Effects of Psychiatric Comorbidities on the Prognosis of New-Onset Pediatric Epilepsy: A Retrospective Nationwide Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
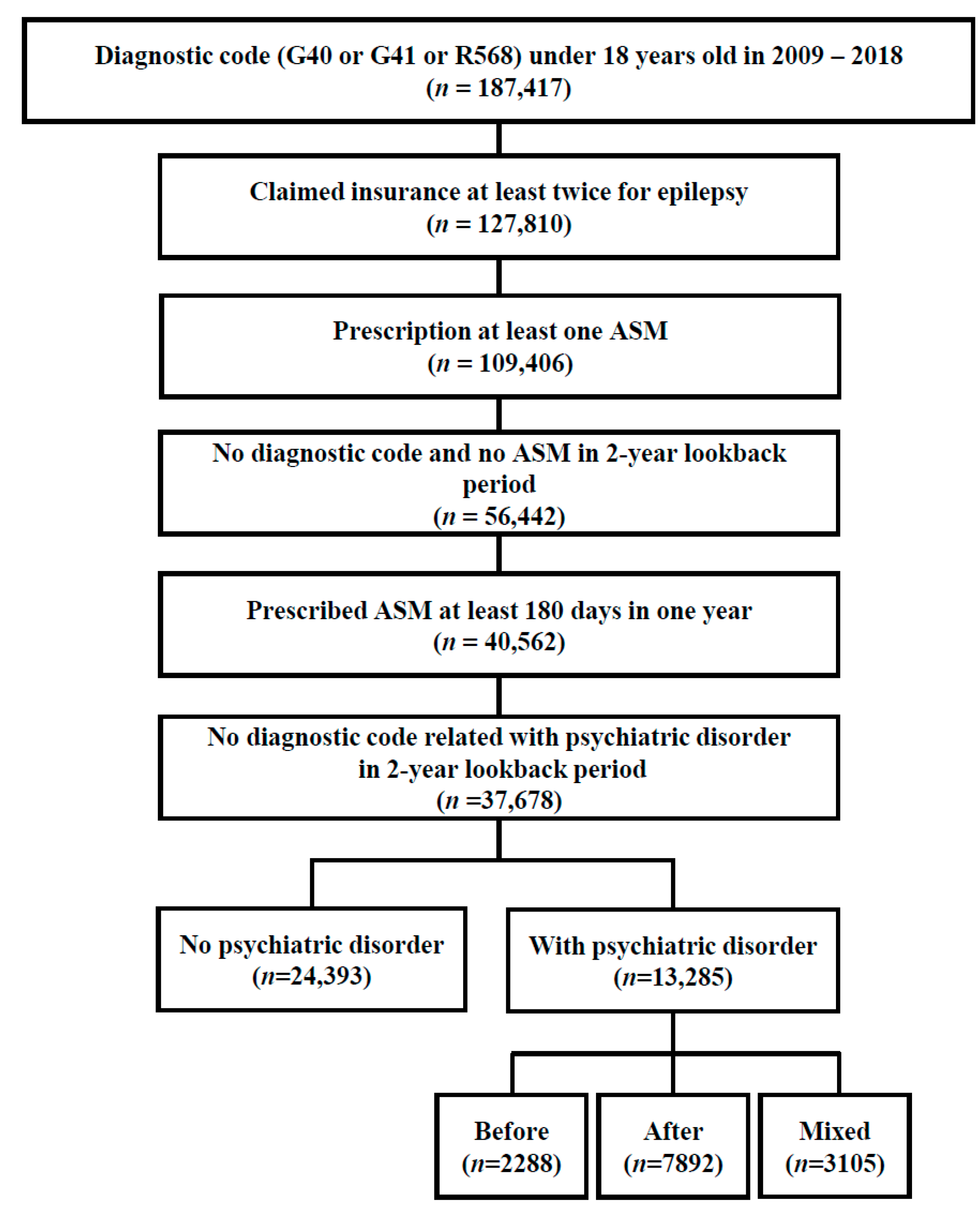
2.2. Study Population: Inclusion and Exclusion Criteria
2.3. Definition of Psychiatric Disorder
2.4. Variables
2.5. Study Outcome
2.6. Statistical Analysis
3. Results
3.1. Participants with Epilepsy and Psychiatric Disorder
3.2. Characteristics of the Four Groups
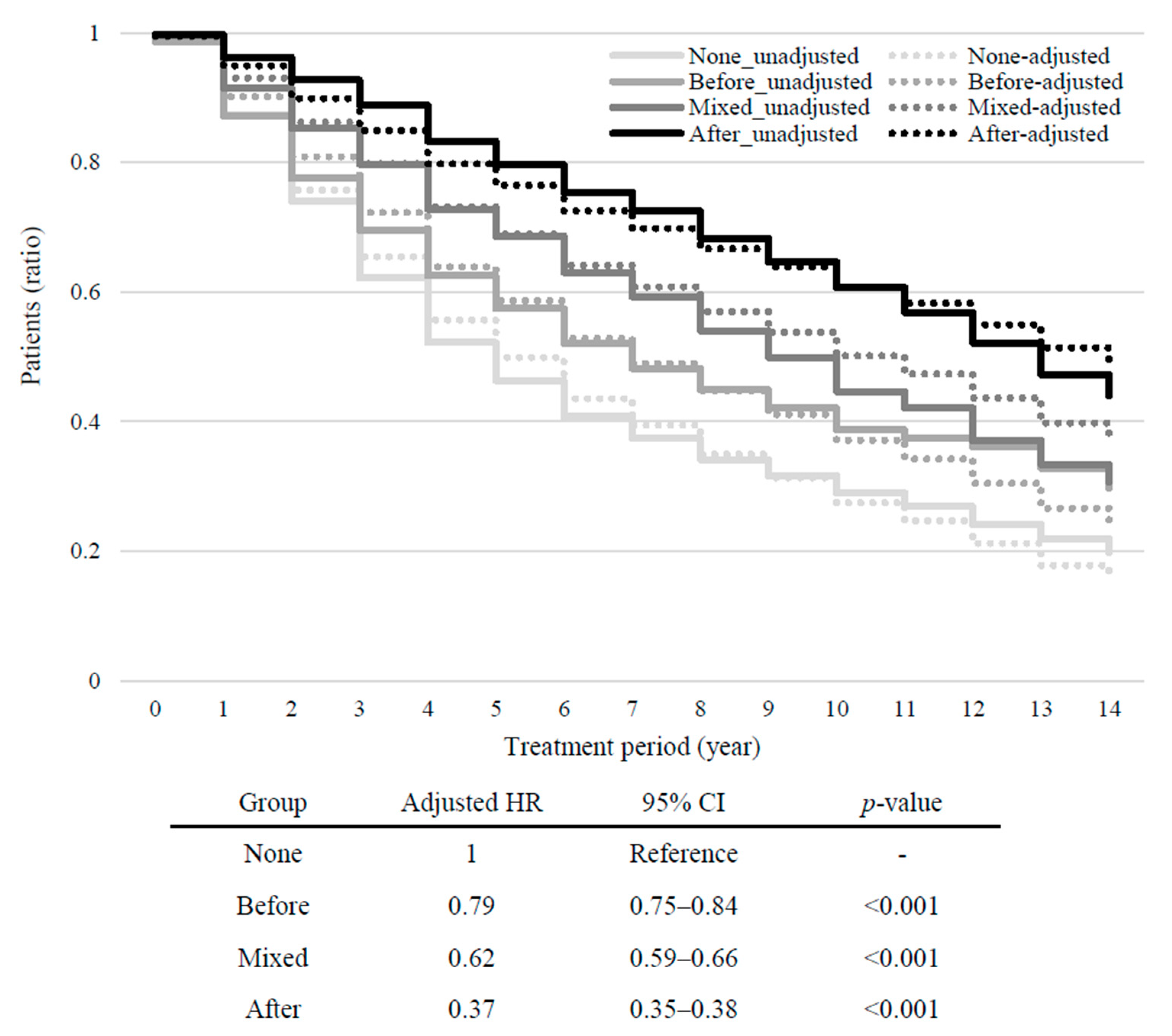
3.3. Intergroup Differences in Treatment Outcomes and Healthcare Utilization
3.4. Regression Analysis on the Healthcare Utilization and Clinical Characteristics for Epilepsy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russ, S.A.; Larson, K.; Halfon, N. A national profile of childhood epilepsy and seizure disorder. Pediatrics 2012, 129, 256–264. [Google Scholar] [CrossRef]
- Bartolini, E.; Ferrari, A.R.; Lattanzi, S.; Pradella, S.; Zaccara, G. Drug-resistant epilepsy at the age extremes: Disentangling the underlying etiology. Epilepsy Behav. 2022, 132, 108739. [Google Scholar] [CrossRef]
- Keezer, M.R.; Sisodiya, S.M.; Sander, J.W. Comorbidities of epilepsy: Current concepts and future perspectives. Lancet Neurol. 2016, 15, 106–115. [Google Scholar] [CrossRef]
- Aaberg, K.M.; Bakken, I.J.; Lossius, M.I.; Lund Søraas, C.; Håberg, S.E.; Stoltenberg, C.; Surén, P.; Chin, R. Comorbidity and childhood epilepsy: A nationwide registry study. Pediatrics 2016, 138, e20160921. [Google Scholar] [CrossRef] [PubMed]
- Hackett, R.; Hackett, L.; Bhakta, P. Psychiatric disorder and cognitive function in children with epilepsy in Kerala, South India. Seizure 1998, 7, 321–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Plioplys, S.; Dunn, D.W.; Caplan, R. 10-year research update review: Psychiatric problems in children with epilepsy. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 1389–1402. [Google Scholar] [CrossRef]
- Salpekar, J.A.; Mula, M. Common psychiatric comorbidities in epilepsy: How big of a problem is it? Epilepsy Behav. 2019, 98, 293–297. [Google Scholar] [CrossRef]
- Tellez-Zenteno, J.F.; Patten, S.B.; Jetté, N.; Williams, J.; Wiebe, S. Psychiatric comorbidity in epilepsy: A population-based analysis. Epilepsia 2007, 48, 2336–2344. [Google Scholar] [CrossRef]
- Åndell Jason, E. Neurodevelopmental and psychiatric comorbidities negatively affect outcome in children with unprovoked seizures-a non-systematic review. Acta Paediatr. 2021, 110, 2944–2950. [Google Scholar] [CrossRef]
