Relationship between Circadian System Status, Child–Pugh Score, and Clinical Outcome in Cirrhotic Patients on Waiting Lists for Liver Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ambulatory Circadian Monitoring (ACM)
2.3. Blood Parameters
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Child–Pugh Score Influences Circadian Rhythm
3.2. Biochemical Parameters Showed Alterations Related to Chronic Liver Disease
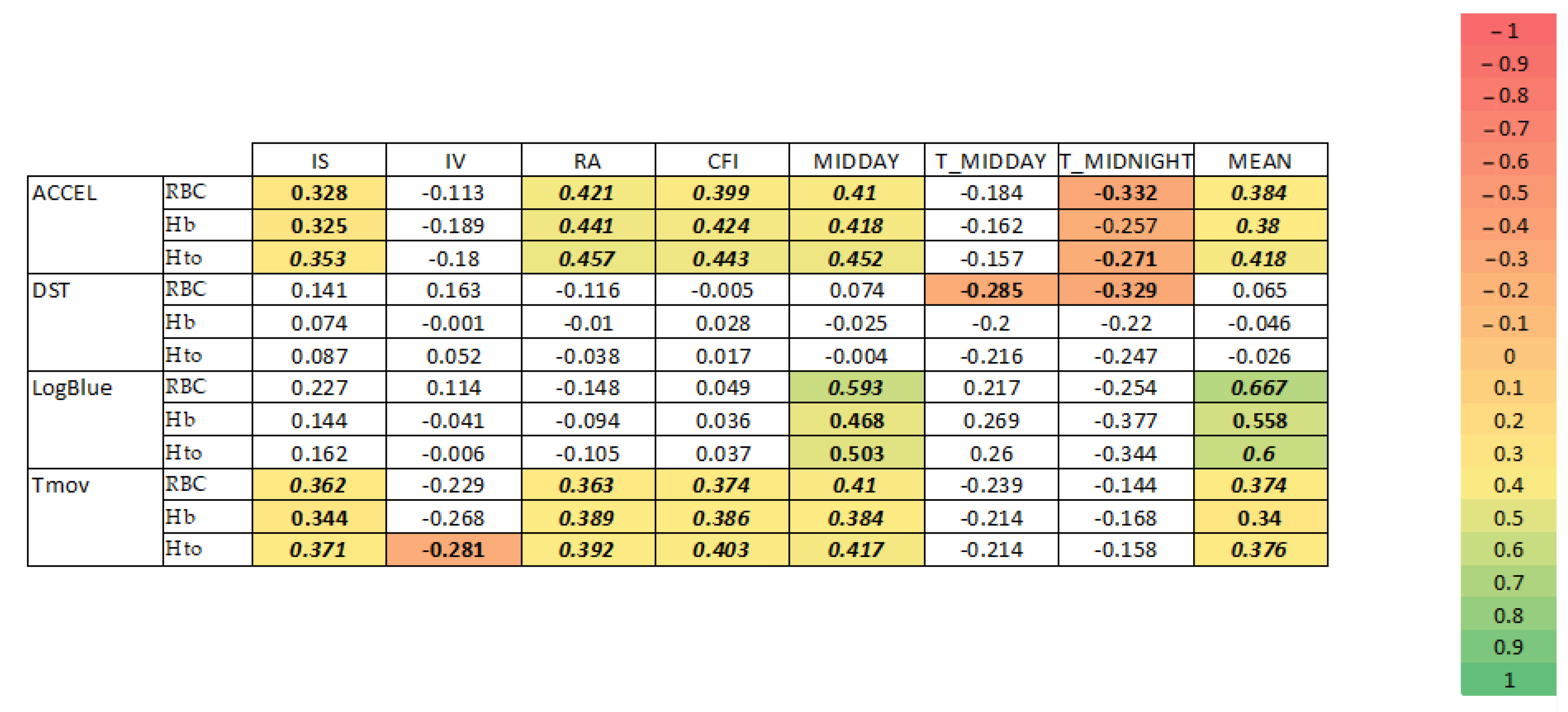
3.3. Correlations between Circadian Parameters and Blood Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelbasset, W.K.; Nambi, G.; Elsayed, S.H.; Moawd, S.A.; Ibrahim, A.A.; Verma, A.; Tantawy, S.A.; Kamel, D.M.; Saleh, A.K.; Aldhafian, O.R.; et al. Prevalence and Nonpharmacological Interventions for Sarcopenia among Cirrhotic Patients. Dis. Markers 2021, 2021, 8866093. [Google Scholar] [CrossRef]
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic Encephalopathy-Definition, Nomenclature, Diagnosis, and Quantification: Final Report of the Working Party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Matsumoto, Y.; Momoki, C.; Yuikawa, M.; Okada, G.; Hamakawa, E.; Kawamura, E.; Hagihara, A.; Toyama, M.; Fujii, H.; et al. Physical Inactivity and Insufficient Dietary Intake Are Associated with the Frequency of Sarcopenia in Patients with Compensated Viral Liver Cirrhosis. Hepatol. Res. 2013, 43, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Arguelles-Prieto, R.; Bonmati-Carrion, M.A.; Rol, M.A.; Madrid, J.A. Determining Light Intensity, Timing and Type of Visible and Circadian Light from an Ambulatory Circadian Monitoring Device. Front. Physiol. 2019, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Mullen, K.D. Mechanisms, Diagnosis and Management of Hepatic Encephalopathy. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Shenoda, B.; Boselli, J. Vascular Syndromes in Liver Cirrhosis. Clin. J. Gastroenterol. 2019, 12, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, S.; Middleton, B.; Mani, A.R.; Skene, D.J.; Morgan, M.Y. On the Origin and the Consequences of Circadian Abnormalities in Patients with Cirrhosis. Am. J. Gastroenterol. 2010, 105, 1773–1781. [Google Scholar] [CrossRef]
- Montagnese, S.; De Pittà, C.; De Rui, M.; Corrias, M.; Turco, M.; Merkel, C.; Amodio, P.; Costa, R.; Skene, D.J.; Gatta, A. Sleep-Wake Abnormalities in Patients with Cirrhosis. Hepatology 2014, 59, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, S.; Middleton, B.; Mani, A.R.; Skene, D.J.; Morgan, M.Y. Sleep and Circadian Abnormalities in Patients with Cirrhosis: Features of Delayed Sleep Phase Syndrome? Metab. Brain Dis. 2009, 24, 427–439. [Google Scholar] [CrossRef]
- Onghena, L.; Develtere, W.; Poppe, C.; Geerts, A.; Troisi, R.; Vanlander, A.; Berrevoet, F.; Rogiers, X.; Van Vlierberghe, H.; Verhelst, X. Quality of Life after Liver Transplantation: State of the Art. World J. Hepatol. 2016, 8, 749–756. [Google Scholar] [CrossRef]
