Confocal Laser Endomicroscopy in Resection of Sinonasal Malignant Melanoma—Preliminary Report on Real-Time Margin Assessment and Support in Surgical Decision-Making
Abstract
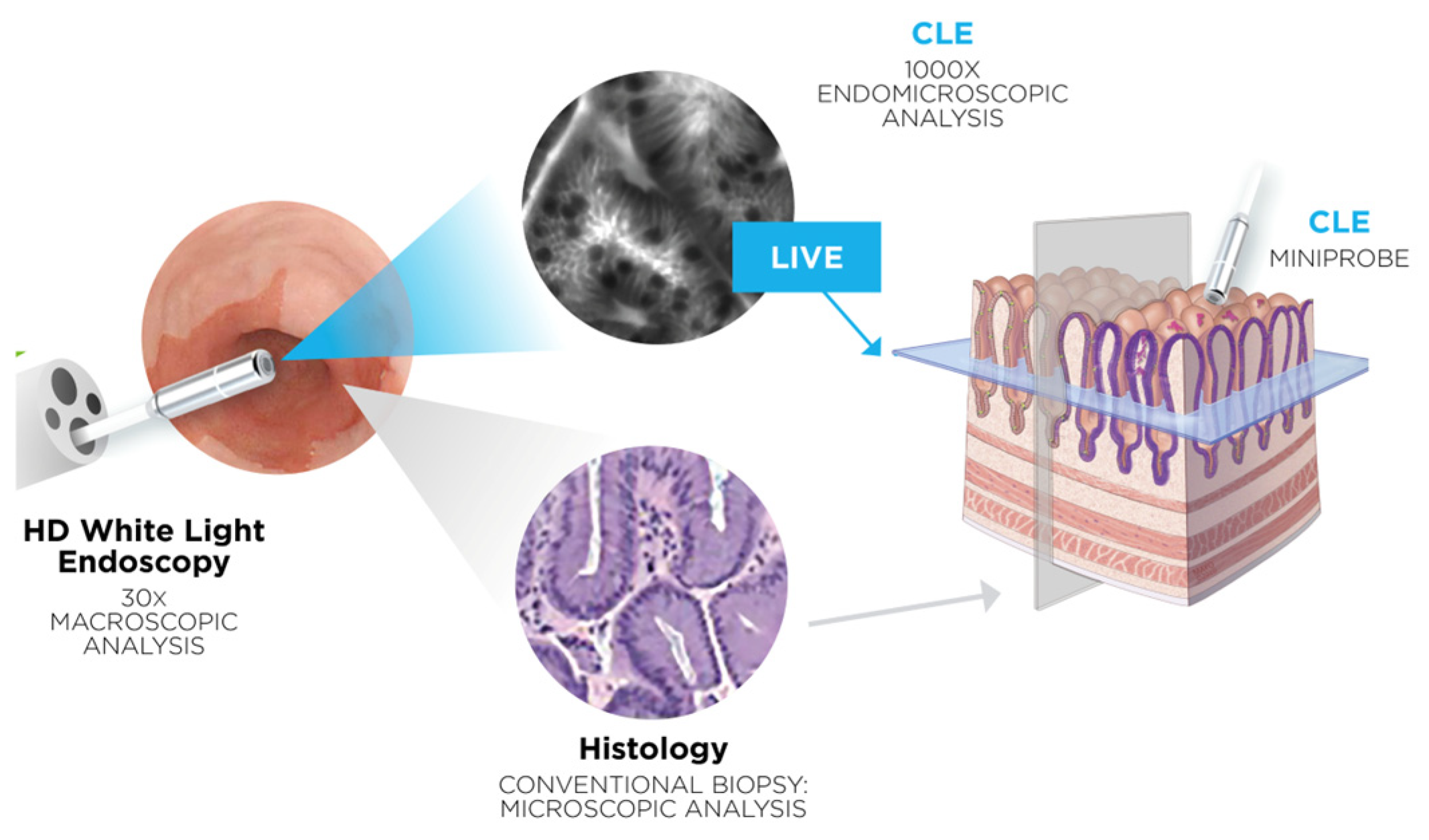
1. Introduction
2. Materials and Methods
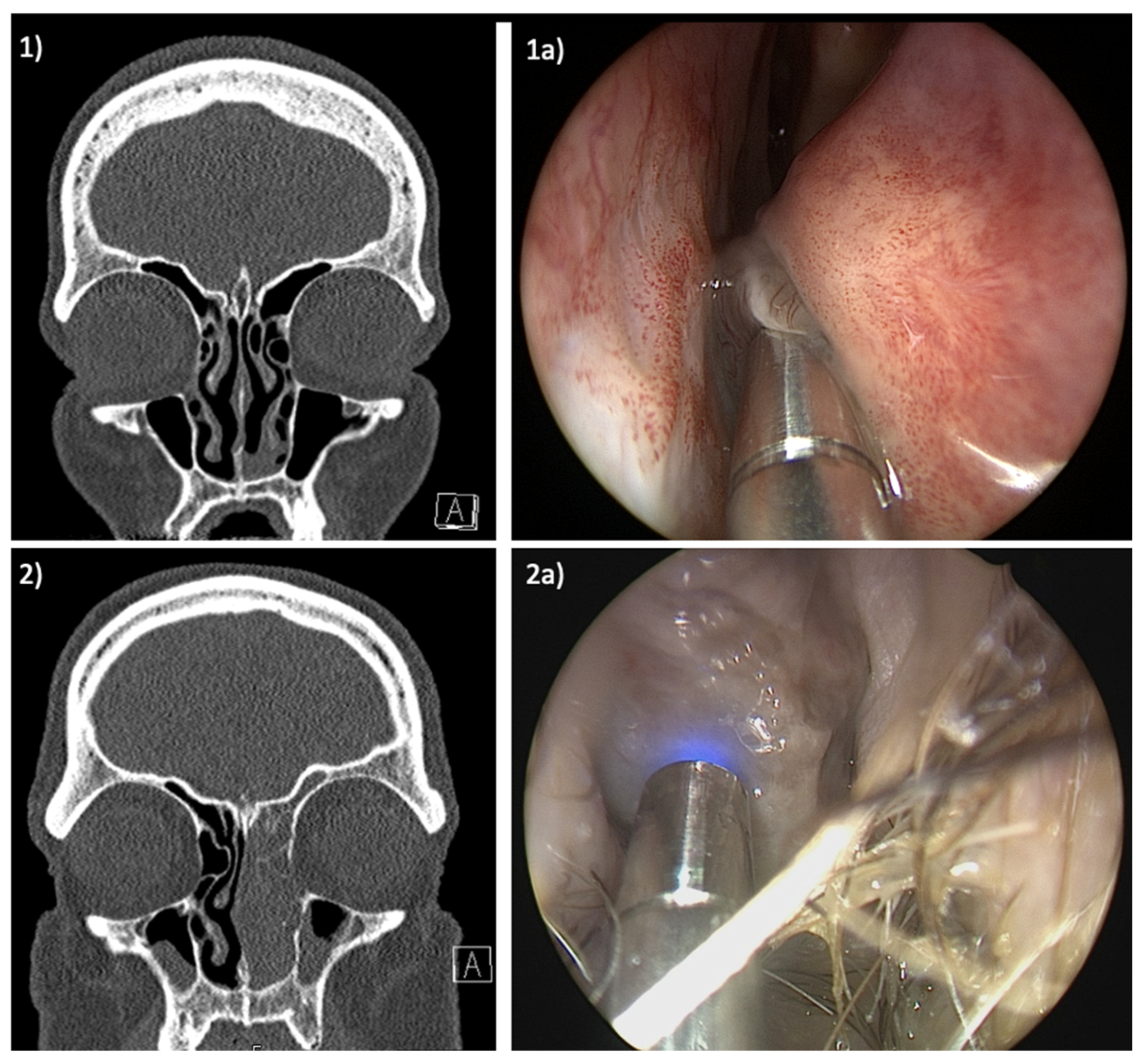
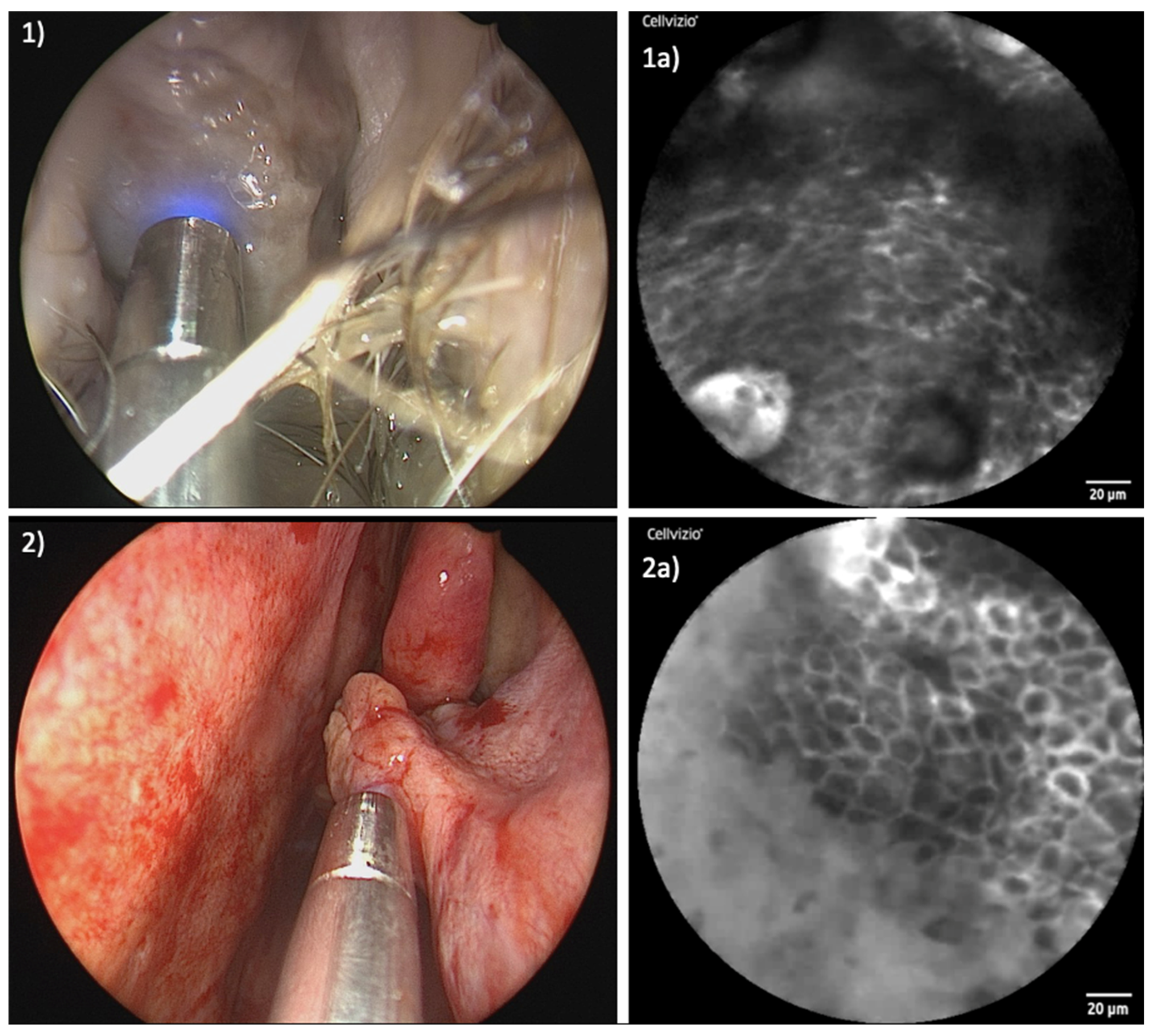
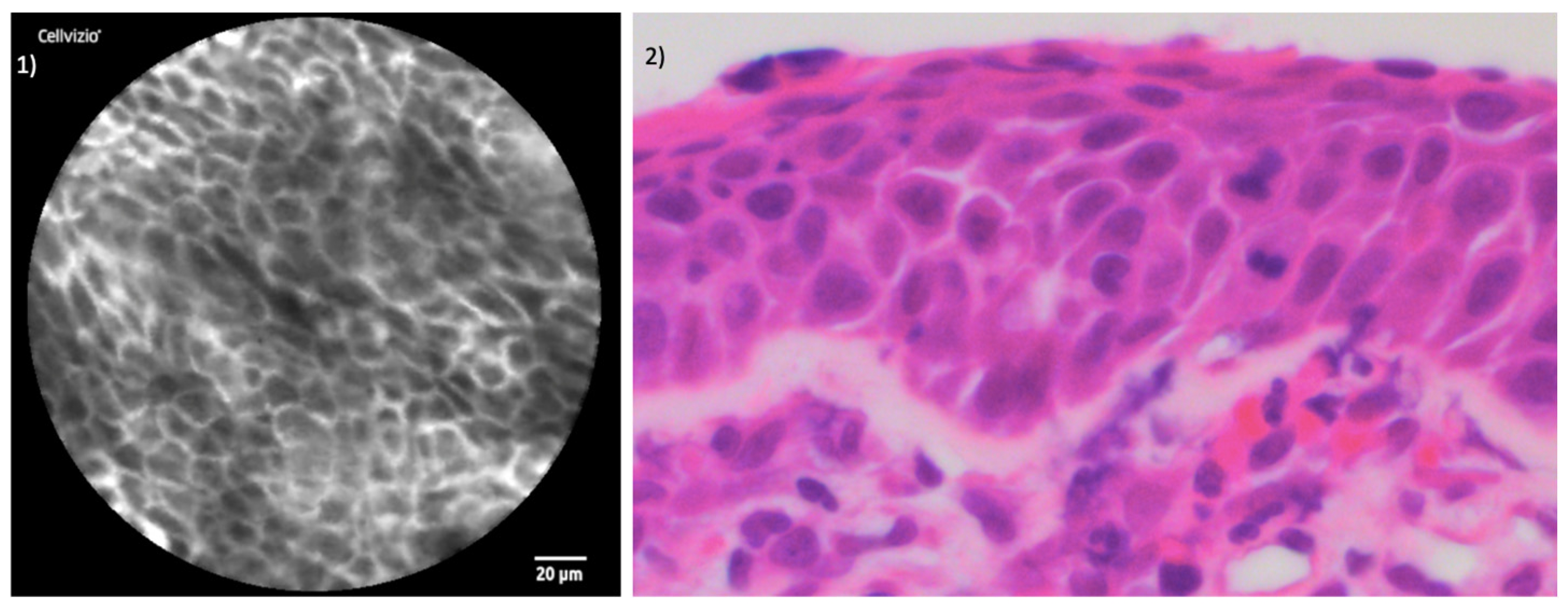
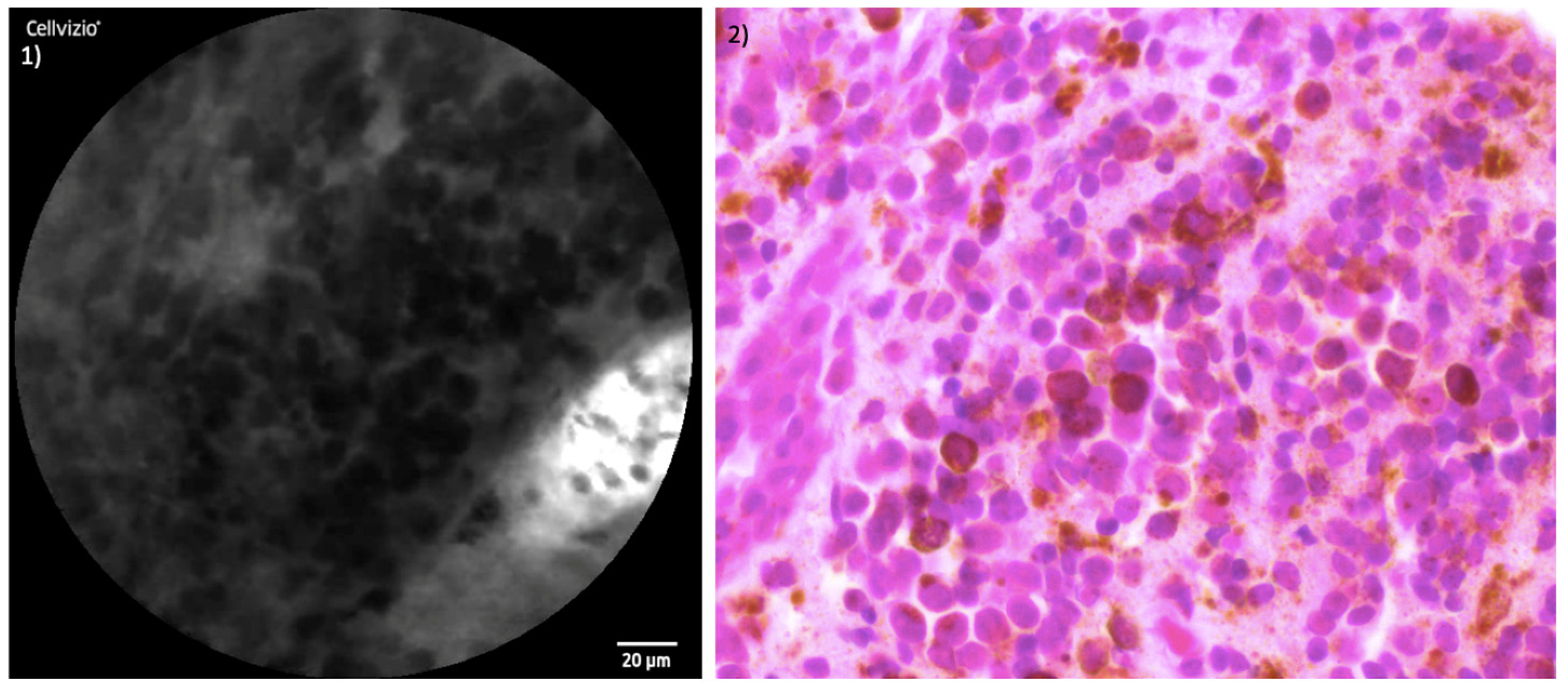
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amit, M.; Na’ara, S.; Hanna, E.Y. Contemporary Treatment Approaches to Sinonasal Mucosal Melanoma. Curr. Oncol. Rep. 2018, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Gilain, L.; Houette, A.; Montalban, A.; Mom, T.; Saroul, N. Mucosal melanoma of the nasal cavity and paranasal sinuses. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2014, 131, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Salari, B.; Foreman, R.K.; Emerick, K.S.; Lawrence, D.P.; Duncan, L.M. Sinonasal Mucosal Melanoma: An Update and Review of the Literature. Am. J. Dermatopathol. 2022, 44, 424–432. [Google Scholar] [CrossRef]