- Hesdorffer, D.C.; Ishihara, L.; Mynepalli, L.; Webb, D.J.; Weil, J.; Hauser, W.A. Epilepsy, suicidality, and psychiatric disorders: A bidirectional association. Ann. Neurol. 2012, 72, 184–191. [Google Scholar] [CrossRef]
- Mula, M.; Monaco, F. Antiepileptic drugs and psychopathology of epilepsy: An update. Epileptic Disord. 2009, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mula, M.; Trimble, M.R.; Lhatoo, S.D.; Sander, J.W.A.S. Topiramate and psychiatric adverse events in patients with epilepsy. Epilepsia 2003, 44, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gistau, V.; Pintor, L.; Sugranyes, G.; Baillés, E.; Carreño, M.; Donaire, A.; Boget, T.; Setoain, X.; Bargalló, N.; Rumia, J. Prevalence of interictal psychiatric disorders in patients with refractory temporal and extratemporal lobe epilepsy in Spain. A comparative study. Epilepsia 2010, 51, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Jason, E.Å.; Tomson, T.; Carlsson, S.; Tedroff, K.; Åmark, P. Neurodevelopmental comorbidities and seizure control 24 months after a first unprovoked seizure in children. Epilepsy Res. 2018, 143, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Tsubouchi, Y.; Tanabe, A.; Saito, Y.; Noma, H.; Maegaki, Y. Long-term prognosis of epilepsy in patients with cerebral palsy. Dev. Med. Child Neurol. 2019, 61, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Baca, C.B.; Vickrey, B.G.; Caplan, R.; Vassar, S.D.; Berg, A.T. Psychiatric and medical comorbidity and quality of life outcomes in childhood-onset epilepsy. Pediatrics 2011, 128, e1532–e1543. [Google Scholar] [CrossRef] [PubMed]
- Camfield, C.S.; Camfield, P.R. Long-term social outcomes for children with epilepsy. Epilepsia 2007, 48, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Vestergaard, M.; Mortensen, P.B.; Sidenius, P.; Agerbo, E. Epilepsy and risk of suicide: A population-based case-control study. Lancet Neurol. 2007, 6, 693–698. [Google Scholar] [CrossRef]
- Kim, L.; Kim, J.A.; Kim, S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef]
