Severe Traumatic Brain Injury and Pulmonary Embolism: Risks, Prevention, Diagnosis and Management
Abstract
:1. Introduction
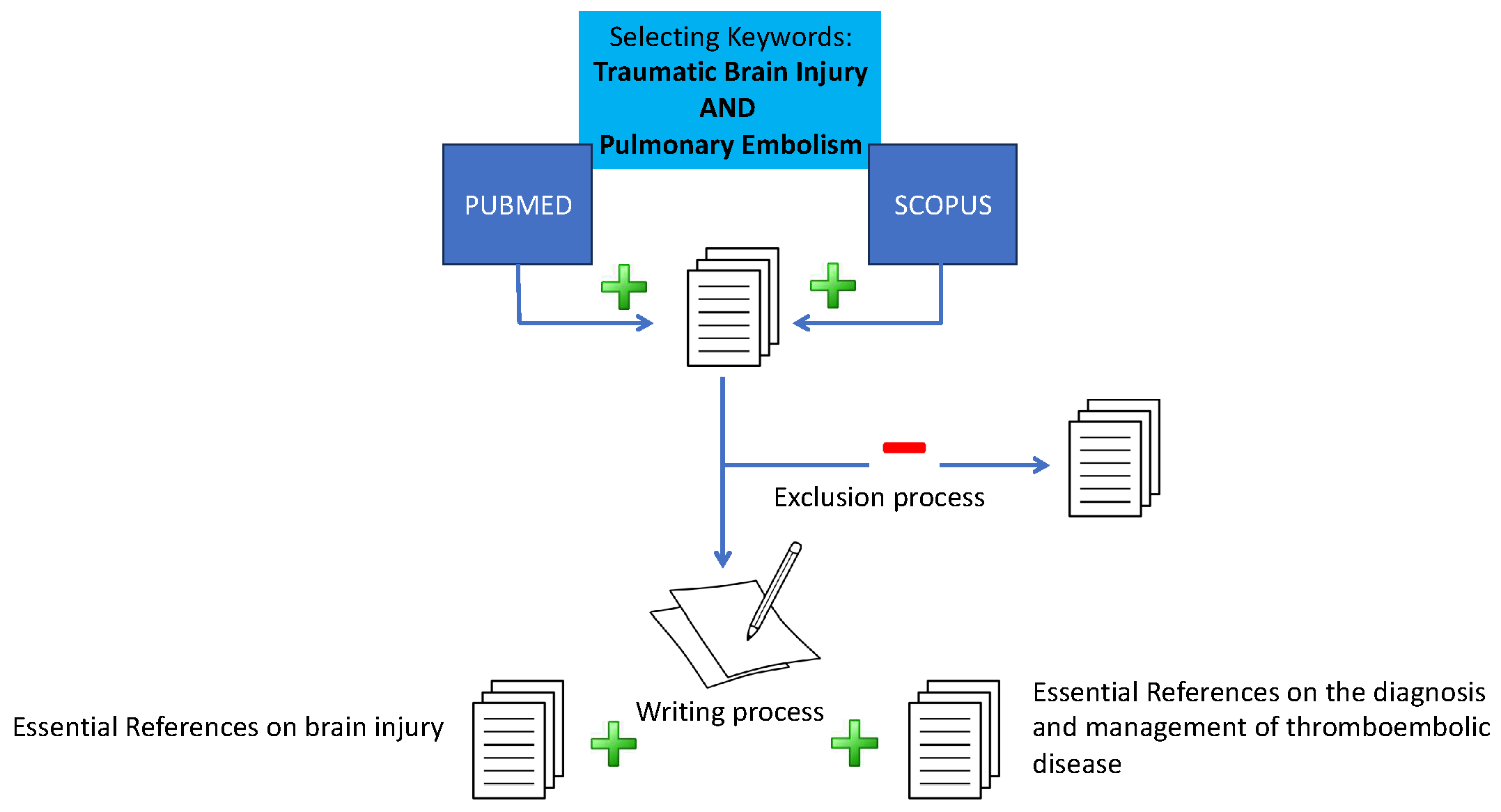
2. Materials and Methods
3. Results
| Authors | Methods | No of Patients | Topic |
|---|---|---|---|
| Song et al., 2024 [27] | SR + MA | TXA does not affect the incidence of PE. | |
| Zhang et al., 2024 [28] | MA | TXA does not affect the incidence of PE. | |
| Cole et al., 2024 [29] | Single center, retrospective | 818 | Risk factors for PE |
| Condon et al., 2024 [30] | Multiple center, retrospective | 14,926 | LMWH was associated with lower mortality risk compared to UH in geriatric patients. |
| Park et al., 2023 [31] | Single center, prospective | 120 | Crcl predicts goal enoxaparin dose |
| Jakob et al., 2023 [32] | Multiple center, retrospective | 75,570 | Risk factors for PE |
| Wu et al., 2023 [33] | Secondary analysis of PCD | 881 | Early vs. late initiation of VTE prophylaxis |
| Chen et al., 2023 [34] | Multiple center, retrospective | 847 | Risk factors for PE |
| Samuel et al., 2023 [35] | Single center, retrospective | 103 | Timing of therapeutic AC in TBI with PE |
| The PATCH-Trauma Investigators and the ANZICS Clinical Trials Group 2023 [36] | Multiple center RCT | 1310 | TXA effect |
| Zheng et al., 2022 [37] | SR + MA | EPO in TBI | |
| Hazelton et al., 2022 [38] | Multicenter prospective observational study | 1623 | WB resuscitation in TBI |
| Ali et al.,2022 [39] | Multiple center, retrospective | 2754 | Risk of PE in THC+ cases |
| Ali Basil Ali et al., 2022 [40] | Multiple center, retrospective | 37,988 | Risk of PE in DC vs. CO |
| Perissier et al., 2022 [41] | Single center, prospective | 120 | Reasons for late initiation of VTE prophylaxis |
| Fletcher-Sandersjöö et al., 2022 [42] | Retrospective observational | 848 | Clinical significance of VTE in TBI |
| Byrne et al., 2022 [43] | Multiple center, retrospective | 4951 | Early vs. late initiation of VTE prophylaxis |
| Rivas et al., 2022 [44] | Multiple center, retrospective | 264 | Early vs. late initiation of VTE prophylaxis |
| El-Menyar et al., 2022 [45] | Multiple center RCT | 220 | Effect of second TXA dose |
| Galaher et al., 2021 [46] | Single center, retrospective | 806 | Diagnostic yield of TTE |
| Gates et al., 2021 [47] | Single center, prospective | 1698 | Titration of prophylactic AC to anti-Xa levels |
| Elkbuli et al., 2021 [48] | Single center, retrospective | 413 | Prophylactic IVC |
| Elkbuli et al., 2020 [49] | Single center, retrospective | 513 | Timing of prophylactic IVC placement |
| Chipman et al., 2020 [50] | Single center, retrospective | 50 | Timing of therapeutic AC in TBI with PE |
| Ahmed et al., 2020 [51] | Multiple center, retrospective | 2370 | Risk of PE in DC |
| Lu et al., 2020 [52] | SR + MA | Early vs. late initiation of VTE prophylaxis | |
| Zhang et al., 2020 [53] | Single center, retrospective | 144 | Risk of pharmaceutical immobilization |
| July et al., 2020 [54] | MA | TXA effect | |
| Chen et al., 2020 [55] | MA | TXA effect | |
| Rabinstein et al., 2019 [56] | Single center, retrospective | 77 | PICC as a risk factor |
3.1. Incidence of PE in sTBI
3.2. Risk Factors for PE in sTBI
3.3. The Role of TXA
3.4. The Role of Transfusions and Transfusion Thresholds
3.5. Timing of Prophylaxis Initiation
3.6. Agents and Dosage of Pharmaceutical VTE Prophylaxis
3.7. The Role of IVCFs
3.8. Timely PE Suspicion and Diagnosis
3.9. Treatment of PE in TBI
3.10. Management of High-Risk PE in sTBI
4. Discussion
5. Limitations
6. Areas of Future Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic Brain Injury: Progress and Challenges in Prevention, Clinical Care, and Research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef] [PubMed]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.J.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Meyfroidt, G.; Bouzat, P.; Casaer, M.P.; Chesnut, R.; Hamada, S.R.; Helbok, R.; Hutchinson, P.; Maas, A.I.R.; Manley, G.; Menon, D.K.; et al. Management of Moderate to Severe Traumatic Brain Injury: An Update for the Intensivist. Intensive Care Med. 2022, 48, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Graziano, F.; Picetti, E.; Åkerlund, C.; Addis, A.; Pastore, G.; Sivero, M.; Rebora, P.; Galimberti, S.; Stocchetti, N.; et al. Early Systemic Insults Following Traumatic Brain Injury: Association with Biomarker Profiles, Therapy for Intracranial Hypertension, and Neurological Outcomes—An Analysis of CENTER-TBI Data. Intensive Care Med. 2024, 50, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Poole, D.; McNett, M.; Asehnoune, K.; Bösel, J.; Bruder, N.; Chieregato, A.; Cinotti, R.; Duranteau, J.; Einav, S.; et al. Mechanical Ventilation in Patients with Acute Brain Injury: Recommendations of the European Society of Intensive Care Medicine Consensus. Intensive Care Med. 2020, 46, 2397–2410. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Deng, M.; Wang, L.; Dong, Y.; Chen, L.; Dai, X. Low vs Standardized Dose Anticoagulation Regimens for Extracorporeal Membrane Oxygenation: A Meta-Analysis. PLoS ONE 2021, 16, e0249854. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Ortu, A.; Bilotta, F.; Lombardo, A.; Sekhon, M.S.; Gallo, F.; Matta, B.F. Extracorporeal Membrane Oxygenation for Adult Respiratory Distress Syndrome in Trauma Patients: A Case Series and Systematic Literature Review. J. Trauma Acute Care Surg. 2017, 82, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Schöchl, H.; Hunt, B.J.; Sauaia, A. Trauma-Induced Coagulopathy. Nat. Rev. Dis. Primers 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- The CRASH-3 trial collaborators. Effects of Tranexamic Acid on Death, Disability, Vascular Occlusive Events and Other Morbidities in Patients with Acute Traumatic Brain Injury (CRASH-3): A Randomised, Placebo-Controlled Trial. Lancet 2019, 394, 1713–1723. [Google Scholar] [CrossRef]
- Abhilash, K.P.; Sivanandan, A. Early Management of Trauma: The Golden Hour. Curr. Med. Issues 2020, 18, 36. [Google Scholar] [CrossRef]
- Kornblith, L.Z.; Moore, H.B.; Cohen, M.J. Trauma-induced Coagulopathy: The Past, Present, and Future. J. Thromb. Haemost. 2019, 17, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Jamjoom, A.A.B.; Chari, A.; Salijevska, J.; Meacher, R.; Brennan, P.; Statham, P. A National Survey of Thromboprophylaxis in Traumatic Brain Injury in the United Kingdom. Br. J. Neurosurg. 2016, 30, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Mayo, P.H.; Copetti, R.; Feller-Kopman, D.; Mathis, G.; Maury, E.; Mongodi, S.; Mojoli, F.; Volpicelli, G.; Zanobetti, M. Thoracic Ultrasonography: A Narrative Review. Intensive Care Med. 2019, 45, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Acute Pulmonary Embolism Developed in Collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Kucher, N.; Goldhaber, S.Z. Cardiac Biomarkers for Risk Stratification of Patients with Acute Pulmonary Embolism. Circulation 2003, 108, 2191–2194. Available online: https://www.ahajournals.org/doi/full/10.1161/01.CIR.0000100687.99687.CE (accessed on 20 July 2024). [CrossRef] [PubMed]
- Murata, M.; Nakagawa, N.; Kawasaki, T.; Yasuo, S.; Yoshida, T.; Ando, K.; Okamori, S.; Okada, Y. Adverse Events during Intrahospital Transport of Critically Ill Patients: A Systematic Review and Meta-Analysis. Am. J. Emerg. Med. 2022, 52, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Frickey, N.; Kraincuk, P.; Zhilla, I.; Binder, T.; Plöchl, W. Fulminant Pulmonary Embolism Treated by Extracorporeal Membrane Oxygenation in a Patient with Traumatic Brain Injury. J. Trauma Inj. Infect. Crit. Care 2008, 64, E41–E43. [Google Scholar] [CrossRef]
- Bouzekraoui, I.; Dahbi, Y.; El Fassi, A.; Garda, Y.; Doumiri, M.; Amor, M. Early Severe Pulmonary Embolism in a Severe Traumatic Brain Injury: A Case Report. Intensive Care 2023, 6, 1457. [Google Scholar]
- Visconti, L.; Celi, A.; Carrozzi, L.; Tinelli, C.; Crocetti, L.; Daviddi, F.; De Caterina, R.; Madonna, R.; Pancani, R. Inferior Vena Cava Filters: Concept Review and Summary of Current Guidelines. Vasc. Pharmacol. 2024, 155, 107375. [Google Scholar] [CrossRef]
- Pieraccini, M.; Guerrini, S.; Laiolo, E.; Puliti, A.; Roviello, G.; Misuraca, L.; Spargi, G.; Limbruno, U.; Breggia, M.; Grechi, M. Acute Massive and Submassive Pulmonary Embolism: Preliminary Validation of Aspiration Mechanical Thrombectomy in Patients with Contraindications to Thrombolysis. Cardiovasc. Interv. Radiol. 2018, 41, 1840–1848. [Google Scholar] [CrossRef]