- Chłopek, M.; Lasota, J.; Thompson, L.D.R.; Szczepaniak, M.; Kuźniacka, A.; Hińcza, K.; Kubicka, K.; Kaczorowski, M.; Newford, M.; Liu, Y.; et al. Alterations in key signaling pathways in sinonasal tract melanoma. A molecular genetics and immunohistochemical study of 90 cases and comprehensive review of the literature. Mod. Pathol. 2022, 35, 1609–1617. [Google Scholar] [CrossRef]
- Crippen, M.M.; Kılıç, S.; Eloy, J.A. Updates in the management of sinonasal mucosal melanoma. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 52–57. [Google Scholar] [CrossRef]
- Chatelet, F.; Simon, F.; Bedarida, V.; Le Clerc, N.; Adle-Biassette, H.; Manivet, P.; Herman, P.; Verillaud, B. Surgical Management of Sinonasal Cancers: A Comprehensive Review. Cancers 2021, 13, 3995. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.D. Confocal laser endomicroscopy in the “in vivo” histological diagnosis of the gastrointestinal tract. World J. Gastroenterol. 2009, 15, 5770–5775. [Google Scholar] [CrossRef]
- Frieling, T.; Gjini, B.; Melchior, I.; Hemmerlein, B.; Kiesslich, R.; Kuhlbusch-Zicklam, R. Eosinophilic esophagitis and duodenal food challenge—Evaluation through endoscopic confocal laser endomicroscopy. Z. Für Gastroenterol. 2023; Epub ahead of print. [Google Scholar]
