One-Month Duration Compared with Twelve-Month Duration of Dual Antiplatelet Therapy in Elective Angioplasty for Coronary Artery Disease: Bleeding and Ischaemic Outcomes
Abstract
1. Introduction
2. Materials and Methods
- Type 3a: Overt bleeding plus haemoglobin drop of 3 to <5 g/dL (provided haemoglobin drop is related to bleed) or transfusion with overt bleeding
- Type 3b: Overt bleeding plus haemoglobin drop <5 g/dL (provided haemoglobin drop is related to bleed), cardiac tamponade, bleeding requiring surgical intervention for control, or bleeding requiring IV vasoactive agents
- Type 3c: Intracranial haemorrhage confirmed by autopsy, imaging or lumbar puncture, or intraocular bleed compressing vision
- Type 4: CABG related bleeding within 48 h
- Type 5a: Probable fatal bleeding
- Type 5b: Definite fatal bleeding (Overt or autopsy or imaging confirmation)
- Any death
- Cerebrovascular event (CVE)
- Any myocardial infarction
- Any revascularisation
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shanmugam, V.B.; Harper, R.; Meredith, I.; Malaiapan, Y.; Psaltis, P.J. An Overview of PCI in the Very Elderly. J. Geriatr. Cardiol. 2015, 12, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.-H.-L.; Xu, P.; Wang, B.; Lu, Y.; Wu, Q.-Y.; Zhou, M.-L.; Wu, J.-R.; Cai, J.-J.; Sun, X.; Yuan, H. Duration of Dual Antiplatelet Therapy after Percutaneous Coronary Intervention with Drug-Eluting Stent: Systematic Review and Network Meta-Analysis. BMJ 2019, 365, l2222. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease Developed in Collaboration with EACTS. Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef] [PubMed]
- Gilard, M.; Hasdai, D.; Hatala, R.; Mahfoud, F.; Masip, J.; Muneretto, C.; Valgimigli, M.; Achenbach, S.; Bax, J.J. European Association of Cardiovascular Imaging (EACVI), European Association of Preventive Cardiology (EAPC), European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart Rhythm Assoc. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Mihatov, N.; Secemsky, E.A.; Kereiakes, D.J.; Steg, G.; Serruys, P.W.; Chichareon, P.; Shen, C.; Yeh, R.W. Utility of the Dual Antiplatelet Therapy Score to Guide Antiplatelet Therapy: A Systematic Review and Meta-Analysis. Catheter. Cardiovasc. Interv. 2021, 97, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Special Report Standardized Bleeding Definitions for Cardiovascular Clinical Trials A Consensus Report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, H.M.; McFadden, E.P.; Farb, A.; Mehran, R.; Stone, G.W.; Spertus, J.; Onuma, Y.; Morel, M.; van Es, G.-A.; Zuckerman, B.; et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation 2018, 137, 2635–2650. [Google Scholar] [CrossRef]