- Wible, B.C.; Buckley, J.R.; Cho, K.H.; Bunte, M.C.; Saucier, N.A.; Borsa, J.J. Safety and Efficacy of Acute Pulmonary Embolism Treated via Large-Bore Aspiration Mechanical Thrombectomy Using the Inari FlowTriever Device. J. Vasc. Interv. Radiol. 2019, 30, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.; Meyer, G. Management of Acute Pulmonary Embolism 2019: What Is New in the Updated European Guidelines? Intern. Emerg. Med. 2020, 15, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Besen, B.A.M.P.; Herridge, M.S. Focus on the Frail and Elderly: Who Should Have a Trial of ICU Treatment? Intensive Care Med. 2020, 46, 1030–1032. [Google Scholar] [CrossRef] [PubMed]
- Baggiani, M.; Guglielmi, A.; Citerio, G. Acute Traumatic Brain Injury in Frail Patients: The next Pandemic. Curr. Opin. Crit. Care 2022, 28, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, M.; Yin, W.; Zhou, L.; Zuo, X. Safety and Efficacy of Low Molecular Weight Heparin for Thromboprophylaxis in the Elderly: A Network Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2021, 12, 783104. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, M.; Bouchaala, K.; Baccouche, N.; Chtara, K.; Chelly, H.; Bouaziz, M. The Role of Cardiac Dysfunction and Post-Traumatic Pulmonary Embolism in Brain-Lung Interactions Following Traumatic Brain Injury. Acute Crit. Care 2022, 37, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-X.; Wu, J.-X.; Zhong, H.; Chen, W.; Zheng, J.-C. Therapeutic Efficacy of Tranexamic Acid on Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Scand. J. Trauma Resusc. Emerg. Med. 2024, 32, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, T. Efficacy and Safety of Tranexamic Acid in Acute Traumatic Brain Injury: A Meta-Analysis of Randomized Controlled Trials. Am. J. Emerg. Med. 2024, 80, 35–43. [Google Scholar] [CrossRef]
- Cole, K.L.; Nguyen, S.; Gelhard, S.; Hardy, J.; Cortez, J.; Nunez, J.M.; Menacho, S.T.; Grandhi, R. Factors Associated with Venous Thromboembolism Development in Patients with Traumatic Brain Injury. Neurocrit. Care 2024, 40, 568–576. [Google Scholar] [CrossRef]
- Condon, F.; Grigorian, A.; Russell, D.; Demetriades, D. Venous Thromboembolism Chemoprophylaxis in Geriatric Trauma Patients with Isolated Severe Traumatic Brain Injury. Eur. J. Trauma Emerg. Surg. 2024, 50, 197–203. [Google Scholar] [CrossRef]
- Park, G.; Dhillon, N.K.; Fierro, N.M.; Drevets, P.; Stupinski, J.; Ley, E.J. Creatinine Clearance Predicts the Goal Enoxaparin Dose in Traumatic Brain Injury. J. Trauma Acute Care Surg. 2024, 96, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Jakob, D.A.; Müller, M.; Lewis, M.; Wong, M.D.; Exadaktylos, A.K.; Demetriades, D. Risk Factors for Thromboembolic Complications in Isolated Severe Head Injury. Eur. J. Trauma Emerg. Surg. 2024, 50, 185–195. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Chien, C.-Y.; Matsushima, K.; Schellenberg, M.; Inaba, K.; Moore, E.E.; Sauaia, A.; Knudson, M.M.; Martin, M.J.; the CLOTT Study Group. Early Venous Thromboembolism Prophylaxis in Patients with Trauma Intracranial Hemorrhage: Analysis of the Prospective Multicenter Consortium of Leaders in Traumatic Thromboembolism Study. J. Trauma Acute Care Surg. 2023, 95, 649–656. [Google Scholar] [CrossRef]
- Chen, D.; Luo, J.; Zhang, C.; Tang, L.; Deng, H.; Chang, T.; Xu, H.; He, M.; Wan, D.; Zhang, F.; et al. Venous Thrombus Embolism in Polytrauma: Special Attention to Patients with Traumatic Brain Injury. J. Clin. Med. 2023, 12, 1716. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Menchaca, C.; Gusdon, A.M. Timing of Anticoagulation for Venous Thromboembolism after Recent Traumatic and Vascular Brain Injury. J. Thromb. Thrombolysis 2023, 55, 289–296. [Google Scholar] [CrossRef]
- The PATCH-Trauma Investigators and the ANZICS Clinical Trials Group. Prehospital Tranexamic Acid for Severe Trauma. N. Engl. J. Med. 2023, 389, 127–136. [Google Scholar] [CrossRef]
- Zheng, Q. Comparative Safety of Multiple Doses of Erythropoietin for the Treatment of Traumatic Brain Injury: A Systematic Review and Network Meta-Analysis. Front. Neurol. 2022, 13, 998320. [Google Scholar] [CrossRef] [PubMed]
- Hazelton, J.P.; Ssentongo, A.E.; Oh, J.S.; Ssentongo, P.; Seamon, M.J.; Byrne, J.P.; Armento, I.G.; Jenkins, D.H.; Braverman, M.A.; Mentzer, C.; et al. Use of Cold-Stored Whole Blood Is Associated with Improved Mortality in Hemostatic Resuscitation of Major Bleeding: A Multicenter Study. Ann. Surg. 2022, 276, 579–588. [Google Scholar] [CrossRef]
- Ali, A.; Tatum, D.; Olubowale, O.O.; McGrew, P.R.; Duchesne, J.; Taghavi, S. Thromboembolic Outcomes in Tetrahydrocannabinol-Positive Trauma Patients with Traumatic Brain Injury. J. Surg. Res. 2022, 275, 194–202. [Google Scholar] [CrossRef]