- Tian, S.; Huang, H.; Zhang, Y.; Shi, H.; Dong, Y.; Zhang, W.; Bai, C. The role of confocal laser endomicroscopy in pulmonary medicine. Eur. Respir. Rev. 2023, 32, 220185. [Google Scholar] [CrossRef]
- Bitaraf, M.; Ghafoori Yazdi, M.; Amini, E. Upper Tract Urothelial Carcinoma (UTUC) Diagnosis and Risk Stratification: A Comprehensive Review. Cancers 2023, 15, 4987. [Google Scholar] [CrossRef]
- Maragkou, T.; Quint, K.; Pollo, B.; Hewer, E. Intraoperative confocal laser endomicroscopy for brain tumors—Potential and challenges from a neuropathological perspective. Free Neuropathol. 2022, 3, 3–24. [Google Scholar] [CrossRef]
- Sievert, M.; Stelzle, F.; Aubreville, M.; Mueller, S.K.; Eckstein, M.; Oetter, N.; Maier, A.; Mantsopoulos, K.; Iro, H.; Goncalves, M. Intraoperative free margins assessment of oropharyngeal squamous cell carcinoma with confocal laser endomicroscopy: A pilot study. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 4433–4439. [Google Scholar] [CrossRef] [PubMed]
- Pogorzelski, B.; Hanenkamp, U.; Goetz, M.; Kiesslich, R.; Gosepath, J. Systematic intraoperative application of confocal endomicroscopy for early detection and resection of squamous cell carcinoma of the head and neck: A preliminary report. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 404–411. [Google Scholar] [PubMed]
- Wenda, N.; Kiesslich, R.; Gosepath, J. Technical Note: First Use of Endonasal Confocal Laser Endomicroscopy—Feasibility and Proof of Concept. Int. Arch. Oto-Rhino-Laryngol. 2021, 26, e396–e400. [Google Scholar] [CrossRef] [PubMed]
- Wenda, N.; Fruth, K.; Fisseler-Eckhoff, A.; Gosepath, J. The Multifaceted Role of Confocal Laser Endomicroscopy in Head and Neck Surgery: Oncologic and Functional Insights. Diagnostics 2023, 13, 3081. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.J.; Howard, D.J.; Harding, L.; Wei, W.I. Management options and survival in malignant melanoma of the sinonasal mucosa. Laryngoscope 1999, 109 Pt 1, 208–211. [Google Scholar] [CrossRef]
- Lai, Y.; Meng, X.; Liu, Q.; Lu, H.; Guo, L.; Wang, S.; Wang, D. Impact of adjuvant therapy on survival for sinonasal mucosal melanoma. Acta Oto-laryngol. 2020, 140, 79–84. [Google Scholar] [CrossRef]