- Gilbert, T.; Neuburger, J.; Kraindler, J.; Keeble, E.; Smith, P.; Ariti, C.; Arora, S.; Street, A.; Parker, S.; Roberts, H.C.; et al. Development and Validation of a Hospital Frailty Risk Score Focusing on Older People in Acute Care Settings Using Electronic Hospital Records: An Observational Study. Lancet 2018, 391, 1775–1782. [Google Scholar] [CrossRef]
- Corballis, N.H.; Wickramarachchi, U.; Vassiliou, V.S.; Eccleshall, S.C. Duration of Dual Antiplatelet Therapy in Elective Drug-coated Balloon Angioplasty. Catheter. Cardiovasc. Interv. 2019, 96, 1016–1020. [Google Scholar] [CrossRef]
- Merinopoulos, I.; Gunawardena, T.; Corballis, N.; Bhalraam, U.; Gilbert, T.; Maart, C.; Richardson, P.; Ryding, A.; Sarev, T.; Sawh, C.; et al. Paclitaxel Drug-Coated Balloon-Only Angioplasty for de Novo Coronary Artery Disease in Elective Clinical Practice. Clin. Res. Cardiol. 2022, 112, 1186–1193. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2023, 82, 833–955. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, T.; Benedetto, U.; Bacchi-Reggiani, L.; Della Riva, D.; Biondi-Zoccai, G.; Feres, F.; Abizaid, A.; Hong, M.-K.; Kim, B.-K.; Jang, Y.; et al. Mortality in Patients Treated with Extended Duration Dual Antiplatelet Therapy after Drug-Eluting Stent Implantation: A Pairwise and Bayesian Network Meta-Analysis of Randomised Trials. Lancet 2015, 385, 2371–2382. [Google Scholar] [CrossRef]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation 2019, 140, 240–261. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, P.-J.; Eggen, A.E.; Steigen, T.; Wilsgaard, T.; Kristensen, A.; Skogsholm, A.; Holme, E.; van den Heuvel, C.; Nordrehaug, J.E.; Bendz, B.; et al. Incidence and Risk Factors for Major Bleeding among Patients Undergoing Percutaneous Coronary Intervention: Findings from the Norwegian Coronary Stent Trial (NORSTENT). PLoS ONE 2021, 16, e0247358. [Google Scholar] [CrossRef] [PubMed]