- Nigatu, A.; Yap, J.E.; Lee Chuy, K.; Go, B.; Doukky, R. Bleeding Risk of Transesophageal Echocardiography in Patients with Esophageal Varices. J. Am. Soc. Echocardiogr. 2019, 32, 674–676.e2. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Nicolas, A.; Ortiz-Tudela, E.; Madrid, J.A.; Rol, M.A. Crosstalk between Environmental Light and Internal Time in Humans. Chronobiol. Int. 2011, 28, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.G.S.; Colares, J.R.; da Fonseca, S.R.B.; Martins, G.d.S.; Miguel, F.M.; Dias, A.S.; Marroni, C.A.; Picada, J.N.; Lehmann, M.; Marroni, N.A.P. Sarcopenia, Oxidative Stress and Inflammatory Process in Muscle of Cirrhotic Rats–Action of Melatonin and Physical Exercise. Exp. Mol. Pathol. 2021, 121, 104662. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Saccardo, D.; De Rui, M.; Vettore, E.; Verardo, A.; Carraro, P.; Di Vitofrancesco, N.; Mani, A.R.; Angeli, P.; Bolognesi, M.; et al. Abnormalities in the 24-Hour Rhythm of Skin Temperature in Cirrhosis: Sleep-Wake and General Clinical Implications. Liver Int. 2017, 37, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Tudela, E.; Martinez-Nicolas, A.; Campos, M.; Rol, M.Á.; Madrid, J.A. A New Integrated Variable Based on Thermometry, Actimetry and Body Position (TAP) to Evaluate Circadian System Status in Humans. PLoS Comput. Biol. 2010, 6, e1000996. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Nicolas, A.; Madrid, J.A.; Rol, M.A. Day-Night Contrast as Source of Health for the Human Circadian System. Chronobiol. Int. 2014, 31, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.; Di Pascoli, M.; Verardo, A.; Gatta, A. Splanchnic Vasodilation and Hyperdynamic Circulatory Syndrome in Cirrhosis. World J. Gastroenterol. 2014, 20, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Mullington, J.M.; Abbott, S.M.; Carroll, J.E.; Davis, C.J.; Dijk, D.J.; Dinges, D.F.; Gehrman, P.R.; Ginsburg, G.S.; Gozal, D.; Haack, M.; et al. Developing Biomarker Arrays Predicting Sleep and Circadian-Coupled Risks to Health. Sleep 2016, 39, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Sacerdoti, D.; Mania, D.; Jiang, H.; Pesce, P.; Gaiani, S.; Gatta, A.; Bolognesi, M. Increased EETs Participate in Peripheral Endothelial Dysfunction of Cirrhosis. Prostaglandins Other Lipid Mediat. 2012, 98, 129–132. [Google Scholar] [CrossRef]
- Lowe, D.; Sanvictores, T.; Zubair, M.; John, S. Alkaline Phosphatase. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459201/ (accessed on 6 June 2024).
- Garcia-Compean, D.; Jacquez-Quintana, J.O.; Gonzalez-Gonzalez, J.A.; Maldonado-Garza, H. Liver Cirrhosis and Diabetes: Risk Factors, Pathophysiology, Clinical Implications and Management. World J. Gastroenterol. 2009, 15, 280–288. [Google Scholar] [CrossRef]