- Ali, A.B.; Khawaja, A.M.; Reilly, A.; Tahir, Z.; Rao, S.S.; Bernstock, J.D.; Chen, P.; Molino, J.; Gormley, W.; Izzy, S. Venous Thromboembolism Risk and Outcomes Following Decompressive Craniectomy in Severe Traumatic Brain Injury: An Analysis of the Nationwide Inpatient Sample Database. World Neurosurg. 2022, 161, e531–e545. [Google Scholar] [CrossRef]
- Perissier, C.; Crespy, T.; Godon, A.; Bosson, J.-L.; Bouzat, P. Reasons for a Late Initiation of Pharmacological Thromboprophylaxis in Severe Trauma Patients: A Prospective Observational Study. Anaesth. Crit. Care Pain. Med. 2022, 41, 101037. [Google Scholar] [CrossRef] [PubMed]
- Fletcher-Sandersjöö, A.; Tatter, C.; Tjerkaski, J.; Bartek, J., Jr.; Svensson, M.; Thelin, E.P.; Bellander, B.-M. Clinical Significance of Vascular Occlusive Events Following Moderate-to-Severe Traumatic Brain Injury: An Observational Cohort Study. Semin. Thromb. Hemost. 2022, 48, 301–308. [Google Scholar] [CrossRef]
- Byrne, J.P.; Mason, S.A.; Gomez, D.; Hoeft, C.; Subacius, H.; Xiong, W.; Neal, M.; Pirouzmand, F.; Nathens, A.B. Timing of Pharmacologic Venous Thromboembolism Prophylaxis in Severe Traumatic Brain Injury: A Propensity-Matched Cohort Study. J. Am. Coll. Surg. 2016, 223, 621–631e5. [Google Scholar] [CrossRef]
- Rivas, L.; Vella, M.; Ju, T.; Fernandez-Moure, J.S.; Sparks, A.; Seamon, M.J.; Sarani, B. Early Chemoprophylaxis Against Venous Thromboembolism in Patients with Traumatic Brain Injury. Am. Surg. 2022, 88, 187–193. [Google Scholar] [CrossRef] [PubMed]
- El-Menyar, A.; Ahmed, K.; Hakim, S.; Kanbar, A.; Mathradikkal, S.; Siddiqui, T.; Jogol, H.; Younis, B.; Taha, I.; Mahmood, I.; et al. Efficacy and Safety of the Second In-Hospital Dose of Tranexamic Acid after Receiving the Prehospital Dose: Double-Blind Randomized Controlled Clinical Trial in a Level 1 Trauma Center. Eur. J. Trauma Emerg. Surg. 2022, 48, 3089–3099. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, J.; Stone, L.; Marquart, G.; Freeman, C.; Zonies, D. Do I Really Need This Transthoracic ECHO? An over-Utilized Test in Trauma and Surgical Intensive Care Units. Injury 2022, 53, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Gates, R.S.; Lollar, D.I.; Collier, B.R.; Smith, J.; Faulks, E.R.; Gillen, J.R. Enoxaparin Titrated by Anti-Xa Levels Reduces Venous Thromboembolism in Trauma Patients. J. Trauma: Acute Care Surg. 2022, 92, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Elkbuli, A.; Ehrhardt, J.D.; Kinslow, K.; McKenney, M. Prophylactic Inferior Vena Cava Filters: Outcomes in Severely Injured Trauma Patients. Am. Surg. 2021, 87, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Elkbuli, A.; Ehrhardt, J.D.; Kinslow, K.; McKenney, M. Timing of Prophylactic Inferior Vena Cava Filter Placement and Trauma Outcomes: Does Early Placement Matter? Am. Surg. 2020, 86, 1741–1747. [Google Scholar] [CrossRef]
- Chipman, A.M.; Radowsky, J.; Vesselinov, R.; Chow, D.; Schwartzbauer, G.; Tesoriero, R.; Stein, D. Therapeutic Anticoagulation in Patients with Traumatic Brain Injuries and Pulmonary Emboli. J. Trauma Acute Care Surg. 2020, 89, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Greenberg, P.; Shin, S.H. Pulmonary Complications and Sepsis Following Severe Acute Subdural Hematoma in Patients Who Underwent Craniotomy versus Craniectomy: A Propensity Score Matched Analysis. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2020, 81, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; Alvi, M.A.; Rovin, R.A.; Kasper, E.M. Clinical Outcomes Following Early versus Late Pharmacologic Thromboprophylaxis in Patients with Traumatic Intracranial Hemorrhage: A Systematic Review and Meta-Analysis. Neurosurg. Rev. 2020, 43, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Parikh, B.; Dirlikov, B.; Cage, T.; Lee, M.; Singh, H. Elevated Risk of Venous Thromboembolism among Post-Traumatic Brain Injury Patients Requiring Pharmaceutical Immobilization. J. Clin. Neurosci. 2020, 75, 66–70. [Google Scholar] [CrossRef] [PubMed]