- Natsuaki, M.; Morimoto, T.; Shiomi, H.; Kadota, K.; Tada, T.; Takeji, Y.; Matsumura-Nakano, Y.; Yoshikawa, Y.; Watanabe, H.; Yamamoto, K.; et al. Effects of Acute Coronary Syndrome and Stable Coronary Artery Disease on Bleeding and Ischemic Risk after Percutaneous Coronary Intervention. Circ. J. 2021, 85, 1928–1941. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Patialiakas, A.; Thury, A.; McFadden, E.; Colangelo, S.; Campo, G.; Tebaldi, M.; Ungi, I.; Tondi, S.; Roffi, M.; et al. Zotarolimus-Eluting Versus Bare-Metal Stents in Uncertain Drug-Eluting Stent Candidates. J. Am. Coll. Cardiol. 2015, 65, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Varenne, O.; Cook, S.; Sideris, G.; Kedev, S.; Cuisset, T.; Carrié, D.; Hovasse, T.; Garot, P.; El Mahmoud, R.; Spaulding, C.; et al. Drug-Eluting Stents in Elderly Patients with Coronary Artery Disease (SENIOR): A Randomised Single-Blind Trial. Lancet 2018, 391, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Meredith, I.T.; Abizaid, A.; Pocock, S.J.; Carrié, D.; Naber, C.; Lipiecki, J.; Richardt, G.; Iñiguez, A.; Brunel, P.; et al. Polymer-Free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2015, 373, 2038–2047. [Google Scholar] [CrossRef]
- Hong, S.-J.; Kim, J.-S.; Hong, S.J.; Lim, D.-S.; Lee, S.-Y.; Yun, K.H.; Park, J.-K.; Kang, W.C.; Kim, Y.H.; Yoon, H.-J.; et al. 1-Month Dual-Antiplatelet Therapy Followed by Aspirin Monotherapy after Polymer-Free Drug-Coated Stent Implantation: One-Month DAPT Trial. JACC Cardiovasc. Interv. 2021, 14, 1801–1811. [Google Scholar] [CrossRef]
- Windecker, S.; Latib, A.; Kedhi, E.; Kirtane, A.J.; Kandzari, D.E.; Mehran, R.; Price, M.J.; Abizaid, A.; Simon, D.I.; Worthley, S.G.; et al. Polymer-Based or Polymer-Free Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2020, 382, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Min, P.-K.; Kang, T.S.; Cho, Y.-H.; Cheong, S.-S.; Kim, B.-K.; Kwon, S.W.; Park, W.J.; Lee, J.-H.; Kim, W.; Lee, W.-S.; et al. P2Y12 Inhibitor Monotherapy vs Dual Antiplatelet Therapy after Deployment of a Drug-Eluting Stent The SHARE Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e240877. [Google Scholar] [CrossRef] [PubMed]
- Natsuaki, M.; Watanabe, H.; Morimoto, T.; Yamamoto, K.; Obayashi, Y.; Nishikawa, R.; Ando, K.; Domei, T.; Suwa, S.; Ogita, M.; et al. An Aspirin-Free Versus Dual Antiplatelet Strategy for Coronary Stenting: STOPDAPT-3 Randomized Trial. Circulation 2024, 149, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Capranzano, P.; Moliterno, D.; Capodanno, D. Aspirin-Free Antiplatelet Strategies after Percutaneous Coronary Interventions. Eur. Heart J. 2024, 45, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Merinopoulos, I.; Bhalraam, U.; Holmes, T.; Tsampasian, V.; Corballis, N.; Gunawardena, T.; Sawh, C.; Maart, C.; Wistow, T.; Ryding, A.; et al. Circulating Intermediate Monocytes CD14++CD16+ are Increased after Elective Percutaneous Coronary Intervention. PLoS ONE 2023, 18, e0294746. [Google Scholar] [CrossRef] [PubMed]