- Buijs, R.M.; La Fleur, S.E.; Wortel, J.; Van Heyningen, C.; Zuiddam, L.; Mettenleiter, T.C.; Kalsbeek, A.; Nagai, K.; Niijima, A. The Suprachiasmatic Nucleus Balances Sympathetic and Parasympathetic Output to Peripheral Organs through Separate Preautonomic Neurons. J. Comp. Neurol. 2003, 464, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Bonmati-Carrion, M.A.; Middleton, B.; Revell, V.; Skene, D.J.; Rol, M.A.; Madrid, J.A. Circadian Phase Asessment by Ambulatory Monitoring in Humans: Correlation with Dim Light Melatonin Onset. Chronobiol. Int. 2014, 31, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Kräuchi, K.; Knoblauch, V.; Wirz-Justice, A.; Cajochen, C. Challenging the Sleep Homeostat Does Not Influence the Thermoregulatory System in Men: Evidence from a Nap vs. Sleep-Deprivation Study. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 290, 1052–1061. [Google Scholar] [CrossRef]
- Peck-Radosavljevic, M. Thrombocytopenia in Chronic Liver Disease. Liver Int. 2017, 37, 778–793. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Mannucci, M.P. The Coagulopathy of Liver Disease. Blood Coagul. Fibrinolysis 2013, 24, 10–17. [Google Scholar] [CrossRef]
- Batinga, H.; Martinez-Nicolas, A.; Zornoza-Moreno, M.; Sánchez-Solis, M.; Larqué, E.; Mondéjar, M.T.; Moreno-Casbas, M.; García, F.J.; Campos, M.; Rol, M.A.; et al. Ontogeny and Aging of the Distal Skin Temperature Rhythm in Humans. Age (Omaha) 2015, 37, 29. [Google Scholar] [CrossRef]
- van Marken Lichtenbelt, W.D.; Daanen, H.A.M.; Wouters, L.; Fronczek, R.; Raymann, R.J.E.M.; Severens, N.M.W.; Van Someren, E.J.W. Evaluation of Wireless Determination of Skin Temperature Using IButtons. Physiol. Behav. 2006, 88, 489–497. [Google Scholar] [CrossRef]
- Lisman, T.; Caldwell, S.H.; Intagliata, N.M. Review Haemostatic Alterations and Management of Haemostasis in Patients with Cirrhosis. J. Hepatol. 2022, 76, 1291–1305. [Google Scholar] [CrossRef]
- Child, C.G. Surgery and Portal Hypertension. Major Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar]
- Pugh, R.N.H.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the Oesophagus for Bleeding Oesophageal Varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Madrid-Navarro, C.J.; Escamilla-Sevilla, F.; Mínguez-Castellanos, A.; Campos, M.; Ruiz-Abellán, F.; Madrid, J.A.; Rol, M.A. Multidimensional Circadian Monitoring by Wearable Biosensors in Parkinson’s Disease. Front. Neurol. 2018, 9, 157. [Google Scholar] [CrossRef]
- Witting, W.; Kwa, I.H.; Eikelenboom, P.; Mirmiran, M.; Swaab, D.F. Alterations in the Circadian Rest-Activity Rhythm in Aging and Alzheimer’s Disease. Biol. Psychiatry 1990, 27, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Nicolas, A.; Madrid, J.A.; García, F.J.; Campos, M.; Moreno-Casbas, M.T.; Almaida-Pagán, P.F.; Lucas-Sánchez, A.; Rol, M.A. Circadian Monitoring as an Aging Predictor. Sci. Rep. 2018, 8, 15027. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Nicolas, A.; Guaita, M.; Santamaría, J.; Montserrat, J.M.; Rol, M.Á.; Madrid, J.A. Circadian Impairment of Distal Skin Temperature Rhythm in Patients with Sleep-Disordered Breathing: The Effect of CPAP. Sleep 2017, 40, 31–37. [Google Scholar] [CrossRef]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Müller, M.J.; Böker, K.; Selberg, O. Metabolism of Energy-Yielding Substrates in Patients with Liver Cirrhosis. Clin. Investig. 1994, 72, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Gattolliat, C.H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef]
