7, 22. [Google Scholar] [CrossRef]
- Garcia-Montoya, L.; Gul, H.; Emery, P. Recent advances in ankylosing spondylitis: Understanding the disease and management. F1000Research 2018, 7, F1000 Faculty Rev-1512. [Google Scholar] [CrossRef]
- Baraliakos, X.; Gensler, L.S.; D’Angelo, S.; Iannone, F.; Favalli, E.G.; de Peyrecave, N.; Auteri, S.E.; Caporali, R. Biologic therapy and spinal radiographic progression in patients with axial spondyloarthritis: A structured literature review. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20906040. [Google Scholar] [CrossRef] [PubMed]
- Danve, A.; Deodhar, A. Treatment of axial spondyloarthritis: An update. Nat. Rev. Rheumatol. 2022, 18, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Rosenø, N.A.L.; Aagaard, D.; Lørup, E.H.; Nielsen, M.-L.; Nymand, L.; Kristensen, L.E.; Thyssen, J.P.; Thomsen, S.F.; Cordtz, R.L.; et al. Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis—A nationwide cohort study from the DANBIO and DERMBIO registries. Semin. Arthritis Rheum. 2022, 53, 151979. [Google Scholar] [CrossRef] [PubMed]
- Glintborg, B.; Østergaard, M.; Krogh, N.S.; Tarp, U.; Manilo, N.; Loft, A.G.R.; Hansen, A.; Schlemmer, A.; Fana, V.; Lindegaard, H.M.; et al. Clinical response, drug survival and predictors thereof in 432 ankylosing spondylitis patients after switching tumour necrosis factor α inhibitor therapy: Results from the Danish nationwide DANBIO registry. Ann. Rheum. Dis. 2013, 72, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Glintborg, B.; Ostergaard, M.; Krogh, N.S.; Dreyer, L.; Kristensen, H.L.; Hetland, M.L. Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti-tumour necrosis factor: Results from 8 years’ surveillance in the Danish nationwide DANBIO registry. Ann. Rheum. Dis. 2010, 69, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Visman, I.M.; Atiqi, S.; Boers, M.; Twisk, J.W.R.; Nurmohamed, M.T. Changes in Tumor Necrosis Factor Inhibitor Drug Survival in Patients with Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis Over 15 Years. J. Rheumatol. 2024, 51, 84–87. [Google Scholar] [CrossRef]
- Jeong, H.; Eun, Y.H.; Kim, I.Y.; Kim, H.; Ahn, J.K.; Lee, J.; Koh, E.-M.; Cha, H.-S. Drug survival of tumor necrosis factor α inhibitors in patients with ankylosing spondylitis in Korea. Korean J. Intern. Med. 2018, 33, 407–416. [Google Scholar] [CrossRef]
- Lam, W.Y.; Fresco, P. Medication Adherence Measures: An Overview. BioMed Res. Int. 2015, 2015, 217047. [Google Scholar] [CrossRef]
- Cramer, J.A.; Roy, A.; Burrell, A.; Fairchild, C.J.; Fuldeore, M.J.; Ollendorf, D.A.; Wong, P.K. Medication compliance and persistence: Terminology and definitions. Value Health 2008, 11, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.M.; Nau, D.P.; Cramer, J.A.; Benner, J.; Gwadry-Sridhar, F.; Nichol, M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007, 10, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.L.; Quan, H.; Rabi, D.M. Measuring medication adherence in patients with incident hypertension: A retrospective cohort study. BMC Health Serv. Res. 2017, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Gendelman, O.; Weitzman, D.; Rosenberg, V.; Shalev, V.; Chodick, G.; Amital, H. Characterization of adherence and persistence profile in a real-life population of patients treated with adalimumab. Br. J. Clin. Pharmacol. 2018, 84, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wen, J.; Alexander, G.C.; Curtis, J.R. Comparative effectiveness of biologics and targeted therapies for psoriatic arthritis. RMD Open 2021, 7, e001399. [Google Scholar] [CrossRef]