- Merinopoulos, I.; Bhalraam, U.; Kasmai, B.; Hewson, D.; Greenwood, R.; Eccleshall, S.C.; Smith, J.; Tsampasian, V.; Vassiliou, V. Myocardial inflammation after elective percutaneous coronary intervention. Hell. J. Cardiol. 2024; in Press. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G.; Marenna, A.; Sperandeo, L.; Manzi, L.; Avvedimento, M.; Simonetti, F.; Canonico, M.E.; Paolillo, R.; Spinelli, A.; Borgia, F.; et al. Pharmacodynamic Effects of Cangrelor in Elective Complex PCI: Insights from the POMPEII Registry. EuroIntervention 2023, 18, 1266–1268. [Google Scholar] [CrossRef] [PubMed]
- Merinopoulos, I.; Gunawardena, T.; Corballis, N.; Bhalraam, U.; Reinhold, J.; Wickramarachchi, U.; Maart, C.; Gilbert, T.; Richardson, P.; Sulfi, S.; et al. Assessment of Paclitaxel Drug-Coated Balloon Only Angioplasty in STEMI. Cardiovasc. Interv. 2023, 16, 771–779. [Google Scholar] [CrossRef]
- Gunawardena, T.D.; Corballis, N.; Merinopoulos, I.; Wickramarachchi, U.; Reinhold, J.; Maart, C.; Sreekumar, S.; Sawh, C.; Wistow, T.; Sarev, T.; et al. Drug-Coated Balloon vs. Drug-Eluting Stents for De Novo Unprotected Left Main Stem Disease: The SPARTAN-LMS Study. J. Cardiovasc. Dev. Dis. 2023, 10, 84. [Google Scholar] [CrossRef]
- Merinopoulos, I.; Gunawardena, T.; Wickramarachchi, U.; Richardson, P.; Maart, C.; Sreekumar, S.; Sawh, C.; Wistow, T.; Sarev, T.; Ryding, A.; et al. Long-Term Safety of Paclitaxel Drug-Coated Balloon-Only Angioplasty for de Novo Coronary Artery Disease: The SPARTAN DCB Study. Clin. Res. Cardiol. 2021, 110, 220–227. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Vos, N.S.; Fagel, N.D.; Amoroso, G.; Herrman, J.-P.R.; Patterson, M.S.; Piers, L.H.; van der Schaaf, R.J.; Slagboom, T.; Vink, M.A. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2019, 12, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B.; Ohlow, M.-A.; Ewen, S.; Kische, S.; Rudolph, T.K.; Clever, Y.P.; Wagner, A.; Richter, S.; El-Garhy, M.; Böhm, M.; et al. Bare Metal or Drug-Eluting Stent versus Drug-Coated Balloon in Non-ST-Elevation Myocardial Infarction: The Randomised PEPCAD NSTEMI Trial. EuroIntervention 2020, 15, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Farah, A.; Ohlow, M.-A.; Mangner, N.; Möbius-Winkler, S.; Leibundgut, G.; Weilenmann, D.; Wöhrle, J.; Richter, S.; Schreiber, M.; et al. Drug-Coated Balloons for Small Coronary Artery Disease (BASKET-SMALL 2): An Open-Label Randomised Non-Inferiority Trial. Lancet 2018, 392, 849–856. [Google Scholar] [CrossRef]