- Moreau, C.A.; Urchs, S.G.W.; Kuldeep, K.; Orban, P.; Schramm, C.; Dumas, G.; Labbe, A.; Huguet, G.; Douard, E.; Quirion, P.O.; et al. Mutations Associated with Neuropsychiatric Conditions Delineate Functional Brain Connectivity Dimensions Contributing to Autism and Schizophrenia. Nat. Commun. 2020, 11, 5272. [Google Scholar] [CrossRef] [PubMed]
- Wullems, J.A.; Verschueren, S.M.P.; Degens, H.; Morse, C.I.; Onambélé, G.L. A Review of the Assessment and Prevalence of Sedentarism in Older Adults, Its Physiology/Health Impact and Non-Exercise Mobility Counter-Measures. Biogerontology 2016, 17, 547–565. [Google Scholar] [CrossRef]
- Zuurbier, L.A.; Luik, A.I.; Hofman, A.; Franco, O.H.; Van Someren, E.J.W.; Tiemeier, H. Fragmentation and Stability of Circadian Activity Rhythms Predict Mortality. Am. J. Epidemiol. 2015, 181, 54–63. [Google Scholar] [CrossRef]
- Giannini, E.G.; Savarino, V. Thrombocytopenia in Liver Disease. Curr. Opin. Hematol. 2008, 15, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, B.; Semmler, G.; Maurer, F.; Schwabl, P.; Bucsics, T.A.; Paternostro, R.; Bauer, D.; Simbrunner, B.; Trauner, M.; Mandorfer, M.; et al. Prevalence of and Risk Factors for Anaemia in Patients with Advanced Chronic Liver Disease. Liver Int. 2020, 40, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Northup, P.G.; Garcia-Pagan, J.C.; Garcia-Tsao, G.; Intagliata, N.M.; Superina, R.A.; Roberts, L.N.; Lisman, T.; Valla, D.C. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients With Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 73, 366–413. [Google Scholar] [CrossRef] [PubMed]
- Manrai, M.; Dawra, S.; Srivastava, S.; Kapoor, R.; Singh, A. Anemia in Cirrhosis: An Underestimated Entity. World J. Clin. Cases 2022, 10, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Parise, G. Skeletal Muscle Erythropoietin Expression Is Responsive to Hypoxia and Exercise. Med. Sci. Sports Exerc. 2016, 48, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, M.; Yu, X.; Nicolas-Metral, V.; Pulido, S.M.; Liu, C.; Ruegg, U.T.; Noguchi, C.T. Erythropoietin Stimulates Proliferation and Interferes with Differentiation of Myoblasts. J. Biol. Chem. 2000, 275, 39754–39761. [Google Scholar] [CrossRef] [PubMed]
- Rundqvist, H.; Rullman, E.; Sundberg, C.J.; Fischer, H.; Eisleitner, K.; Ståhlberg, M.; Sundblad, P.; Jansson, E.; Gustafsson, T. Activation of the Erythropoietin Receptor in Human Skeletal Muscle. Eur. J. Endocrinol. 2009, 161, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Mae, Y.; Yamada, K.; Taniguchi, S.; Hamada, S.; Yamamoto, M.; Iyama, T.; Isomoto, H. Skeletal Muscle Mass Is Associated with Erythropoietin Response in Hemodialysis Patients. BMC Nephrol. 2021, 22, 4–9. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, P.; Strahan, O.; Ferguson, D.; Monaghan, A.; Kennedy, M.; Forde, C.; Melo, A.M.; Doherty, D.G.; O’Brien, K.K.; McKiernan, S.; et al. Improvement in Cognitive Impairment Following a 12-Week Aerobic Exercise Intervention in Individuals with Non-Cirrhotic Chronic Hepatitis C. J. Viral Hepat. 2021, 28, 637–650. [Google Scholar] [CrossRef]