- July, J.; Pranata, R. Tranexamic Acid Is Associated with Reduced Mortality, Hemorrhagic Expansion, and Vascular Occlusive Events in Traumatic Brain Injury—Meta-Analysis of Randomized Controlled Trials. BMC Neurol. 2020, 20, 119. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, M. The Efficacy of Tranexamic Acid for Brain Injury: A Meta-Analysis of Randomized Controlled Trials. Am. J. Emerg. Med. 2020, 38, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Rabinstein, A.A.; Hellickson, J.D.; Macedo, T.A.; Lewis, B.D.; Mandrekar, J.; McBane, R.D. Sequential Pneumatic Compression in the Arm in Neurocritical Patients with a Peripherally Inserted Central Venous Catheter: A Randomized Trial. Neurocrit Care 2020, 32, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Seifi, A.; Dengler, B.; Martinez, P.; Godoy, D.A. Pulmonary Embolism in Severe Traumatic Brain Injury. J. Clin. Neurosci. 2018, 57, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, M.; Chan, N.; Bhagirath, V.; Ginsberg, J. Prevention of Venous Thromboembolism in 2020 and Beyond. J. Clin. Med. 2020, 9, 2467. [Google Scholar] [CrossRef]
- Nguyen, S.; Grandhi, R.; Cole, K.L. Response to “Additional Associated Factors for Venous Thromboembolism in Patients with Traumatic Brain Injury”. Neurocrit Care 2023, 39, 734–735. [Google Scholar] [CrossRef]
- Yorkgitis, B.K.; Berndtson, A.E.; Cross, A.; Kennedy, R.; Kochuba, M.P.; Tignanelli, C.; Tominaga, G.T.; Jacobs, D.G.; Marx, W.H.; Ashley, D.W.; et al. American Association for the Surgery of Trauma/American College of Surgeons-Committee on Trauma Clinical Protocol for Inpatient Venous Thromboembolism Prophylaxis after Trauma. J. Trauma Acute Care Surg. 2022, 92, 597–604. [Google Scholar] [CrossRef]
- Rogers, M.A.M.; Levine, D.A.; Blumberg, N.; Flanders, S.A.; Chopra, V.; Langa, K.M. Triggers of Hospitalization for Venous Thromboembolism. Circulation 2012, 125, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.A.; Spencer, F.A. Risk Factors for Venous Thromboembolism. Circulation 2003, 107, I9–I16. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.R.; Fromentin, M.; Ronot, M.; Gauss, T.; Harrois, A.; Duranteau, J.; Paugam-Burtz, C. Femoral Arterial and Central Venous Catheters in the Trauma Resuscitation Room. Injury 2018, 49, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Valle, E.J.; Van Haren, R.M.; Allen, C.J.; Jouria, J.M.; Bullock, M.R.; Schulman, C.I.; Namias, N.; Livingstone, A.S.; Proctor, K.G. Does Traumatic Brain Injury Increase the Risk for Venous Thromboembolism in Polytrauma Patients? J. Trauma Acute Care Surg. 2014, 77, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.H.; Satterwhite, T.; McConnell, B.J.; Costin, B.; Borit, A.; Gould, L.; Pruessner, J.; Bernstein, D.; Gildenberg, P.L. Deep Vein Thrombosis and Pulmonary Embolism in Head Injured Patients. Angiology 1983, 34, 627–638. [Google Scholar] [CrossRef]
- Ekeh, A.P.; Dominguez, K.M.; Markert, R.J.; McCarthy, M.C. Incidence and Risk Factors for Deep Venous Thrombosis after Moderate and Severe Brain Injury. J. Trauma 2010, 68, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Dengler, B.A.; Mendez-Gomez, P.; Chavez, A.; Avila, L.; Michalek, J.E.; Hernandez, B.; Grandhi, R.; Seifi, A. Safety of Chemical DVT Prophylaxis in Severe Traumatic Brain Injury with Invasive Monitoring Devices. Neurocritical Care 2016, 25, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Booth, K.; Rivet, J.; Flici, R.; Harvey, E.; Hamill, M.; Hundley, D.; Holland, K.; Hubbard, S.; Trivedi, A.; Collier, B. Progressive Mobility Protocol Reduces Venous Thromboembolism Rate in Trauma Intensive Care Patients: A Quality Improvement Project. J. Trauma Nurs. 2016, 23, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Blebea, N.M.; Pricopie, A.I.; Vlad, R.-A.; Hancu, G. Phytocannabinoids: Exploring Pharmacological Profiles and Their Impact on Therapeutical Use. Int. J. Mol. Sci. 2024, 25, 4204. [Google Scholar] [CrossRef]
- Chakroun-Walha, O.; Samet, A.; Jerbi, M.; Nasri, A.; Talbi, A.; Kanoun, H.; Souissi, B.; Chtara, K.; Bouaziz, M.; Ksibi, H.; et al. Benefits of the Tranexamic Acid in Head Trauma with No Extracranial Bleeding: A Prospective Follow-up of 180 Patients. Eur. J. Trauma Emerg. Surg. 2019, 45, 719–726. [Google Scholar] [CrossRef]
- Rowell, S.E.; Meier, E.N.; McKnight, B.; Kannas, D.; May, S.; Sheehan, K.; Bulger, E.M.; Idris, A.H.; Christenson, J.; Morrison, L.J.; et al. Effect of Out-of-Hospital Tranexamic Acid vs Placebo on 6-Month Functional Neurologic Outcomes in Patients with Moderate or Severe Traumatic Brain Injury. JAMA 2020, 324, 961. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, A.F.; Fergusson, D.A.; Clayton, L.; Patton, M.-P.; Neveu, X.; Walsh, T.S.; Docherty, A.; Malbouisson, L.M.; Pili-Floury, S.; English, S.W.; et al. Liberal or Restrictive Transfusion Strategy in Patients with Traumatic Brain Injury. N. Engl. J. Med. 2024, NEJMoa2404360. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.P.; Witiw, C.D.; Schuster, J.M.; Pascual, J.L.; Cannon, J.W.; Martin, N.D.; Reilly, P.M.; Nathens, A.B.; Seamon, M.J. Association of Venous Thromboembolism Prophylaxis After Neurosurgical Intervention for Traumatic Brain Injury with Thromboembolic Complications, Repeated Neurosurgery, and Mortality. JAMA Surg. 2022, 157, e215794. [Google Scholar] [CrossRef] [PubMed]