- Rissanen, T.T.; Uskela, S.; Eränen, J.; Mäntylä, P.; Olli, A.; Romppanen, H.; Siljander, A.; Pietilä, M.; Minkkinen, M.J.; Tervo, J.; et al. Drug-Coated Balloon for Treatment of de-Novo Coronary Artery Lesions in Patients with High Bleeding Risk (DEBUT): A Single-Blind, Randomised, Non-Inferiority Trial. Lancet 2019, 394, 230–239. [Google Scholar] [CrossRef]
- Corballis, N.H.; Nyi, T.H.; Vassiliou, V.S.; Eccleshall, S.C. Drug-Coated Balloons or Drug-Eluting Stents—Determining an Optimum Strategy for Patients with High Bleeding Risk. Heart Int. 2020, 14, 100. [Google Scholar] [CrossRef]
- Ludman, P. BCIS Audit Data 2015; BCIS: Coventry, UK, 2015. [Google Scholar]

| Patient Characteristics | One-Month DAPT (n = 340) | Twelve-Month DAPT (n = 685) | p Value |
|---|---|---|---|
| Female sex | 78 (22.9) | 154 (22.5) | 0.9 |
| Age (mean, SD) | 68 (10) | 67 (10) | 0.087 |
| Frailty score (median, IQR) | 0.0 (0.0–0.7) | 0.0 (0.0–0.6) | 0.35 |
Frailty
| 323 (99.7) 1 (0.3) 0 (0) | 642 (99.4) 4 (0.6) 0 (0) | 0.4 |
| Hypertension | 186 (54.7) | 392 (57.2) | 0.4 |
| Dyslipidaemia | 115 (33.8) | 231 (33.7) | 0.9 |
| Previous CVE | 23 (6.8) | 32 (4.7) | 0.1 |
| Peripheral vascular disease | 14 (4.1) | 29 (4.2) | 0.7 |
| Previous MI | 45 (13.2) | 116 (16.9) | 0.1 |
| Previous PCI | 51 (15.0) | 86 (12.6) | 0.3 |
| Previous CABG | 29 (8.5) | 49 (7.2) | 0.4 |
| COPD | 11 (3.2) | 42 (6.1) | 0.05 |
| Family history of coronary disease | 91 (26.8) | 178 (26.0) | 0.8 |
| Diabetic | 78 (22.9) | 155 (22.6) | 0.9 |
| Current/ex-smoker | 212 (62.7) | 457 (67.3) | 0.1 |
| Dual antiplatelet use | 340 (100) | 685 (100) | >0.99 |
| DAPT Risk Score | |||
| <2 | 296 (87.6) | 545 (80.3) | 0.05 |
| ≥2 | 42 (12.4) | 134 (19.7) | 0.04 |
| DAPT used | |||
| Aspirin and clopidogrel | 329 (96.7) | 630 (92) | |
| Aspirin and ticagrelor | 7 (2.1) | 50 (7.3) | |
| Aspirin and prasugrel | 4 (1.2) | 5 (0.7) |
| Lesion Characteristics | Duration of DAPT | p-Value | |
|---|---|---|---|
| 1 Month (n = 340) | 12 Months (n = 685) | ||
| Vessel treated, n (%) | 0.054 1 | ||
| LMS | 12 (3.5) | 25 (3.6) | |
| LAD | 192 (56) | 375 (55) | |
| Cx | 64 (19) | 96 (14) | |
| RCA | 69 (20) | 170 (25) | |
| Graft | 3 (0.9) | 19 (2.8) | |