- Lang, E.; Gatidis, S.; Freise, N.F.; Bock, H.; Kubitz, R.; Lauermann, C.; Orth, H.M.; Klindt, C.; Schuier, M.; Keitel, V.; et al. Conjugated Bilirubin Triggers Anemia by Inducing Erythrocyte Death. Hepatology 2015, 61, 275–284. [Google Scholar] [CrossRef]
- Herman, J.; Baram, M. Blood and Volume Resuscitation for Variceal Hemorrhage. Ann. Am. Thorac. Soc. 2015, 12, 1100–1102. [Google Scholar] [CrossRef]
- Marušić, M.; Klemenčić, A.; Međugorac, M. When to Decide for Transfusion in Patients with Liver Cirrhosis and Acute Bleeding from Esophageal Varices? Liberal versus Restrictive Approach. AME Med. J. 2019, 4, 7. [Google Scholar] [CrossRef]
- Tripathi, D.; Stanley, A.J.; Hayes, P.C.; Patch, D.; Millson, C.; Mehrzad, H.; Austin, A.; Ferguson, J.W.; Olliff, S.P.; Hudson, M.; et al. UK Guidelines on the Management of Variceal Haemorrhage in Cirrhotic Patients. Gut 2015, 64, 1680–1704. [Google Scholar] [CrossRef]
- Ballester-Clau, R.; Torres Vicente, G.; Cucala Ramos, M.; Aracil Blanch, C.; Miñana Calafat, J.M.; Pijoan Comas, E.; Reñé Espinet, J.M.; Planella de Rubinat, M. Efficacy and Safety of Treatment With Ferric Carboxymaltose in Patients With Cirrhosis and Gastrointestinal Bleeding. Front. Med. 2020, 7, 128. [Google Scholar] [CrossRef]
- Rashidi-Alavijeh, J.; Nuruzade, N.; Frey, A.; Huessler, E.M.; Hörster, A.; Zeller, A.C.; Schütte, A.; Schmidt, H.; Willuweit, K.; Lange, C.M. Implications of Anaemia and Response to Anaemia Treatment on Outcomes in Patients with Cirrhosis. JHEP Rep. 2023, 5, 100688. [Google Scholar] [CrossRef]
- Schwabl, P.; Seeland, B.A.; Riedl, F.; Schubert, T.L.; Königshofer, P.; Brusilovskaya, K.; Petrenko, O.; Hofer, B.; Schiefer, A.I.; Trauner, M.; et al. Splenectomy Ameliorates Portal Pressure and Anemia in Animal Models of Cirrhotic and Non-Cirrhotic Portal Hypertension. Adv. Med. Sci. 2022, 67, 154–162. [Google Scholar] [CrossRef]
- Kedia, S.; Goyal, R.; Mangla, V.; Kumar, A.; Shalimar, S.; Das, P.; Pal, S.; Sahni, P.; Acharya, S.K. Splenectomy in Cirrhosis with Hypersplenism: Improvement in Cytopenias, Child’s Status and Institution of Specific Treatment for Hepatitis C with Success. Ann. Hepatol. 2012, 11, 921–929. [Google Scholar] [CrossRef]
- Shrestha, A.; Pradhananga, S. Holistic Approach in the Management of Nonalcoholic Fatty Liver Disease. Euroasian J. Hepato-Gastroenterol. 2022, 12, S51–S58. [Google Scholar] [CrossRef]
- Zou, D.; Yang, Z.; Qi, X.; Fan, D. Towards Holistic Integrative Medicine Based Management Strategy of Liver Cirrhosis. Expert Rev. Gastroenterol. Hepatol. 2024, 18, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Marchesini, G.; Nicolino, F.; Graziani, R.; Sgarbi, D.; Loguercio, C.; Abbiati, R.; Zoli, M. Psychological Status and Depression in Patients with Liver Cirrhosis. Dig. Liver Dis. 2005, 37, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, S.; Middleton, B.; Skene, D.J.; Morgan, M.Y. Night-Time Sleep Disturbance Does Not Correlate with Neuropsychiatric Impairment in Patients with Cirrhosis. Liver Int. 2009, 29, 1372–1382. [Google Scholar] [CrossRef]