- Ley, E.J.; Brown, C.V.R.; Moore, E.E.; Sava, J.A.; Peck, K.; Ciesla, D.J.; Sperry, J.L.; Rizzo, A.G.; Rosen, N.G.; Brasel, K.J.; et al. Updated Guidelines to Reduce Venous Thromboembolism in Trauma Patients: A Western Trauma Association Critical Decisions Algorithm. J. Trauma Acute Care Surg. 2020, 89, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Geerts, W.H.; Jay, R.M.; Code, K.I.; Chen, E.; Szalai, J.P.; Saibil, E.A.; Hamilton, P.A. A Comparison of Low-Dose Heparin with Low- Molecular-Weight Heparin as Prophylaxis against Venous Thromboembolism after Major Trauma. N. Engl. J. Med. 1996, 335, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Fareed, J.; Hoppensteadt, D.; Walenga, J.; Iqbal, O.; Ma, Q.; Jeske, W.; Sheikh, T. Pharmacodynamic and Pharmacokinetic Properties of Enoxaparin: Implications for Clinical Practice. Clin. Pharmacokinet. 2003, 42, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.; Harada, M.Y.; Barmparas, G.; Chung, K.; Mason, R.; Yim, D.A.; Dhillon, N.; Margulies, D.R.; Gewertz, B.L.; Ley, E.J. Association Between Enoxaparin Dosage Adjusted by Anti–Factor Xa Trough Level and Clinically Evident Venous Thromboembolism After Trauma. JAMA Surg. 2016, 151, 1006. [Google Scholar] [CrossRef]
- Greenfield, L.J.; Proctor, M.C.; Rodriguez, J.L.; Luchette, F.A.; Cipolle, M.D.; Cho, J. Posttrauma Thromboembolism Prophylaxis. J. Trauma: Acute Care Surg. 1997, 42, 100. [Google Scholar] [CrossRef]
- Gearhart, M.M.; Luchette, F.A.; Proctor, M.C.; Lutomski, D.M.; Witsken, C.; James, L.; Davis, K.; Johannigman, J.A.; Hurst, J.M.; Frame, S.B. The Risk Assessment Profile Score Identifies Trauma Patients at Risk for Deep Vein Thrombosis. Surgery 2000, 128, 631–640. [Google Scholar] [CrossRef]
- Helms, J.; Carrier, M.; Klok, F.A. High-Risk Pulmonary Embolism in the Intensive Care Unit. Intensiv. Care Med. 2023, 49, 579–582. Available online: https://link.springer.com/article/10.1007/s00134-023-07011-0 (accessed on 21 July 2024). [CrossRef]
- Piazza, G. Advanced Management of Intermediate- and High-Risk Pulmonary Embolism. J. Am. Coll. Cardiol. 2020, 76, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Ius, F.; Hoeper, M.M.; Fegbeutel, C.; Kühn, C.; Olsson, K.; Koigeldiyev, N.; Tudorache, I.; Warnecke, G.; Optenhöfel, J.; Puntigam, J.O.; et al. Extracorporeal Membrane Oxygenation and Surgical Embolectomy for High-Risk Pulmonary Embolism. Eur. Respir. J. 2019, 53, 1801773. [Google Scholar] [CrossRef] [PubMed]
- Costantini, T.W.; Bulger, E.; Price, M.A.; Haut, E.R.; the National Trauma Research Action Plan (NTRAP) Investigators Group. Research Priorities in Venous Thromboembolism after Trauma: Secondary Analysis of the National Trauma Research Action Plan. J. Trauma Acute Care Surg. 2023, 95, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Chopard, R.; Nielsen, P.; Ius, F.; Cebotari, S.; Ecarnot, F.; Pilichowski, H.; Schmidt, M.; Kjaergaard, B.; Sousa-Casasnovas, I.; Ghoreishi, M.; et al. Optimal Reperfusion Strategy in Acute High-Risk Pulmonary Embolism Requiring Extracorporeal Membrane Oxygenation Support: A Systematic Review and Meta-Analysis. Eur. Respir. J. 2022, 60, 2102977. [Google Scholar] [CrossRef] [PubMed]
- Assouline, B.; Assouline-Reinmann, M.; Giraud, R.; Levy, D.; Saura, O.; Bendjelid, K.; Combes, A.; Schmidt, M. Manag. Management of High-Risk Pulmonary Embolism: What Is the Place of Extracorporeal Membrane Oxygenation? J. Clin. Med. 2022, 11, 4734. [Google Scholar] [CrossRef]
- Toma, C.; Jaber, W.; Weinberg, M.; Bunte, M.; Khandhar, S.; Stegman, B.; Gondi, S.; Chambers, J.; Amin, R.; Leung, D.; et al. Acute Outcomes for the Full US Cohort of the FLASH Mechanical Thrombectomy Registry in Pulmonary Embolism. Available online: https://eurointervention.pcronline.com/article/acute-outcomes-for-the-full-us-cohort-of-the-flash-mechanical-thrombectomy-registry-in-pulmonary-embolism (accessed on 16 July 2024).
