Efficacy and Safety of Magnesium Sulfate as an Adjunct to Ropivacaine Wound Infiltration in Thyroid Surgery: A Prospective, Double-Blind, Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Randomization and Masking
2.3. Anesthesia and Surgical Procedure
2.4. Assessment of Postoperative Pain
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Clinical and Demographic Characteristics of Study Patients
3.2. Total Analgesic Requirements
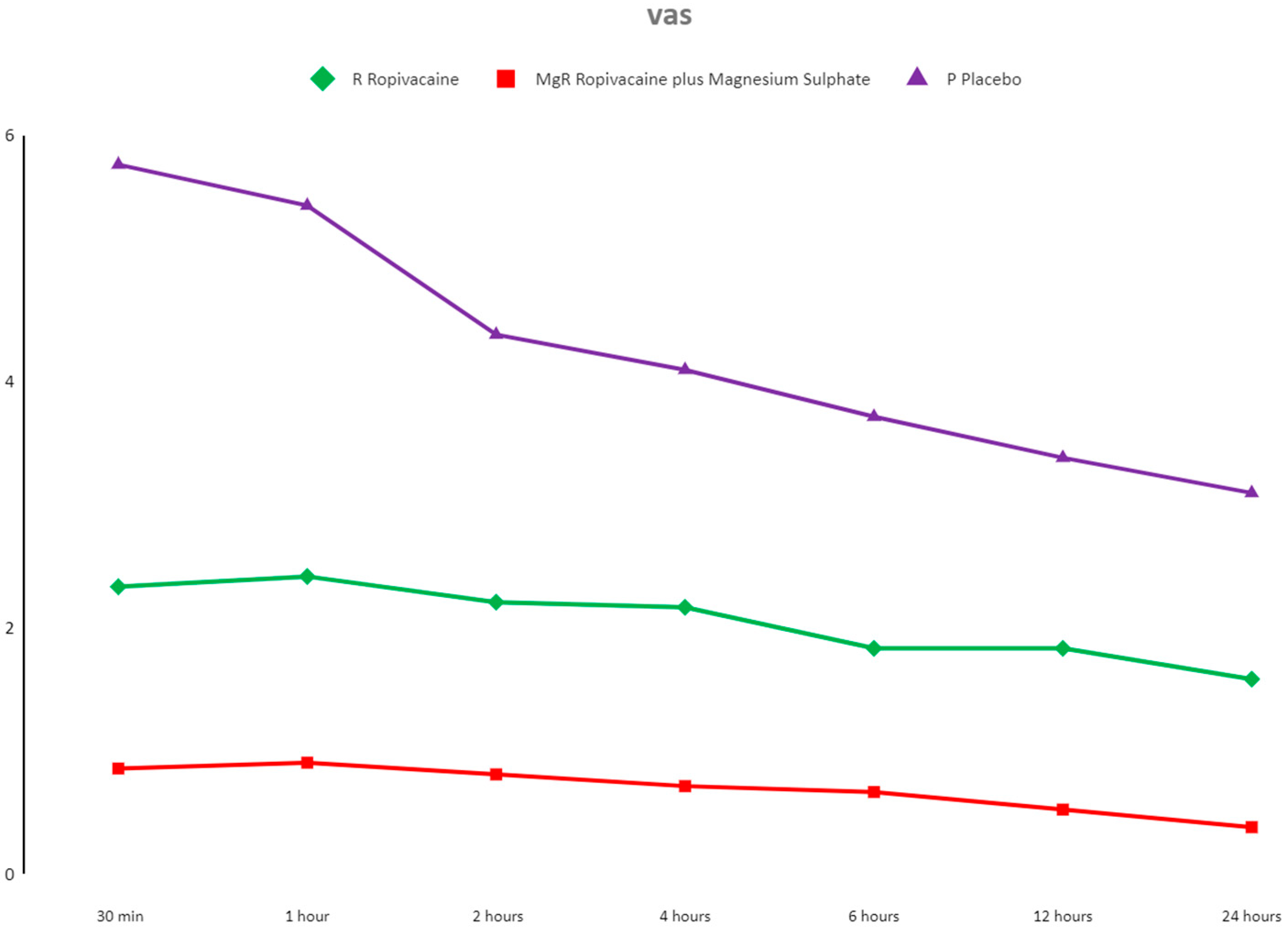
3.3. Pain Evaluation
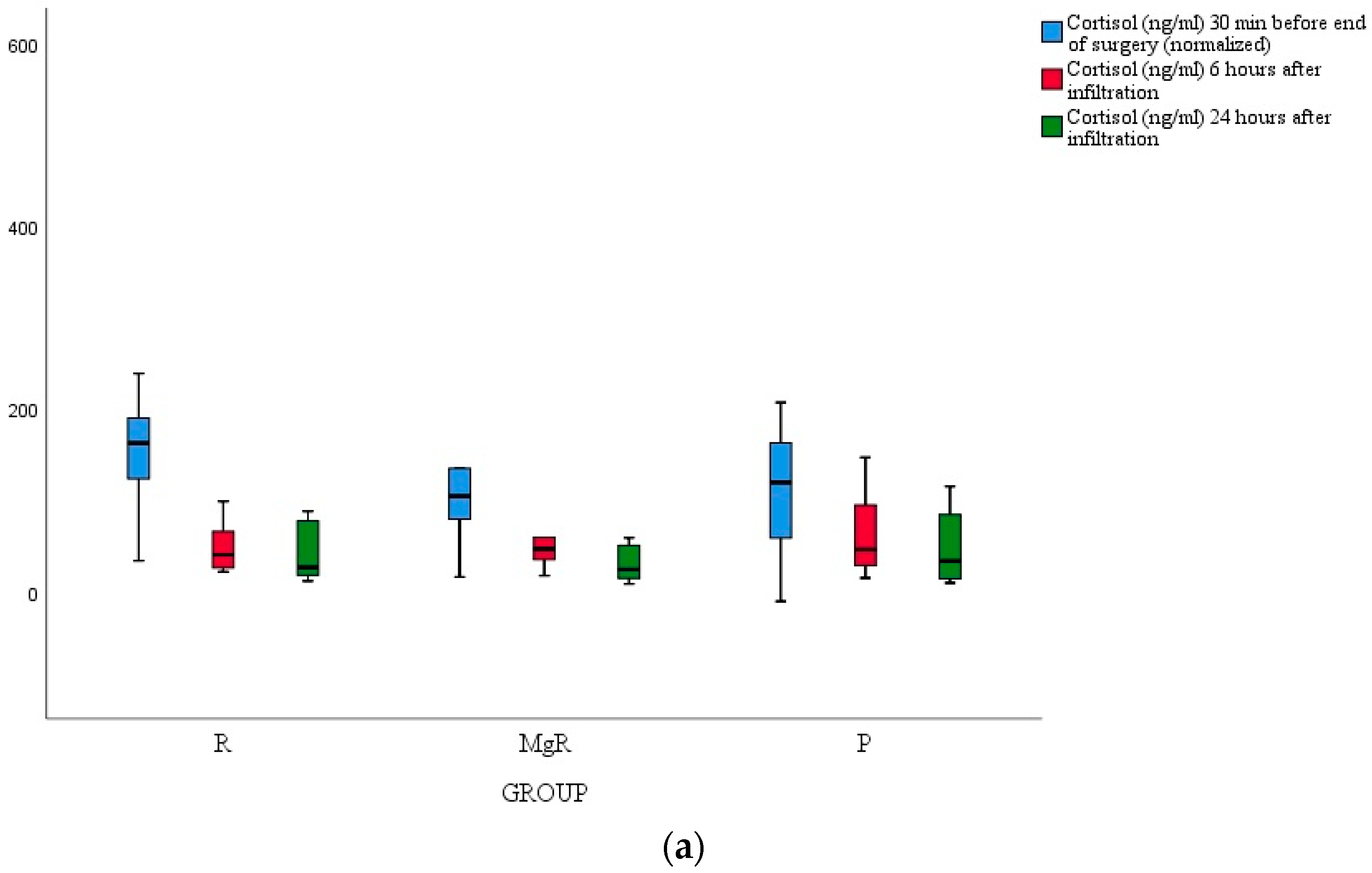
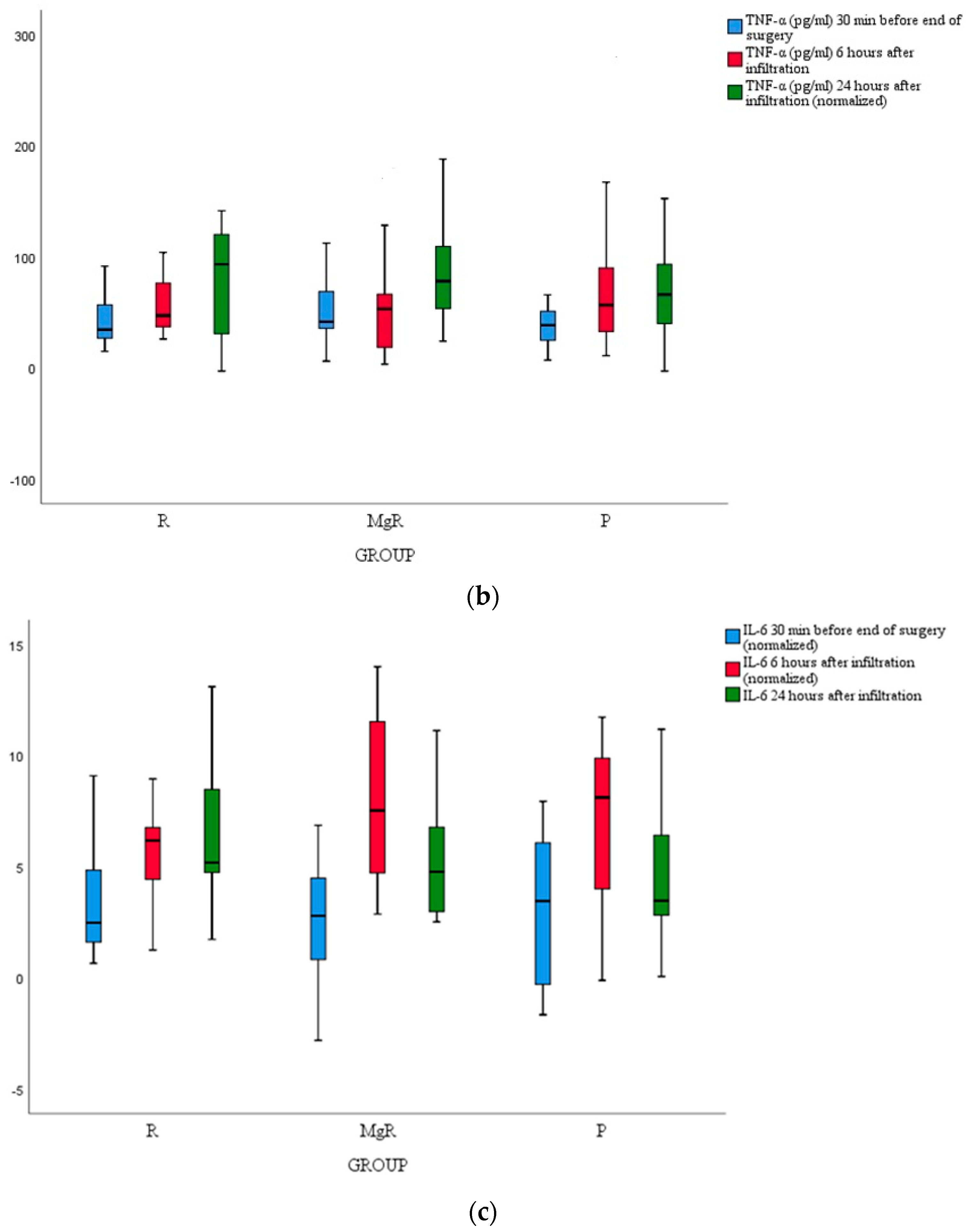
3.4. Inflammatory Marker Release
3.5. Postoperative Complications
3.6. Patients’ Satisfaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terris, D.J.; Snyder, S.; Carneiro-Pla, D.; Iii, W.B.I.; Kandil, E.; Orloff, L.; Shindo, M.; Tufano, R.P.; Tuttle, R.M.; Urken, M.; et al. American Thyroid Association statement on outpatient thyroidectomy. Thyroid 2013, 23, 1193–1202. [Google Scholar] [CrossRef]
- Bianchini, C.; Malago, M.; Crema, L.; Aimoni, C.; Matarazzo, T.; Bortolazzi, S.; Ciorba, A.; Pelucchi, S.; Pastore, A. Post-operative pain management in head and neck cancer patients: Predictive factors and efficacy of therapy. Acta Otorhinolaryngol. Ital. 2016, 36, 91. [Google Scholar] [CrossRef]
- Nabata, K.J.; Guo, R.; Nguyen, A.; Osborn, J.A.; Wiseman, S.M. Superiority of non-opioid postoperative pain management after thyroid and parathyroid operations: A systematic review and meta-analysis. Surg. Oncol. 2022, 41, 101731. [Google Scholar] [CrossRef]
- Laskou, S.; Tsaousi, G.; Pourzitaki, C.; Loukipoudi, L.; Papazisis, G.; Kesisoglou, I.; Sapalidis, K. Local Wound Infiltration for Thyroidectomized Patients in the Era of Multimodal Analgesia. Medicina (Kaunas) 2023, 59, 1662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tauzin-Fin, P.; Sesay, M.; Svartz, L.; Krol-Houdek, M.C.; Maurette, P. Wound infiltration with magnesium sulphate and ropivacaine mixture reduces postoperative tramadol requirements after radical prostatectomy. Acta Anaesthesiol. Scand. 2009, 53, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Eldaba, A.A.; Amr, Y.M.; Sobhy, R.A. Effect of wound infiltration with bupivacaine or lower dose bupivacaine/magnesium versus placebo for postoperative analgesia after cesarean section. Anesth. Essays Res. 2013, 7, 336–340. [Google Scholar]