- Han, S.H.; Lee, H.; Shin, J.Y.; Moon, H.J.; Lee, S.Y. Real-world prescribing trends of valproate in women with epilepsy in Korea. Epilepsy Behav. 2021, 115, 107700. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, H.S.; Kim, M.S.; Lee, J.; Jung, K.Y. Anti-seizure medication prescription in epilepsy patients in South Korea: A seven-year population-based retrospective cohort study. Seizure 2023, 109, 70–76. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chung, S.E.; Kim, D.W.; Eun, S.H.; Kang, H.C.; Cho, Y.W.; Yi, S.D.; Kim, H.D.; Jung, K.Y.; Cheong, H.K.; et al. Estimating the prevalence of treated epilepsy using administrative health data and its validity: ESSENCE Study. J. Clin. Neurol. 2016, 12, 434–440. [Google Scholar] [CrossRef]
- Reid, A.Y.; St Germaine-Smith, C.; Liu, M.; Sadiq, S.; Quan, H.; Wiebe, S.; Faris, P.; Dean, S.; Jetté, N. Development and validation of a case definition for epilepsy for use with administrative health data. Epilepsy Res. 2012, 102, 173–179. [Google Scholar] [CrossRef]
- Tu, K.; Wang, M.; Jaakkimainen, R.L.; Butt, D.; Ivers, N.M.; Young, J.; Green, D.; Jetté, N. Assessing the validity of using administrative data to identify patients with epilepsy. Epilepsia 2014, 55, 335–343. [Google Scholar] [CrossRef]
- Houghton, R.; Ong, R.C.; Bolognani, F. Psychiatric comorbidities and use of psychotropic medications in people with autism spectrum disorder in the United States. Autism Res. 2017, 10, 2037–2047. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, J.K.; Jhon, M.; Lee, H.J.; Kim, H.; Kim, J.W.; Lee, J.Y.; Kim, J.M.; Shin, I.S. Mindlink: A stigma-free youth-friendly community-based early-intervention centre in Korea. Early Interv. Psychiatry 2021, 15, 1389–1394. [Google Scholar] [CrossRef]
- Ogilvie, J.M.; Tzoumakis, S.; Allard, T.; Thompson, C.; Kisely, S.; Stewart, A. Prevalence of psychiatric disorders for Indigenous Australians: A population-based birth cohort study. Epidemiol. Psychiatr. Sci. 2021, 30, e21. [Google Scholar] [CrossRef]
- Rodrigues, R.; MacDougall, A.G.; Zou, G.; Lebenbaum, M.; Kurdyak, P.; Li, L.; Shariff, S.Z.; Anderson, K.K. Involuntary hospitalization among young people with early psychosis: A population-based study using health administrative data. Schizophr. Res. 2019, 208, 276–284. [Google Scholar] [CrossRef]
- van Fenema, E.; Giltay, E.; van Noorden, M.; van Hemert, A.; Zitman, F. Assessing adherence to guidelines with administrative data in psychiatric outpatients. J. Eval. Clin. Pract. 2017, 23, 5–13. [Google Scholar] [CrossRef]