- Ismayl, M.; Machanahalli Balakrishna, A.; Aboeata, A.; Gupta, T.; Young, M.N.; Altin, S.E.; Aronow, H.D.; Goldsweig, A.M. Meta-Analysis Comparing Catheter-Directed Thrombolysis Versus Systemic Anticoagulation Alone for Submassive Pulmonary Embolism. Am. J. Cardiol. 2022, 178, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, E.D.; Liang, N.L.; El-Shazly, O.M.; Toma, C.; Singh, M.J.; Makaroun, M.S.; Chaer, R.A. Improved Early Right Ventricular Function Recovery but Increased Complications with Catheter-Directed Interventions Compared with Anticoagulation Alone for Submassive Pulmonary Embolism. J. Vasc. Surg. Venous Lymphat. Disord. 2016, 4, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Gould, M.K.; Louie, J.D.; Rosenberg, J.K.; Sze, D.Y.; Hofmann, L.V. Catheter-Directed Therapy for the Treatment of Massive Pulmonary Embolism: Systematic Review and Meta-Analysis of Modern Techniques. J. Vasc. Interv. Radiol. 2009, 20, 1431–1440. [Google Scholar] [CrossRef]
- Meyer, R.M.; Larkin, M.B.; Szuflita, N.S.; Neal, C.J.; Tomlin, J.M.; Armonda, R.A.; Bailey, J.A.; Bell, R.S. Early Venous Thromboembolism Chemoprophylaxis in Combat-Related Penetrating Brain Injury. J. Neurosurg. 2017, 126, 1047–1055. [Google Scholar] [CrossRef]
- Piazza, G.; Hohlfelder, B.; Jaff, M.R.; Ouriel, K.; Engelhardt, T.C.; Sterling, K.M.; Jones, N.J.; Gurley, J.C.; Bhatheja, R.; Kennedy, R.J.; et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc. Interv. 2015, 8, 1382–1392. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chakraborty, A.; Weinberg, I.; Kadakia, M.; Wilensky, R.L.; Sardar, P.; Kumbhani, D.J.; Mukherjee, D.; Jaff, M.R.; Giri, J. Thrombolysis for Pulmonary Embolism and Risk of All-Cause Mortality, Major Bleeding, and Intracranial Hemorrhage: A Meta-Analysis. JAMA 2014, 311, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Marti, C.; John, G.; Konstantinides, S.; Combescure, C.; Sanchez, O.; Lankeit, M.; Meyer, G.; Perrier, A. Systemic Thrombolytic Therapy for Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. Eur. Heart J. 2015, 36, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Hong Son, P.D.; Uoc, N.H.; Lu, P.H.; Hung, D.Q.; Vo, H.-L. Surgical Pulmonary Embolectomy in a Multi-Trauma Patient: One-Center Experience in the Resource-Limited Setting. SAGE Open Med. Case Rep. 2020, 8, 2050313X2095375. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.S.; Salottolo, K.; Bar-Or, R.; Offner, P.; Mains, C.; Sullivan, M.; Bar-Or, D. Pharmacologic Thromboprophylaxis Is a Risk Factor for Hemorrhage Progression in a Subset of Patients With Traumatic Brain Injury. J. Trauma Inj. Infect. Crit. Care 2010, 68, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Morgano, G.P.; Bennett, C.; Dentali, F.; Francis, C.W.; Garcia, D.A.; Kahn, S.R.; Rahman, M.; Rajasekhar, A.; Rogers, F.B.; et al. American Society of Hematology 2019 Guidelines for Management of Venous Thromboembolism: Prevention of Venous Thromboembolism in Surgical Hospitalized Patients. Blood Adv. 2019, 3, 3898–3944. [Google Scholar] [CrossRef] [PubMed]
- Phelan, H.A.; Eastman, A.L.; Madden, C.J.; Aldy, K.; Berne, J.D.; Norwood, S.H.; Scott, W.W.; Bernstein, I.H.; Pruitt, J.; Butler, G.; et al. TBI Risk Stratification at Presentation: A Prospective Study of the Incidence and Timing of Radiographic Worsening in the Parkland Protocol. J. Trauma Acute Care Surg. 2012, 73, S122–S127. [Google Scholar] [CrossRef] [PubMed]
- Pastorek, R.A.; Cripps, M.W.; Bernstein, I.H.; Scott, W.W.; Madden, C.J.; Rickert, K.L.; Wolf, S.E.; Phelan, H.A. The Parkland Protocol’s Modified Berne-Norwood Criteria Predict Two Tiers of Risk for Traumatic Brain Injury Progression. J. Neurotrauma 2014, 31, 1737–1743. [Google Scholar] [CrossRef]
- Ferenczy, A.M.; Strawn, D.; Strawn, D.; Johns, T.; Parel, R.J.; Ashley, D.W. Safety of the Trauma Quality Improvement Program Guideline for Venous Thromboembolism Prophylaxis in Traumatic Brain Injury. Am. Surg. 2023, 89, 3460–3464. [Google Scholar] [CrossRef]
- Honeybul, S.; Kolias, A.G. (Eds.) Traumatic Brain Injury: Science, Practice, Evidence and Ethics; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-78074-6. [Google Scholar]
- Bova, C.; Greco, F.; Misuraca, G.; Serafini, O.; Crocco, F.; Greco, A.; Noto, A. Diagnostic Utility of Echocardiography in Patients with Suspected Pulmonary Embolism. Am. J. Emerg. Med. 2003, 21, 180–183. [Google Scholar] [CrossRef]