- Kundra, S.; Singh, R.M.; Singh, G.; Singh, T.; Jarewal, V.; Katyal, S. Efficacy of Magnesium Sulphate as an Adjunct to Ropivacaine in Local Infiltration for Postoperative Pain Following Lower Segment Caesarean Section. J. Clin. Diagn. Res. 2016, 10, 18–22. [Google Scholar] [CrossRef]
- Donadi, P.; Moningi, S.; Gopinath, R. Comparison of bupivacaine anδ bupivacaine plus magnesium sulphate infiltration for postoperative analgesia in patients undergoing lumbar laminectomy: A prospective randomised double-blinded controlled study. J. Neuroanaesth. Crit. Care 2014, 1, 183–187. [Google Scholar]
- Miu, M.; Royer, C.; Gaillat, C.; Schaup, B.; Menegaux, F.; Langeron, O.; Riou, B.; Aubrun, F. Lack of Analgesic Effect Induced by Ropivacaine Wound Infiltration in Thyroid Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. Anesth. Analg. 2016, 122, 559–564. [Google Scholar] [CrossRef]
- Lacoste, L.; Thomas, D.; Kraimps, J.L.; Chabin, M.; Ingrand, P.; Barbier, J.; Fusciardi, J. Postthyroidectomy analgesia: Morphine, buprenorphine, or bupivacaine? J. Clin. Anesth. 1997, 9, 189–193. [Google Scholar] [CrossRef]
- Ayman, M.; Materazzi, G.; Bericotti, M.; Rago, R.; Nidal, Y.; Miccoli, P. Bupivacaine 0.5% versus ropivacaine 0.75% wound infiltration to decrease postoperative pain in total thyroidectomy, a prospective controlled study. Minerva Chir. 2012, 67, 511–516. [Google Scholar]
- Li, X.; Yu, L.; Yang, J.; Tan, H. Multimodal analgesia with ropivacaine wound infiltration and intravenous flurbiprofen axetil provides enhanced analgesic effects after radical thyroidectomy: A randomized controlled trial. BMC Anesthesiol. 2019, 19, 167. [Google Scholar]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) [Updated 2023 May 1]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547742/ (accessed on 15 May 2024).