- Wagner, C.J.; Metzger, F.G.; Sievers, C.; Marschall, U.; L’hoest, H.; Stollenwerk, B.; Stock, S. Depression-related treatment and costs in Germany: Do they change with comorbidity? A claims data analysis. J. Affect. Disord. 2016, 193, 257–266. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Hill, C.E.; Lin, C.C.; Terman, S.W.; Rath, S.; Parent, J.M.; Skolarus, L.E.; Burke, J.F. Definitions of drug-resistant epilepsy for administrative claims data research. Neurology 2021, 97, e1343–e1350. [Google Scholar] [CrossRef]
- Strzelczyk, A.; Griebel, C.; Lux, W.; Rosenow, F.; Reese, J.P. The burden of severely drug-refractory epilepsy: A comparative longitudinal evaluation of mortality, morbidity, resource use, and cost using German health insurance data. Front. Neurol. 2017, 8, 712. [Google Scholar] [CrossRef]
- Sullivan-Baca, E.; Rehman, R.; Towne, A.R.; Haneef, Z. Psychiatric co-morbidity of drug-resistant epilepsy in Veterans. Epilepsy Behav. 2023, 139, 109059. [Google Scholar] [CrossRef]
- Teneralli, R.E.; Cepeda, M.S.; Kern, D.M.; Novak, G.P. Individuals who develop drug-resistant epilepsy within a year after initial diagnosis have higher burden of mental and physical diseases one-year prior to epilepsy diagnosis as compared to those whose seizures were controlled during the same interval. Epilepsy Behav. 2021, 123, 108243. [Google Scholar] [CrossRef]
- Christensen, J.; Dreier, J.W.; Sun, Y.; Linehan, C.; Tomson, T.; Marson, A.; Forsgren, L.; Granbichler, C.A.; Trinka, E.; Illiescu, C.; et al. Estimates of epilepsy prevalence, psychiatric co-morbidity and cost. Seizure 2023, 107, 162–171. [Google Scholar] [CrossRef]
- Swaab, D.F.; Bao, A.-M. Sex differences in stress-related disorders: Major depressive disorder, bipolar disorder, and posttraumatic stress disorder. Handb. Clin. Neurol. 2020, 175, 335–358. [Google Scholar] [CrossRef]
- Bölte, S.; Marschik, P.B.; Williams, Z.J.; Lai, M.-C.; Gallagher, L.; Neufeld, J. Sex and gender in neurodevelopmental conditions. Nat. Rev. Neurol. 2023, 19, 136–159. [Google Scholar] [CrossRef]
- Chuah, A.; Ding, K.; Morgado, A.; McCreary, M.; Zuberi, F.; Agostini, M.; Doyle, A. Effects of psychiatric comorbidities in persons with epilepsy on recurrent emergency department visits. Epilepsy Behav. 2022, 136, 108909. [Google Scholar] [CrossRef]
- Hennig, T.; Jaya, E.S.; Koglin, U.; Lincoln, T.M. Associations of attention-deficit/hyperactivity and other childhood disorders with psychotic experiences and disorders in adsolescence. Eur. Child Adolesc. Psychiatry 2017, 26, 421–431. [Google Scholar] [CrossRef]