| Multivessel PCI, n (%) | 32 (9.4) | 88 (13) | 0.11 1 |
| Heavy calcification, n (%) | 104 (31) | 153 (22) | 0.0041 |
| Severe tortuosity, n (%) | 79 (23) | 86 (13) | <0.0011 |
| Diffuse disease | 118 (35) | 139 (20) | <0.0011 |
| DCB/DES use, n (%) | <0.0011 | ||
| DCB | 339 (99.7) | 45 (6.6) | |
| DES | 1 (0.3) | 640 (93.4) | |
| Vessel diameter, median (IQR) | 3.00 (2.75–3.50) | 3.50 (3.00–4.00) | <0.0011 |
| Vessel length, median (IQR) | 20 (20–30) | 24 (18–38) | 0.0191 |
| Bifurcation lesion | 104 (31) | 143 (21) | <0.0011 |
| Intravascular imaging | 8 (2.4) | 61 (8.9) | 0.041 |
| Outcomes, n (%) | Duration of DAPT 1 Month, n = 340 | 12 Months, n = 685 | p-Value |
|---|---|---|---|
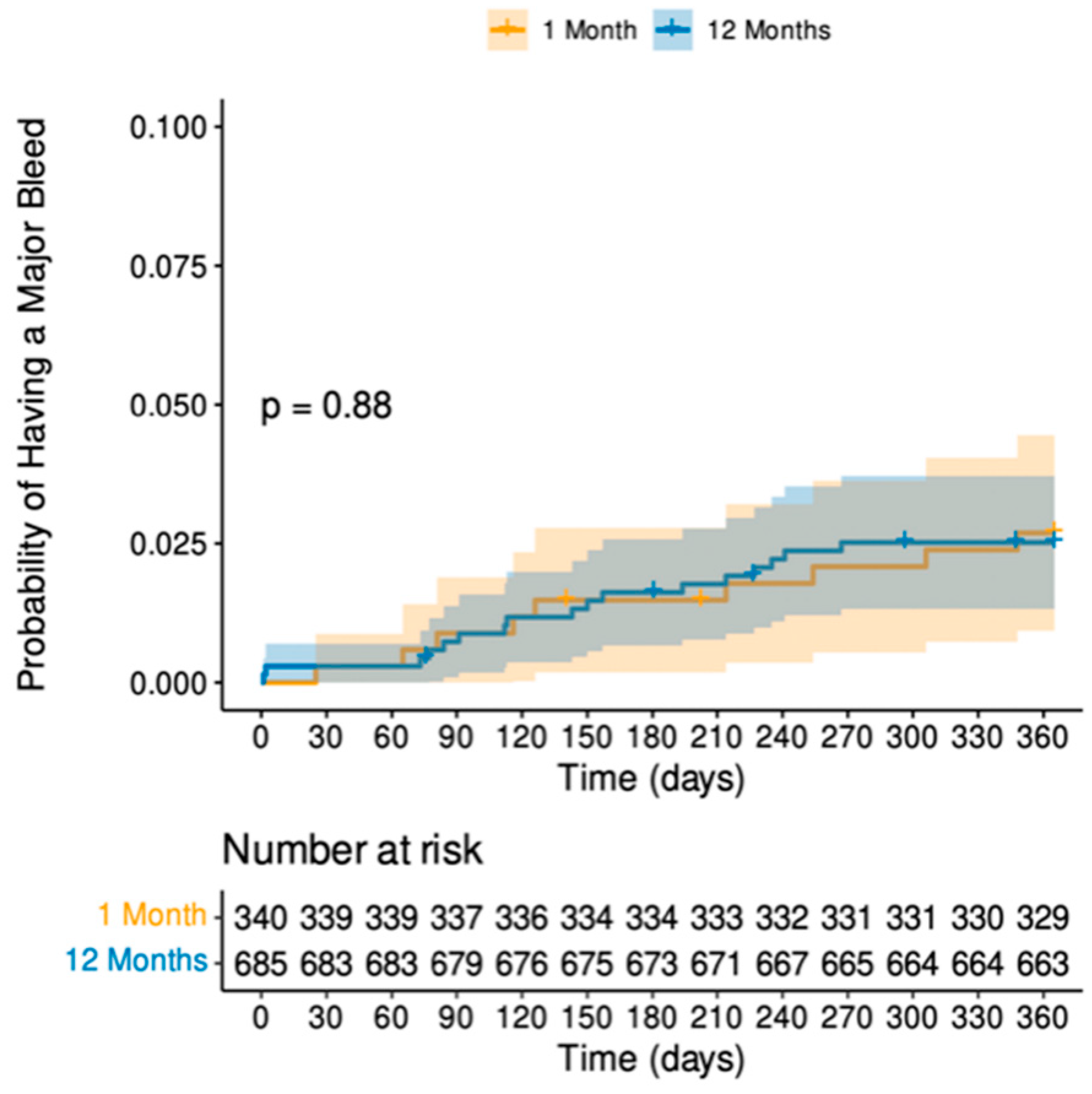
| Major bleeding | 9 (2.6) | 17 (2.5) | 0.87 1 |
| All-cause mortality | 3 (0.9) | 6 (0.9) | >0.99 2 |
| Cardiovascular mortality | 1 (0.3) | 1 (0.1) | 0.55 2 |
| ACS | 8 (2.4) | 9 (1.3) | 0.22 1 |
| Cerebrovascular event | 2 (0.6) | 2 (0.3) | 0.60 2 |
| Any revascularisation | 31 (9.1) | 72 (11) | 0.48 1 |
| Composite of mortality, ACS, CVE and revascularisation | 38 (11) | 85 (12) | 0.57 1 |
| Characteristic | Hazard Ratio (95% CI) | p Value |
|---|---|---|
| Age | 1.02 (0.99–1.06) | 0.21 |
| Sex [Female] | 0.42 (0.13–1.37) | 0.15 |
| DCB/DES use [DES] | 1.07 (0.51–2.24) | 0.86 |
| Frailty score | 0.77 (0.45–1.32) | 0.34 |
| Dyslipidaemia | 0.84 (0.38–1.86) | 0.67 |
| Hypertension | 0.61 (0.29–1.27) | 0.19 |
| Cerebrovascular event | 0.57 (0.08–4.21) | 0.58 |
| Myocardial infarction | 1.58 (0.67–3.69) | 0.29 |
| Coronary artery bypass grafting | 1.92 (0.67–5.50) | 0.23 |
| Heart failure | 1.56 (0.21–11.4) | 0.66 |
| Chronic obstructive pulmonary disease | 0.69 (0.09–5.11) | 0.72 |
| Diabetes mellitus | 1.06 (0.45–2.48) | 0.89 |
| Previous/current smoker | 0.89 (0.42–1.88) | 0.76 |
| Creatinine | 1.00 (0.99–1.02) | 0.55 |
| Multivessel disease | 2.03 (0.83–4.98) | 0.12 |
| Bifurcation lesion | 0.71 (0.27–1.87) | 0.49 |
| Heavy calcification | 0.74 (0.30–1.81) | 0.50 |
| Diffuse disease | 0.74 (0.30–1.82) | 0.51 |
| Severe tortuosity | 0.60 (0.18–2.00) | 0.41 |
| Vessel diameter | 0.63 (0.34–1.17) | 0.14 |
| Lesion length | 1.00 (0.97–1.02) | 0.78 |
| Characteristic | Hazard Ratio (95% CI) | p Value |
|---|---|---|
| Age | 1.01 (1.00–1.03) | 0.16 |
| Sex [Female] | 0.61 (0.40–0.92) | 0.01 |
| DCB/DES use [DES] | 0.84 (0.62–1.13) | 0.24 |
| Frailty score | 1.08 (0.94–1.25) | 0.27 |