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Physician 2008, 11 (Suppl. S2), S105–S120. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Song, Y.-K.; Jeong, H.-M.; Park, S.-N. The effects of magnesium sulfate infiltration on perioperative opioid consumption and opioid-induced hyperalgesia in patients undergoing robot-assisted laparoscopic prostatectomy with remifentanil-based anesthesia. Korean J. Anesthesiol. 2011, 61, 244–250. [Google Scholar] [CrossRef]
- Razavi, S.S.; Peyvandi, H.; Jam, A.R.B.; Safari, F.; Teymourian, H.; Mohajerani, S.A. Versus Bupivacaine Infiltration in Controlling Postoperative Pain in Inguinal Hernia Repair. Anesth. Pain Med. 2015, 5, e30643. [Google Scholar] [CrossRef]
- Karaaslan, K.; Yilmaz, F.; Gulcu, N.; Sarpkaya, A.; Colak, C.; Kocoglu, H. The effects of levobupivacaine versus levobupivacaine plus magnesium infiltration on postoperative analgesia and laryngospasm in pediatric tonsillectomy patients. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 675–681. [Google Scholar] [CrossRef]
- Derbel, R.; Achour, I.; Thabet, W.; Chakroun, A.; Zouch, I.; Charfeddine, I. Addition of magnesium sulfate to bupivacaine improves analgesic efficacy after tonsillectomy: A randomized trial and a CONSORT analysis. Eur. Ann. Otorhinolaryngol. Head Neck. Dis. 2022, 139, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, X.; Zhao, X.; Chen, F.; Wang, W. Pre-emptive peritonsillar infiltration of magnesium sulphate and ropivacaine vs. ropivacaine or magnesium alone for relief of post-adenotonsillectomy pain in children. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 499–503. [Google Scholar] [CrossRef]
- Sane, S.; Mahdkhah, A.; Golabi, P.; Hesami, S.A.; Kazemi Haki, B. Comparison the effect of bupivacaine plus magnesium sulfate with ropivacaine plus magnesium sulfate infiltration on postoperative pain in patients undergoing lumbar laminectomy with general anesthesia. Br. J. Neurosurg. 