- Yin, G.; Guo, W.; Chen, X.; Huang, Z. Prognosis of endoscopic surgery and traditional open resection in mucosal melanoma of the nasal cavity and paranasal sinus. Melanoma Res. 2019, 29, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.D.; Friedland, S.; Sahbaie, P.; Soetikno, R.; Hsiung, P.L.; Liu, J.T.; Crawford, J.M.; Contag, C.H. Functional imaging of colonic mucosa with a fibered confocal microscope for real-time in vivo pathology. Clin. Gastroenterol. Hepatol. 2007, 5, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Lipson, B.K.; Yannuzzi, L.A. Complications of intravenous fluorescein injections. Int. Ophthalmol. Clin. 1989, 29, 200–205. [Google Scholar] [CrossRef]
- Abbaci, M.; Casiraghi, O.; Vergez, S.; Maillard, A.; Lakhdar, A.B.; De Leeuw, F.; Crestani, S.; Ngo, C.; Koscielny, S.; Ferchiou, M.; et al. Diagnostic accuracy of in vivo early tumor imaging from probe-based confocal laser endomicroscopy versus histologic examination in head and neck squamous cell carcinoma. Clin. Oral Investig. 2022, 26, 1823–1833. [Google Scholar] [CrossRef]
- Sethi, S.; Ju, X.; Logan, R.M.; Sambrook, P.; McLaughlin, R.A.; Jamieson, L.M. Diagnostic Accuracy of Confocal Laser Endomicroscopy for the Diagnosis of Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 12390. [Google Scholar] [CrossRef] [PubMed]