- Haddad, A.E.I.; Mouhib, E.A.; Hattab, O.; Assamti, M.; Mojahid, A.; Bkiyer, H.; Nasri, S.; Skiker, I.; Ouafi, E.; Housni, B. Early Bilateral Pulmonary Embolism in a Polytrauma Patient: About a Case Report. Ann. Med. Surg. 2022, 78, 103868. [Google Scholar] [CrossRef]
- Iaccarino, A.; Frati, G.; Schirone, L.; Saade, W.; Iovine, E.; D’Abramo, M.; De Bellis, A.; Sciarretta, S.; Greco, E. Surgical Embolectomy for Acute Massive Pulmonary Embolism: State of the Art. J. Thorac. Dis. 2018, 10, 5154–5161. [Google Scholar] [CrossRef] [PubMed]
- Rosovsky, R.; Zhao, K.; Sista, A.; Rivera-Lebron, B.; Kabrhel, C. Pulmonary Embolism Response Teams: Purpose, Evidence for Efficacy, and Future Research Directions. Res. Pr. Thromb. Haemost. 2019, 3, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Rosovsky, R.; Borges, J.; Kabrhel, C.; Rosenfield, K. Pulmonary Embolism Response Team: Inpatient Structure, Outpatient Follow-up, and Is It the Current Standard of Care? Clin. Chest Med. 2018, 39, 621–630. [Google Scholar] [CrossRef]
- Barnes, G.D.; Kabrhel, C.; Courtney, D.M.; Naydenov, S.; Wood, T.; Rosovsky, R.; Rosenfield, K.; Giri, J.; National PERT Consortium Research Committee. Diversity in the Pulmonary Embolism Response Team Model: An Organizational Survey of the National PERT Consortium Members. Chest 2016, 150, 1414–1417. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.; Giri, J.; Courtney, D.M.; Naydenov, S.; Wood, T.; Rosovsky, R.; Rosenfield, K.; Kabrhel, C.; National PERTTM Consortium Research Committee. Nuts and Bolts of Running a Pulmonary Embolism Response Team: Results from an Organizational Survey of the National PERTTM Consortium Members. Hosp. Pr. 2017, 45, 76–80. [Google Scholar] [CrossRef]
- Bejjani, A.; Khairani, C.D.; Campia, U.; Piazza, G. Pulmonary Embolism Response Teams: Theory, Implementation, and Unanswered Questions. J. Clin. Med. 2022, 11, 6129. [Google Scholar] [CrossRef]
- Hobohm, L.; Farmakis, I.T.; Keller, K.; Scibior, B.; Mavromanoli, A.C.; Sagoschen, I.; Münzel, T.; Ahrens, I.; Konstantinides, S. Pulmonary Embolism Response Team (PERT) Implementation and Its Clinical Value across Countries: A Scoping Review and Meta-Analysis. Clin. Res. Cardiol. 2023, 112, 1351–1361. [Google Scholar] [CrossRef]
- Pandya, V.; Chandra, A.A.; Scotti, A.; Assafin, M.; Schenone, A.L.; Latib, A.; Slipczuk, L.; Khaliq, A. Evolution of Pulmonary Embolism Response Teams in the United States: A Review of the Literature. J. Clin. Med. 2024, 13, 3984. [Google Scholar] [CrossRef]

| Modified Berne-Norwood Criteria | VTE Risk/RAP Score Points | |
|---|---|---|
| Consider: IVC filter, lower-extremity duplex screening for DVT | High risk ICP monitor in situ CO Evidence of progression at 72 h | High BMI, cancer, coagulation abnormalities, CVC in the femoral vein, RBC transfusion > 4 units, post-operative, chest/head and/or abdomen AIS >2, 40–60 y.o. 2 points |
| CTH stable: Initiate pharmacologic prophylaxis at 72 h | Moderate risk SDH > 8 mm EDH > 8 mm Cerebral contusion or IVH >2 cm Multiple contusions in a single lobe SAH with abnormal CTA Evidence of progression at 24 h | VTE Hx, major venous repair, spinal fractures, GCS < 8, 60–75 y.o. 3 points |
| CTH stable: Initiate pharmacologic prophylaxis at 24 h | Low risk No moderate- or high-risk criteria | Spinal cord injury, severe lower extremity and/or pelvic fracture, ≥75 y.o. 4 points |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrettou, C.S.; Dima, E.; Karela, N.R.; Sigala, I.; Korfias, S. Severe Traumatic Brain Injury and Pulmonary Embolism: Risks, Prevention, Diagnosis and Management. J. Clin. Med. 2024, 13, 4527. https://doi.org/10.3390/jcm13154527
Vrettou CS, Dima E, Karela NR, Sigala I, Korfias S. Severe Traumatic Brain Injury and Pulmonary Embolism: Risks, Prevention, Diagnosis and Management. Journal of Clinical Medicine. 2024; 13(15):4527. https://doi.org/10.3390/jcm13154527
Chicago/Turabian StyleVrettou, Charikleia S., Effrosyni Dima, Nina Rafailia Karela, Ioanna Sigala, and Stefanos Korfias. 2024. "Severe Traumatic Brain Injury and Pulmonary Embolism: Risks, Prevention, Diagnosis and Management" Journal of Clinical Medicine 13, no. 15: 4527. https://doi.org/10.3390/jcm13154527