2020, 17, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, R.; Parua, S.; Choudhury, D.; Barooah, R.K. Comparison of Bupivacaine Plus Magnesium Sulfate and Ropivacaine Plus Magnesium Sulfate Infiltration for Postoperative Analgesia in Patients Undergoing Lumbar Laminectomy: A Randomized Double-blinded Study. Anesth. Essays Res. 2017, 11, 686–691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dave, S.; Gopalakrishnan, K.; Krishnan, S.; Natarajan, N. Analgesic Efficacy of Addition of Magnesium Sulfate to Bupivacaine in Wound Infiltration Technique in Perianal Surgeries. Anesth. Essays Res. 2022, 16, 250–254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Q.; Zhao, C.; Hu, J.; Ma, T.; Yang, J.; Kang, P. Efficacy of a Modified Cocktail for Periarticular Local Infiltration Analgesia in Total Knee Arthroplasty: A Prospective, Double-Blinded, Randomized Controlled Trial. J. Bone Jt. Surg. 2023, 105, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, L.; Chen, L.; Wang, Q.; Kang, P. Effects of magnesium sulfate on periarticular infiltration analgesia in total knee arthroplasty: A prospective, double-blind, randomized controlled trial. J. Orthop. Surg. Res. 2023, 18, 301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- George, A.M.; Liu, M. Ropivacaine. [Updated 2023 Jul 31]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532924/ (accessed on 16 June 2024).
- Graf, B.M.; Abraham, I.; Eberbach, N.; Kunst, G.; Stowe, D.F.; Martin, E. Differences in cardiotoxicity of bupivacaine and ropivacaine are the result of physicochemical and stereoselective properties. Anesthesiology 2002, 96, 1427–1434. [Google Scholar] [CrossRef]
- Motamed, C.; Merle, J.C.; Combes, X.; Yahkou, L.; Saidi, N.E.; Degranges, P.; Dhonneur, G. Postthyroidectomy pain control using ropivacaine wound infiltration after intraoperative remifentanil: A prospective double blind randomized controlled study. Acute Pain. 2007, 9, 119–123. [Google Scholar] [CrossRef]
- Karamanlioglu, B.; Turan, A.; Memis, D.; Kaya, G.; Ozata, S.; Ture, M. Infiltration with ropivacaine plus lornoxicam reduces postoperative pain and opioid consumption. Can. J. Anaesth. 2005, 52, 1047–1053. [Google Scholar] [CrossRef]
- Kilbas, Z.; Mentes, M.Ö.; Harlak, A.; Yigit, T.; Balkan, S.M.; Cosar, A.; Öztürk, E.; Kozak, O.; Tufan, C.T. Efficacy of wound infiltration with lornoxicam for postoperative analgesia following thyroidectomy: A prospective, randomized, double-blind study. Turk. J. Med. Sci. 2015, 45, 700–705. [Google Scholar] [CrossRef]