| Dyslipidaemia | 1.12 (0.82–1.52) | 0.48 |
| Hypertension | 1.01 (0.75–1.37) | 0.93 |
| Cerebrovascular event | 1.48 (0.86–2.55) | 0.16 |
| Myocardial infarction | 1.14 (0.78–1.67) | 0.49 |
| Coronary artery bypass grafting | 1.02 (0.59–1.76) | 0.95 |
| Heart failure | 1.33 (0.55–3.24) | 0.53 |
| Chronic obstructive pulmonary disease | 0.67 (0.30–1.51) | 0.33 |
| Diabetes mellitus | 1.36 (0.98–1.86) | 0.06 |
| Previous/current smoker | 0.73 (0.54–0.99) | 0.04 |
| Creatinine | 1.00 (1.00–1.01) | 0.12 |
| Multivessel disease | 1.04 (0.66–1.65) | 0.85 |
| Bifurcation lesion | 1.19 (0.85–1.65) | 0.85 |
| Heavy calcification | 1.76 (1.29–2.39) | <0.001 |
| Diffuse disease | 1.14 (0.82–1.59) | 0.42 |
| Severe tortuosity | 1.06 (0.71–1.58) | 0.77 |
| Vessel diameter | 1.00 (0.78–1.28) | >0.99 |
| Lesion length | 1.00 (0.99–1.01) | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corballis, N.; Bhalraam, U.; Merinopoulos, I.; Gunawardena, T.; Tsampasian, V.; Wickramarachchi, U.; Eccleshall, S.; Vassiliou, V.S. One-Month Duration Compared with Twelve-Month Duration of Dual Antiplatelet Therapy in Elective Angioplasty for Coronary Artery Disease: Bleeding and Ischaemic Outcomes. J. Clin. Med. 2024, 13, 4521. https://doi.org/10.3390/jcm13154521
Corballis N, Bhalraam U, Merinopoulos I, Gunawardena T, Tsampasian V, Wickramarachchi U, Eccleshall S, Vassiliou VS. One-Month Duration Compared with Twelve-Month Duration of Dual Antiplatelet Therapy in Elective Angioplasty for Coronary Artery Disease: Bleeding and Ischaemic Outcomes. Journal of Clinical Medicine. 2024; 13(15):4521. https://doi.org/10.3390/jcm13154521
Chicago/Turabian StyleCorballis, Natasha, U. Bhalraam, Ioannis Merinopoulos, Tharusha Gunawardena, Vasiliki Tsampasian, Upul Wickramarachchi, Simon Eccleshall, and Vassilios S. Vassiliou. 2024. "One-Month Duration Compared with Twelve-Month Duration of Dual Antiplatelet Therapy in Elective Angioplasty for Coronary Artery Disease: Bleeding and Ischaemic Outcomes" Journal of Clinical Medicine 13, no. 15: 4521. https://doi.org/10.3390/jcm13154521
APA StyleCorballis, N., Bhalraam, U., Merinopoulos, I., Gunawardena, T., Tsampasian, V., Wickramarachchi, U., Eccleshall, S., & Vassiliou, V. S. (2024). One-Month Duration Compared with Twelve-Month Duration of Dual Antiplatelet Therapy in Elective Angioplasty for Coronary Artery Disease: Bleeding and Ischaemic Outcomes. Journal of Clinical Medicine, 13(15), 4521. https://doi.org/10.3390/jcm13154521