- Lee, Y.K.; Ah, Y.M.; Choi, Y.J.; Cho, Y.S.; Kim, K.J.; Lee, J.Y. Antiepileptic drug adherence and persistence in children with epilepsy attending a large tertiary care Children’s Hospital. Epileptic Disord. 2016, 18, 408–417. [Google Scholar] [CrossRef] [PubMed]
| Before Index Date | After Index Date | Total | |
|---|---|---|---|
| Schizophrenia spectrum disorder and other psychotic disorders (F20 to F29) | 451 (1.35) | 1483 (4.44) | 1934 (5.79) |
| Bipolar disorder (F30 and F31) | 490 (1.47) | 2687 (8.05) | 3177 (9.51) |
| Depressive disorder (F32 and F33) | 1423 (4.26) | 3472 (10.4) | 4895 (14.66) |
| Anxiety disorder (F40 and F41) | 1434 (4.29) | 4317 (12.93) | 5751 (17.22) |
| Obsessive–compulsive disorder (F42) | 116 (0.35) | 357 (1.07) | 473 (1.42) |
| Post-traumatic stress disorder (F43) | 399 (1.19) | 804 (2.41) | 1203 (3.60) |
| Sleep disorder (F51) | 191 (0.57) | 1108 (3.32) | 1299 (3.89) |
| Intellectual disability (F70 to F79) | 1193 (3.57) | 2782 (8.33) | 3975 (11.9) |
| Communication disorder (F80) | 859 (2.57) | 825 (2.47) | 1684 (5.04) |
| Specific learning disorder (F81) | 84 (0.25) | 171 (0.51) | 255 (0.76) |
| Autism spectrum disorder (F84) | 996 (2.98) | 1066 (3.19) | 2062 (6.17) |
| Attention-deficit hyperactivity disorder (F90) | 1761 (5.27) | 2494 (7.47) | 4255 (12.74) |
| Oppositional defiant disorder/conduct disorder (F91 and F92) | 352 (1.05) | 767 (2.30) | 1119 (3.35) |
| Tic disorder (F95) | 574 (1.72) | 739 (2.21) | 1313 (3.93) |
| Variables | Total (n = 37,678) | None (n = 24,393) | Before (n = 2288) | After (n = 7892) | Mixed (n = 3105) | p * |
|---|---|---|---|---|---|---|
| Sex | <0.001 | |||||
| Male | 20,569 (54.59) | 12,939 (53.04) | 1377 (60.18) | 4334 (54.92) | 1919 (61.80) | - |
| Age at diagnosis of epilepsy | <0.001 | |||||
| ≥0 and <12 months | 4470 (11.86) | 3300 (73.83) | 27 (0.60) | 1124 (25.15) | 19 (0.43) | - |
| ≥12 months and <5 years | 6172 (15.43) | 4030 (65.29) | 308 (4.99) | 1487 (24.09) | 347 (5.62) | - |
| ≥5 and <13 years | 15,958 (42.35) | 10,763 (67.45) | 1124 (7.04) | 2759 (17.29) | 1312 (8.22) | - |
| ≥13 and <18 years | 11,078 (29.40) | 6300 (56.87) | 829 (7.48) | 2522 (22.77) | 1427 (12.88) | - |
| Variables | None | Before | After | Mixed | p * |
|---|---|---|---|---|---|
| Total participants | 24,393 (64.74) | 2288 (6.07) | 7892 (20.95) | 3105 (8.24) | |
| Treatment duration (years) | 5.36 ± 3.59 | 5.59 ± 3.33 | 8.31 ± 3.65 | 6.54 ± 3.43 | <0.001 |
| No. of outpatient visits | 39.97 ± 63.38 | 44.72 ± 68.14 | 80.43 ± 127.06 | 52.09 ± 80.65 | <0.001 |