- Yücel, A.; Yazıcı, A.; Müderris, T.; Gül, F. Comparison of lornoxicam and low-dose tramadol for management of post-thyroidectomy pain. Agriculture 2016, 28, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T.; Ang, B.; Schmarbeck, T.; Rhodes, J. Monitoring of sympathetic tone to assess postoperative pain: Skin conductance vs surgical stress index. Anaesthesia 2009, 64, 727e31. [Google Scholar] [CrossRef]
- Jensen, E.W.; Valencia, J.F.; López, A.; Anglada, T.; Agustí, M.; Ramos, Y.; Serra, R.; Jospin, M.; Pineda, P.; Gambus, P. Monitoring hypnotic effect and nociception with two EEG-derived indices, qCON and qNOX, during general anaesthesia. Acta Anaesthesiol. Scand 2014, 58, 933e41. [Google Scholar] [CrossRef]
- Jakuscheit, A.; Weth, J.; Lichtner, G.; Jurth, C.; Rehberg, B.; von Dincklage, F. Intraoperative monitoring of analgesia using nociceptive reflexes correlates with delayed extubation and immediate postoperative pain: A prospective observational study. Eur. J. Anaesthesiol. 2017, 34, 297e305. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T. Objective monitoring of nociception: A review of current commercial solutions. Br. J. Anaesth. 2019, 123, e312–e321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirose, M.; Okutani, H.; Hashimoto, K.; Ueki, R.; Shimode, N.; Kariya, N.; Takao, Y.; Tatara, T. Intraoperative Assessment of Surgical Stress Response Using Nociception Monitor under General Anesthesia and Postoperative Complications: A Narrative Review. J. Clin. Med. 2022, 11, 6080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Group R | Group RMg | Group P | |
|---|---|---|---|
| Sex (M:F) | 4:20 | 5:16 | 5:16 |
| Age (years) BMI (kg/m2) | 54.78 ± 15.08 | 51.48 ± 15.56 | 62.14 ± 10.29 |
| BMI (kg/m2) | 27.49 ± 4.44 | 28.06 ± 6.25 | 27.52 ± 5.70 |
| Hamilton Anxiety Scale | 13.17 ± 5.44 | 14.05 ± 4.19 | 12.86 ± 3.55 |
| Duration of Surgery (min) | 108.54 ± 38.16 | 123.52 ± 27.11 | 125.05 ± 32.23 |