- Mostacci, B.; Ferlisi, M.; Baldi Antognini, A.; Sama, C.; Morelli, C.; Mondini, S.; Cirignotta, F. Sleep Disturbance and Daytime Sleepiness in Patients with Cirrhosis: A Case Control Study. Neurol. Sci. 2008, 29, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Samanta, J.; Dhiman, R.K.; Khatri, A.; Thumburu, K.K.; Grover, S.; Duseja, A.; Chawla, Y. Correlation between Degree and Quality of Sleep Disturbance and the Level of Neuropsychiatric Impairment in Patients with Liver Cirrhosis. Metab. Brain Dis. 2013, 28, 249–259. [Google Scholar] [CrossRef]
- Reja, M.; Phelan, L.P.; Senatore, F.; Rustgi, V.K. Social Impact of Hepatic Encephalopathy. Clin. Liver Dis. 2020, 24, 291–301. [Google Scholar] [CrossRef]
- Vaughn-Sandler, V.; Sherman, C.; Aronsohn, A.; Volk, M.L. Consequences of Perceived Stigma among Patients with Cirrhosis. Dig. Dis. Sci. 2014, 59, 681–689. [Google Scholar] [CrossRef]
- Dasarathy, S. Consilience in Sarcopenia of Cirrhosis. J. Cachexia Sarcopenia Muscle 2012, 3, 225–237. [Google Scholar] [CrossRef]
- Dasarathy, S.; Merli, M. Sarcopenia from Mechanism to Diagnosis and Treatment in Liver Disease. J. Hepathol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef]
- Bellar, A.; Welch, N.; Dasarathy, S. REVIEW Synthesis Exercise and Physical Activity in Cirrhosis: Opportunities or Perils. J. Appl. Physiol. 2020, 128, 1547–1567. [Google Scholar] [CrossRef]
- Duarte-rojo, A.; Ruiz-margáin, A.; Montaño-loza, A.J.; Macías-Rodríguez, R.U.; Ferrando, A.; Kim, W.R. Exercise and Physical Activity for Patients with ESLD: Improving Functional Status and Sarcopenia While on the Transplant Waitlist. Liver Transplant. 2018, 21, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Montano-Loza, A.J.; Lai, J.C.; Dasarathy, S.; Merli, M. Sarcopenia and Frailty in Decompensated Cirrhosis. J. Hepatol. 2021, 75, S147–S162. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, M.R.; de Lima Pace, F.H.; Limongi, T.M.; Henrique, D.M.N.; de Carvalho Mira, P.A.; de Oliveira, T.M.D.; Oliveira, C.C.; de Aguiar, A.S.; Malaguti, C. Factors Associated with the Perceived Benefits and Barriers to Physical Activity in Liver Cirrhosis. Rev. Assoc. Med. Bras. 2021, 67, 271–276. [Google Scholar] [CrossRef]
- Tandon, P.; Ismond, K.P.; Riess, K.; Duarte-Rojo, A.; Al-Judaibi, B.; Dunn, M.A.; Holman, J.; Howes, N.; Haykowsky, M.J.F.; Josbeno, D.A.; et al. Exercise in Cirrhosis: Translating Evidence and Experience to Practice. J. Hepatol. 2018, 69, 1164–1177. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzakis, E.; Josefsson, A.; Castedal, M.; Henfridsson, P.; Bengtsson, M.; Hugosson, I.; Andersson, B.; Björnsson, E. Factors Related to Fatigue in Patients With Cirrhosis Before and After Liver Transplantation. Clin. Gastroenterol. Hepatol. 2012, 10, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Jopson, L.; Dyson, J.K.; Jones, D.E.J. Understanding and Treating Fatigue in Primary Biliary Cirrhosis and Primary Sclerosing Cholangitis. Clin. Liver Dis. 2016, 20, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Danford, C.J.; Trivedi, H.D.; Tapper, E.B.; Patwardhan, V.R.; Bonder, A. Treatment of Fatigue in Primary Biliary Cholangitis: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2019, 64, 2338–2350. [Google Scholar] [CrossRef]