- Weddell, J.; Din, N.R.A.; Harrison, S.R.; Michelena, X.; McGonagle, D.; Barr, A.; Vandevelde, C.; Freeston, J.; Marzo-Ortega, H. Real-world experience of IL-17Ai drug survival in a large cohort of axial spondyloarthritis and psoriatic arthritis. Rheumatol. Adv. Pract. 2024, 8, rkae018. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Villa, I.; Fernández, S.; Martín, J.L.; Charca, L.; Pino, M.; Riancho, L.; Morante, I.; Santos, M.; Brandy, A.; et al. Multicenter Study of Secukinumab Survival and Safety in Spondyloarthritis and Psoriatic Arthritis: SEcukinumab in Cantabria and ASTURias Study. Front. Med. 2021, 8, 679009. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, K.; Bodnár, N.; Nagy, Z.; Szamosi, S.; Horváth, Á.; Váncsa, A.; Szűcs, G.; Szekanecz, Z.; Szántó, S.Z. Real-life experience with switching TNF-α inhibitors in ankylosing spondylitis. Eur. J. Health Econ. 2014, 15, S93–S100. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-L.; Yang, C.-H.; Chi, C.-C. Drug Survival of Biologics in Treating Ankylosing Spondylitis: A Systematic Review and Meta-analysis of Real-World Evidence. BioDrugs 2020, 34, 669–679. [Google Scholar] [CrossRef]
- Diaconu, A.-D.; Pomîrleanu, C.; Russu, M.; Strugariu, G.; Ancuța, E.; Ciortescu, I.; Bologa, C.; Morărașu, B.C.; Constantin, M.; Ceasovschih, A.; et al. Drug Survival, Effectiveness and Safety of Secukinumab in Axial Spondyloarthritis up to 4 Years: A Real-Life Single Center Experience. J. Pers. Med. 2024, 14, 417. [Google Scholar] [CrossRef]
- Deodhar, A.; Yu, D. Switching tumor necrosis factor inhibitors in the treatment of axial spondyloarthritis. Semin. Arthritis Rheum. 2017, 47, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Manica, S.R.; Sepriano, A.; Pimentel-Santos, F.; Gouveia, N.; Barcelos, A.; Branco, J.C.; Bernardes, M.; Ferreira, R.M.; Vieira-Sousa, E.; Barreira, S.; et al. Effectiveness of switching between TNF inhibitors in patients with axial spondyloarthritis: Is the reason to switch relevant? Arthritis Res. Ther. 2020, 22, 195. [Google Scholar] [CrossRef] [PubMed]
- Micheroli, R.; Tellenbach, C.; Scherer, A.; Bürki, K.; Niederman, K.; Nissen, M.J.; Zufferey, P.; Exer, P.; Möller, B.; Kyburz, D.; et al. Effectiveness of secukinumab versus an alternative TNF inhibitor in patients with axial spondyloarthritis previously exposed to TNF inhibitors in the Swiss Clinical Quality Management cohort. Ann. Rheum. Dis. 2020, 79, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, F.; Andras, L.; Pereira-Gillion, C.; Goupille, P.; Salliot, C. Drug maintenance of a second tumor necrosis factor alpha inhibitor in spondyloarthritis patients: A real-life multicenter study. Jt. Bone Spine 2019, 86, 761–767. [Google Scholar] [CrossRef]
- Arturi, P.; Schneeberger, E.E.; Sommerfleck, F.; Buschiazzo, E.; Ledesma, C.; Maldonado Cocco, J.A.; Citera, G. Adherence to treatment in patients with ankylosing spondylitis. Clin. Rheumatol. 2013, 32, 1007–1015. [Google Scholar] [CrossRef]
- Larid, G.; Baudens, G.; Tiemdjo-Djimaffo, G.; Coquerelle, P.; Goeb, V.; Guyot, M.H.; Marguerie, L.; Maury, F.; Veillard, E.; Houvenagel, E.; et al. Retention rate of subcutaneous TNF inhibitors in axial spondyloarthritis in a multicentre study from the RIC-FRANCE network. Sci. Rep. 2024, 14, 1374. [Google Scholar] [CrossRef]
- Nissen, M.J.; Ciurea, A.; Bernhard, J.; Tamborrini, G.; Mueller, R.; Weiss, B.; Toniolo, M.; Exer, P.; Gabay, C.; Finckh, A.; et al. The Effect of Comedication with a Conventional Synthetic Disease-Modifying Antirheumatic Drug on Drug Retention and Clinical Effectiveness of Anti-Tumor Necrosis Factor Therapy in Patients with Axial Spondyloarthritis. Arthritis Rheumatol. 2016, 68, 2141–2150. [Google Scholar] [CrossRef]
- Wallman, J.K.; Kapetanovic, M.C.; Petersson, I.F.; Geborek, P.; Kristensen, L.E. Comparison of non-radiographic axial spondyloarthritis and ankylosing spondylitis patients--baseline characteristics, treatment adherence, and development of clinical variables during three years of anti-TNF therapy in clinical practice. Arthritis Res. Ther. 2015, 17, 378. [Google Scholar] [CrossRef]
- Corli, J.; Flipo, R.-M.; Philippe, P.; Bera-Louville, A.; Béhal, H.; Wibaux, C.; Paccou, J. Tumor Necrosis Factor-α Inhibition in Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis: Treatment Response, Drug Survival, and Patient Outcome. J. Rheumatol. 2015, 42, 2376–2382. [Google Scholar] [CrossRef]
- Lin, P.-T.; Wang, S.-H.; Chi, C.-C. Drug survival of biologics in treating psoriasis: A meta-analysis of real-world evidence. Sci. Rep. 2018, 8, 16068. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).