| Anesthesia Interruption to Extubation Time (min) | 5.75 ± 1.87 | 6.19 ± 3.37 | 6.43 ± 2.40 |
| Incision Length (cm) | 4.39 ± 1.05 | 4.50 ± 0.62 | 4.54 ± 0.72 |
| Remifentanil TCI (μg) | 795.96 ± 287.16 | 729.96 ± 389.40 | 1064.36 ± 457.94 |
| Group R | Group RMg | Group P | |
|---|---|---|---|
| 1st analgesic requirement (min) | 140.60 ± 127.56 * | 184.13 ± 131.58 *^ | 53.75 ± 60.62 |
| Total analgesic requirements in morphine equivalents (mg) | 18.30 ± 13.16 * | 7.53 ± 14.55 *^ | 39.35 ± 19.93 |
| Group R | Group RMg | Group P | |
|---|---|---|---|
| PONV | 2 (3.3%) | 2 (3.3%) | 3 (4.9%) |
| Shiver | 1 (6.1%) | 1 (1.5%) | 0 (0%) |
| Cough | 4 (6.1%) | 2 (3%) | 2 (3%) |
| Sore Throat | 9 (13.6%) | 6 (9.1%) | 9 (13.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskou, S.; Tsaousi, G.; Pourzitaki, C.; Papazisis, G.; Kesisoglou, I.; Sapalidis, K. Efficacy and Safety of Magnesium Sulfate as an Adjunct to Ropivacaine Wound Infiltration in Thyroid Surgery: A Prospective, Double-Blind, Randomized Controlled Trial. J. Clin. Med. 2024, 13, 4499. https://doi.org/10.3390/jcm13154499
Laskou S, Tsaousi G, Pourzitaki C, Papazisis G, Kesisoglou I, Sapalidis K. Efficacy and Safety of Magnesium Sulfate as an Adjunct to Ropivacaine Wound Infiltration in Thyroid Surgery: A Prospective, Double-Blind, Randomized Controlled Trial. Journal of Clinical Medicine. 2024; 13(15):4499. https://doi.org/10.3390/jcm13154499
Chicago/Turabian StyleLaskou, Stiliani, Georgia Tsaousi, Chryssa Pourzitaki, Georgios Papazisis, Isaak Kesisoglou, and Konstantinos Sapalidis. 2024. "Efficacy and Safety of Magnesium Sulfate as an Adjunct to Ropivacaine Wound Infiltration in Thyroid Surgery: A Prospective, Double-Blind, Randomized Controlled Trial" Journal of Clinical Medicine 13, no. 15: 4499. https://doi.org/10.3390/jcm13154499
APA StyleLaskou, S., Tsaousi, G., Pourzitaki, C., Papazisis, G., Kesisoglou, I., & Sapalidis, K. (2024). Efficacy and Safety of Magnesium Sulfate as an Adjunct to Ropivacaine Wound Infiltration in Thyroid Surgery: A Prospective, Double-Blind, Randomized Controlled Trial. Journal of Clinical Medicine, 13(15), 4499. https://doi.org/10.3390/jcm13154499