| No. of ER visits | 2.68 ± 3.87 | 2.64 ± 4.32 | 5.39 ± 8.12 | 3.41 ± 6.69 | <0.001 |
| No. of admissions | 3.71 ± 28.10 | 3.13 ± 18.29 | 10.76 ± 66.10 | 3.92 ± 33.69 | <0.001 |
| Total no. of medications | 2.31 ± 1.83 | 2.46 ± 1.93 | 4.05 ± 2.83 | 2.88 ± 2.07 | <0.001 |
| Status epilepticus | 1354 (5.55) | 154 (6.73) | 1108 (14.04) | 207 (6.67) | <0.001 |
| Drug-resistant epilepsy | 7516 (30.81) | 767 (33.52) | 4389 (55.61) | 1116 (35.94) | <0.001 |
| None (Reference) | Before | After | Mixed | |
|---|---|---|---|---|
| Number of outpatient visits * | 1.00 | 1.32 (1.29–1.35) | 2.83 (2.80–2.85) | 1.89 (1.85–1.93) |
| Number of ER visits * | 1.00 | 1.16 (1.13–1.19) | 2.03 (2.00–20.5) | 1.59 (1.56–1.63) |
| Number of admissions * | 1.00 | 1.32 (1.29–1.35) | 2.83 (2.80–2.85) | 1.89 (1.85–1.93) |
| Total number of medications * | 1.00 | 1.13 (1.10–1.16) | 1.76 (1.74–1.79) | 1.35 (1.32–1.38) |
| Status epilepticus + | 1.00 | 1.69 (1.41–2.01) | 2.92 (2.68–3.18) | 1.86 (1.59–2.18) |
| Drug-resistant epilepsy + | 1.00 | 1.34 (1.22–1.47) | 3.01 (2.85–3.17) | 1.59 (1.47–1.73) |
| None | Before | After | Mixed | |
|---|---|---|---|---|
| Total number of medications * | 0.74 (0.72–0.76) | 0.83 (0.81–0.86) | 1.30 (1.27–1.34) | 1.00 (Reference) |
| Status epilepticus + | 0.54 (0.46–0.63) | 0.91 (0.73–1.13) | 1.57 (1.34–1.84) | 1.00 (Reference) |
| Drug-resistant epilepsy + | 0.63 (0.58–0.68) | 0.84 (0.75–0.94) | 1.89 (1.73–2.06) | 1.00 (Reference) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Choi, A.; Kim, S. Effects of Psychiatric Comorbidities on the Prognosis of New-Onset Pediatric Epilepsy: A Retrospective Nationwide Cohort Study. J. Clin. Med. 2024, 13, 4500. https://doi.org/10.3390/jcm13154500
Lee J, Choi A, Kim S. Effects of Psychiatric Comorbidities on the Prognosis of New-Onset Pediatric Epilepsy: A Retrospective Nationwide Cohort Study. Journal of Clinical Medicine. 2024; 13(15):4500. https://doi.org/10.3390/jcm13154500
Chicago/Turabian StyleLee, Jooyoung, Arum Choi, and Sukil Kim. 2024. "Effects of Psychiatric Comorbidities on the Prognosis of New-Onset Pediatric Epilepsy: A Retrospective Nationwide Cohort Study" Journal of Clinical Medicine 13, no. 15: 4500. https://doi.org/10.3390/jcm13154500
APA StyleLee, J., Choi, A., & Kim, S. (2024). Effects of Psychiatric Comorbidities on the Prognosis of New-Onset Pediatric Epilepsy: A Retrospective Nationwide Cohort Study. Journal of Clinical Medicine, 13(15), 4500. https://doi.org/10.3390/jcm13154500