- Ebadi, M.; Bhanji, R.A.; Mazurak, V.C.; Montano-Loza, A.J. Sarcopenia in Cirrhosis: From Pathogenesis to Interventions. J. Gastroenterol. 2019, 54, 845–859. [Google Scholar] [CrossRef]

| Child–Pugh | A | B | C | p-Value * | |||
|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Female | Male | Female | |
| N | 14 | 4 | 22 | 7 | 10 | 6 | |
| Age (yr) | 59.5 ± 1.7 | 53.7 ± 5.7 | 58.2 ± 2.3 | 59.7 ± 2.3 | 54.4 ± 3.5 | 60 ± 3.8 | 0.660 |
| Weight (Kg) | 73.2 ± 3.6 | 65.9 ± 7.8 | 86 ± 4.5 | 62.9 ± 5.4 | 75.6 ± 4.1 | 67.5 ± 5.9 | 0.060 |
| Height (cm) | 167.9 ± 1.9 | 162 ± 0.7 | 173.1 ± 1.7 | 158.3 ± 2.4 | 172.8 ± 2.1 | 160 ± 2.9 | 0.401 |
| IBM (Kgm−2) | 25.9 ± 1.1 | 25.1 ± 3 | 28.6 ± 1.4 | 25.0 ± 1.8 | 25.2 ± 1.2 | 26.9 ± 1.9 | 0.209 |
| IS | IV | RA | CFI | MIDDAY | TMIDDAY | MIDNIGHT | TMIDNIGHT | MEAN | ||
|---|---|---|---|---|---|---|---|---|---|---|
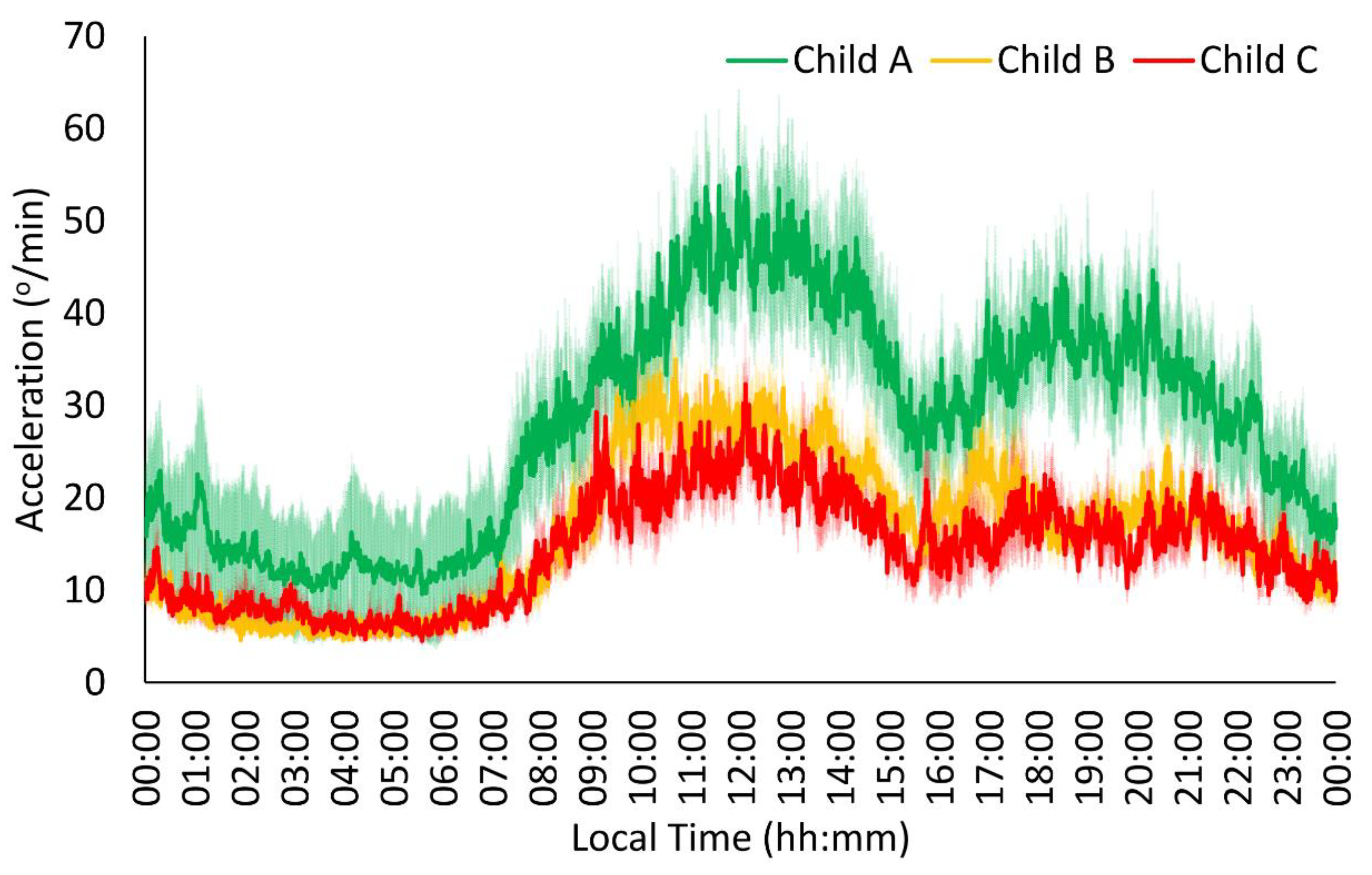
| ACCEL | Child A | 0.31 ± 0.02 | 0.35 ± 0.03 | 0.73 ± 0.04 | 0.63 ± 0.02 | 17.46 ± 1.32 | 14:38 ± 00:23 | 2.48 ± 0.23 | 04:09 ± 00:19 | 11.14 ± 0.78 |
| Child B | 0.30 ± 0.02 | 0.39 ± 0.02 | 0.58 ± 0.03 * | 0.57 ± 0.02 * | 11.51 ± 1.07 * | 14:24 ± 00:19 | 2.82 ± 0.18 | 04:10 ± 00:16 | 7.30 ± 0.64 * | |
| Child C | 0.27 ± 0.02 | 0.44 ± 0.03 | 0.51 ± 0.04 * | 0.51 ± 0.02 * | 9.09 ± 1.36 * | 13:54 ± 00:24 | 2.75 ± 0.23 | 04:10 ± 00:21 | 6.77 ± 0.78 * | |
| p | 0.223 | 0.059 | 0.001 | 0.000 | 0.000 | 0.419 | 0.481 | 0.999 | 0.000 | |
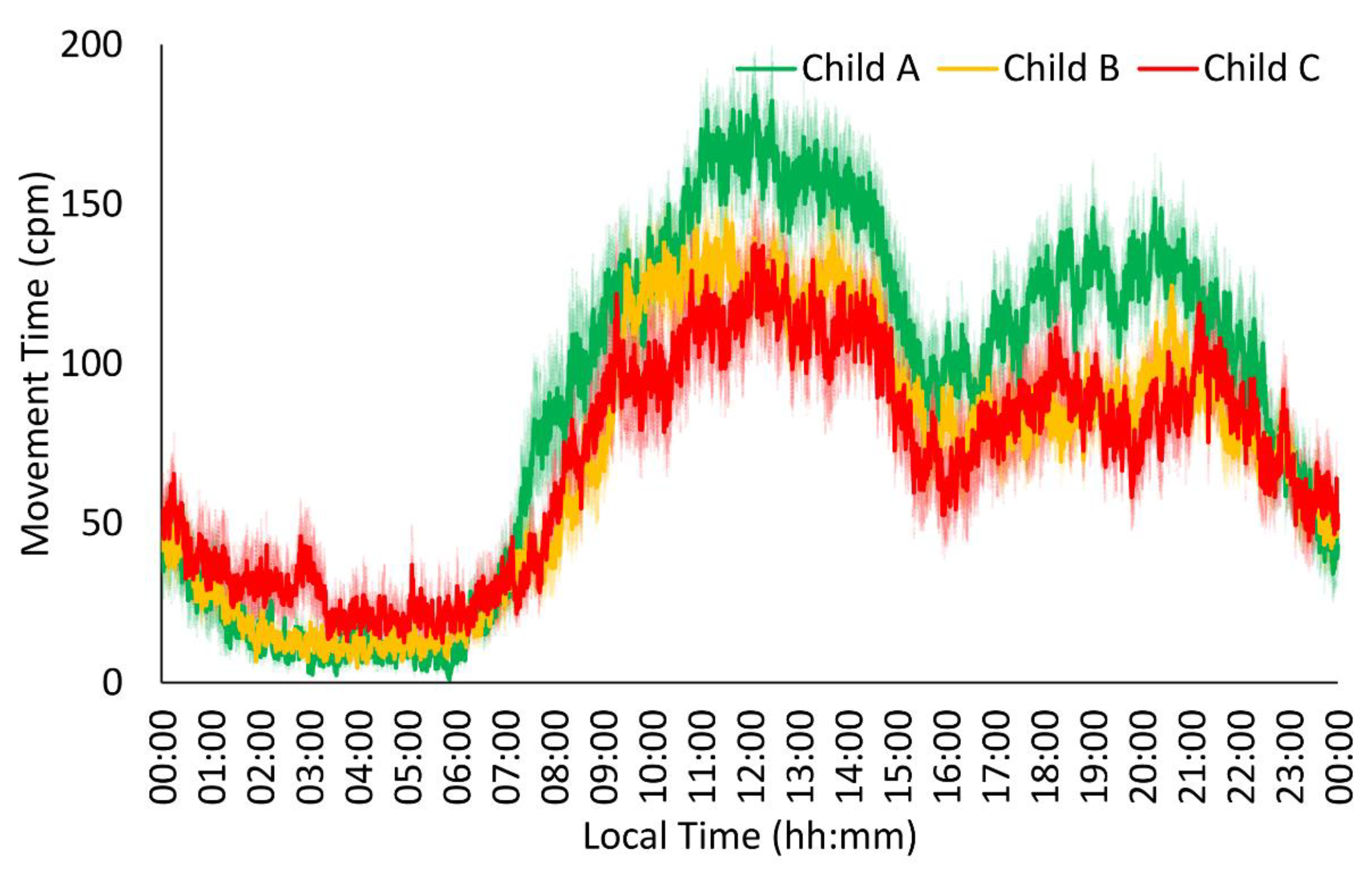
| Tmov | Child A | 0.44 ± 0.03 | 0.26 ± 0.02 | 0.88 ± 0.03 | 0.73 ± 0.02 | 139.45 ± 8.19 | 14:24 ± 00:23 | 8.70 ± 1.61 | 03:32 ± 00:16 | 86.67 ± 5.27 |
| Child B | 0.37 ± 0.02 | 0.33 ± 0.02 * | 0.81 ± 0.03 | 0.67 ± 0.02 | 110.05 ± 6.72 * | 14:38 ± 00:18 | 11.06 ± 1.30 | 04:11 ± 00:13 | 67.77 ± 4.41 * | |
| Child C | 0.32 ± 0.03 * | 0.37 ± 0.02 * | 0.69 ± 0.03 *# | 0.60 ± 0.02 *# | 98.26 ± 9.05 * | 14:32 ± 00:24 | 13.99 ± 1.66 | 04:03 ± 00:17 | 60.67 ± 5.64 * | |
| p | 0.008 | 0.003 | 0.001 | 0.000 | 0.003 | 0.886 | 0.081 | 0.154 | 0.003 | |
| DST | Child A | 0.51 ± 0.04 | 0.02 ± 0.00 | 0.04 ± 0.01 | 0.52 ± 0.02 | 31.48 ± 0.33 | 15:09 ± 00:50 | 34.23 ± 0.18 | 03:03 ± 00:43 | 32.68 ± 0.21 |
| Child B | 0.50 ± 0.03 | 0.02 ± 0.00 | 0.04 ± 0.00 | 0.51 ± 0.01 | 31.76 ± 0.26 | 15:35 ± 00:42 | 34.45 ± 0.15 | 04:04 ± 00:35 | 32.87 ± 0.17 | |
| Child C | 0.36 ± 0.04 *# | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.46 ± 0.02 | 32.56 ± 0.34 | 15:27 ± 00:50 | 34.44 ± 0.19 | 02:05 ± 00:44 | 33.40 ± 0.22 | |
| p | 0.025 | 0.141 | 0.037 | 0.045 | 0.064 | 0.920 | 0.591 | 0.116 | 0.056 | |