- Frenken, A.K.; Sievert, M.; Panuganti, B.; Aubreville, M.; Meyer, T.; Scherzad, A.; Gehrke, T.; Scheich, M.; Hackenberg, S.; Goncalves, M. Feasibility of Optical Biopsy During Endoscopic Sinus Surgery with Confocal Laser Endomicroscopy: A Pilot Study. Laryngoscope, 2024; Epub ahead of print. [Google Scholar]
- Sievert, M.; Mantsopoulos, K.; Mueller, S.K.; Eckstein, M.; Rupp, R.; Aubreville, M.; Stelzle, F.; Oetter, N.; Maier, A.; Iro, H.; et al. Systematic interpretation of confocal laser endomicroscopy: Larynx and pharynx confocal imaging score. Acta Otorhinolaryngol. Ital. 2022, 42, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Sievert, M.; Oetter, N.; Mantsopoulos, K.; Gostian, A.O.; Mueller, S.K.; Koch, M.; Balk, M.; Thimsen, V.; Stelzle, F.; Eckstein, M.; et al. Systematic classification of confocal laser endomicroscopy for the diagnosis of oral cavity carcinoma. Oral Oncol. 2022, 132, 105978. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenda, N.; Fruth, K.; Wagner, S.; Fisseler-Eckhoff, A.; Gosepath, J. Confocal Laser Endomicroscopy in Resection of Sinonasal Malignant Melanoma—Preliminary Report on Real-Time Margin Assessment and Support in Surgical Decision-Making. J. Clin. Med. 2024, 13, 4483. https://doi.org/10.3390/jcm13154483
Wenda N, Fruth K, Wagner S, Fisseler-Eckhoff A, Gosepath J. Confocal Laser Endomicroscopy in Resection of Sinonasal Malignant Melanoma—Preliminary Report on Real-Time Margin Assessment and Support in Surgical Decision-Making. Journal of Clinical Medicine. 2024; 13(15):4483. https://doi.org/10.3390/jcm13154483
Chicago/Turabian StyleWenda, Nina, Kai Fruth, Sebastian Wagner, Annette Fisseler-Eckhoff, and Jan Gosepath. 2024. "Confocal Laser Endomicroscopy in Resection of Sinonasal Malignant Melanoma—Preliminary Report on Real-Time Margin Assessment and Support in Surgical Decision-Making" Journal of Clinical Medicine 13, no. 15: 4483. https://doi.org/10.3390/jcm13154483
APA StyleWenda, N., Fruth, K., Wagner, S., Fisseler-Eckhoff, A., & Gosepath, J. (2024). Confocal Laser Endomicroscopy in Resection of Sinonasal Malignant Melanoma—Preliminary Report on Real-Time Margin Assessment and Support in Surgical Decision-Making. Journal of Clinical Medicine, 13(15), 4483. https://doi.org/10.3390/jcm13154483