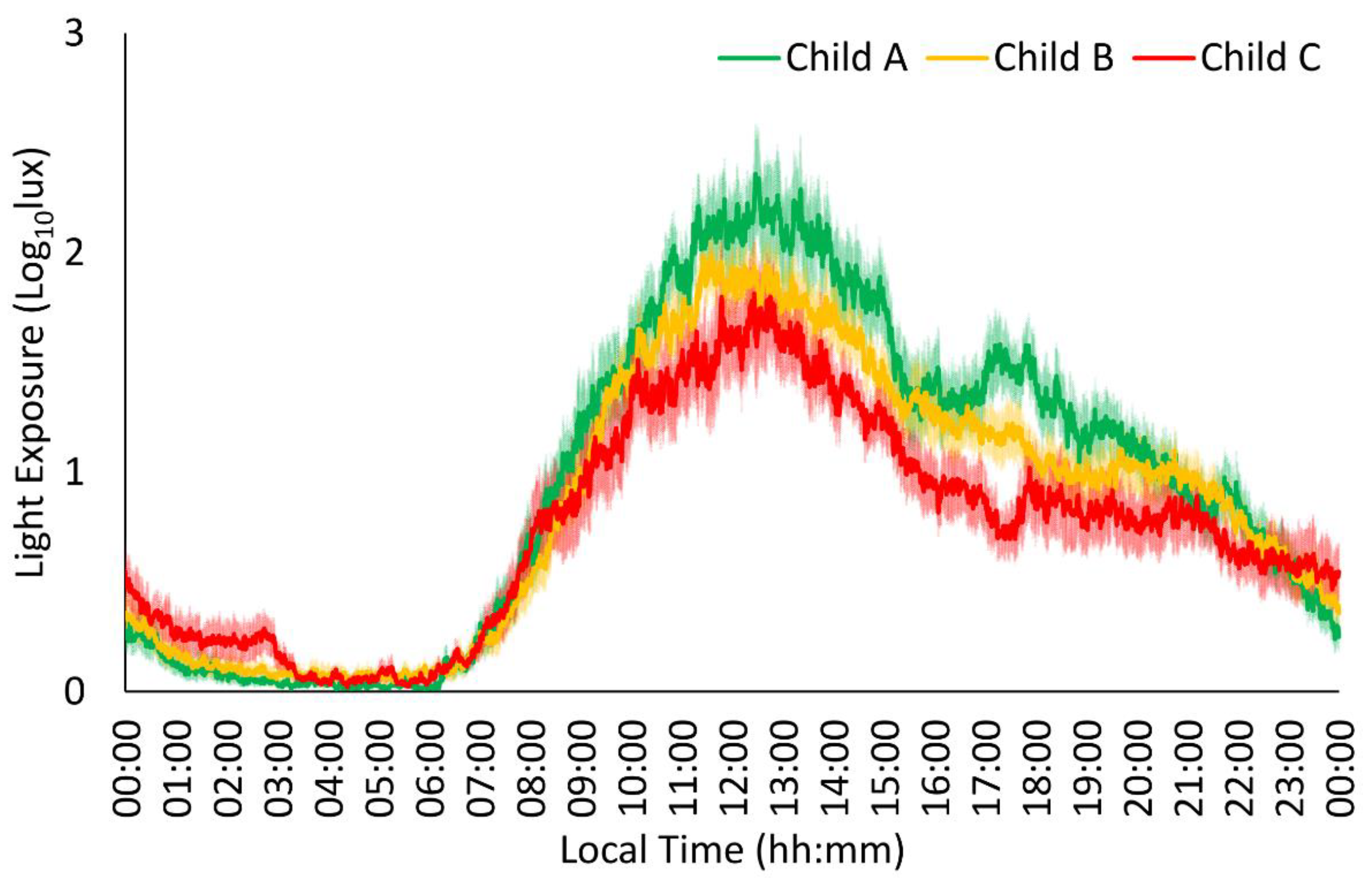
| LE | Child A | 0.61 ± 0.03 | 0.07 ± 0.01 | 0.97 ± 0.02 | 0.85 ± 0.01 | 1.76 ± 0.10 | 13:57 ± 00:22 | 0.01 ± 0.02 | 03:50 ± 00:13 | 0.97 ± 0.07 |
| Child B | 0.58 ± 0.03 | 0.08 ± 0.01 | 0.95 ± 0.02 | 0.84 ± 0.01 | 1.56 ± 0.08 | 14:00 ± 00:18 | 0.05 ± 0.02 | 03:55 ± 00:11 | 0.89 ± 0.05 | |
| Child C | 0.47 ± 0.03 *# | 0.07 ± 0.01 | 0.92 ± 0.02 | 0.78 ± 0.01 *# | 1.22 ± 0.10 *# | 15:03 ± 00:22 | 0.05 ± 0.02 | 04:27 ± 00:14 | 0.72 ± 0.07 * | |
| p | 0.009 | 0.854 | 0.140 | 0.002 | 0.002 | 0.053 | 0.154 | 0.129 | 0.022 | |
| Parameters\Child | Child A | Child B | Child C | p-Value | Normal Rank |
|---|---|---|---|---|---|
| Glu (mg/dL) | 127.5 ± 10.4 | 138.1 ± 16.3 | 125.1 ± 5.5 | NS | 74–106 |
| Ure (mg/dL) | 41.5 ± 4.4 | 38.8 ± 5.1 | 47.3 ± 6.3 | NS | 17–48 |
| Sod (mEq/L) | 141.1 ± 0.9 a | 137.1 ± 0.9 b | 136.5 ± 1.4 b | 0.01 | 136–145 |
| Crea (mg/dL) | 0.8 ± 0.1 | 1.2 ± 0.3 | 1.1 ± 0.2 | NS | 0.7–1.2 |
| Pprot (g/dL) | 6.9 ± 0.3 | 7.2 ± 0.2 | 6.7 ± 0.1 | NS | 6.4–8.3 |
| Alb (g/dL) | 5.1 ± 1.0 | 3.6 ± 0.1 | 3.5 ± 0.2 | NS | 3.5–5.2 |
| Tbil (mg/dL) | 1.1 ± 0.3 | 1.8 ± 0.3 | 2.7 ± 0.7 | 0.077 | 0.05–1.2 |
| DirBil (mg/dL) | 2.0 ± 0.6 | 1.3 ± 0.3 | 2.1 ± 0.8 | NS | <0.3 |
| AFP (U/L) | 4.1 ± 0.7 | 55.0 ± 51.6 | 3.5 ± 0.9 | NS | 20–130 |
| Cholin (U/L) | 5451.0 ± 1010.0 | 3738.0 ± 466.0 | 2519.2 ± 853.0 | 0.061 | 8000–18000 |
| Chol (mg/dL) | 165.2 ± 12.1 | 154.1 ± 8.9 | 122.3 ± 19.0 | NS | <190 |
| TG (mg/dL) | 131.3 ± 20.3 | 95.2 ± 7.1 | 98.0 ± 22.6 | NS | <150 |
| HDLC (mg/dL) | 62.6 ± 7.7 a | 49.3 ± 4.9 ab | 35.6 ± 5.1 b | 0.027 | >46 |
| LDLC (mg/dL) | 75.5 ± 15.5 | 104.0 ± 11.2 | 76.6 ± 17.9 | NS | <70 |
| GOT-AST (U/L) | 60.8 ± 7.3 | 47.4 ± 7.6 | 58.2 ± 9.7 | NS | 5–40 |
| GPT-ALT (U/L) | 70.1 ± 12.7 | 39.6 ± 8.8 | 38.7 ± 8.5 | 0.067 | 5–41 |
| ALP (U/L) | 171.1 ± 26.4 | 168.3 ± 18.6 | 212.9 ± 37.3 | NS | 40–130 |
| GGT (U/L) | 215.6 ± 44.5 a | 99.9 ± 15.8 b | 63.6 ± 16.7 b | 0.001 | 10–71 |
| Trans (mg/L) | 299.8 ± 46.6 a | 226.4 ± 18.4 ab | 181.3 ± 28.9 b | 0.053 | 200–360 |
| Ferr (ng/mL) | 113.8 ± 55.4 | 293.6 ± 82.8 | 252.6 ± 93.9 | NS | 30–400 |
| Parameters\Child | Child A | Child B | Child C | p-Value | Normal Rank |
|---|---|---|---|---|---|
| RBC (106/µL) | 4.4 ± 0.2 a | 3.8 ± 0.1 b | 3.4 ± 0.2 b | 0.001 | 4.5–5.9 |
| Hb (g/dL) | 12.6 ± 0.7 a | 11.5 ± 0.4 ab | 9.9 ± 0.4 b | 0.004 | 13.5–17.5 |
| Hto (%) | 37.2 ± 2.2 a | 33.7 ± 1.1 ab | 29.9 ± 1.2 b | 0.009 | 41–53 |
| MCV (fL) | 86.3 ± 3.9 | 87.3 ± 3.6 | 90.2 ± 2.3 | NS | 80–100 |
| MCH (pg/cell) | 33.0 ± 2.9 | 30.9 ± 0.7 | 30.4 ± 1.1 | NS | 26–34 |
| MHCH (g/dL) | 33.1 ± 0.6 | 34.1 ± 0.3 | 33.2 ± 0.3 | NS | 31–36 |
| RDW (%) | 15.9 ± 1.1 | 15.2 ± 0.4 | 16.6 ± 0.6 | NS | 11.5–14.5 |
| PLT (103/µL) | 101.4 ± 14.6 | 101.0 ± 10.4 | 115.8 ± 13.4 | NS | 150–350 |
| MPV (fL) | 11.7 ± 0.3 | 10.9 ± 0.2 | 10.9 ± 0.4 | 0.086 | 6.4–11.0 |
| PDW (%) | 15.9 ± 0.8 a | 13.1 ± 0.5 ab | 14.3 ± 0.9 b | 0.017 | 9–17 |
| WBC (103/µL) | 5.7 ± 0.5 | 5.1 ± 0.5 | 5.0 ± 0.5 | NS | 4.5–11.0 |
| NEU (103/µL) | 3.6 ± 0.5 | 3.3 ± 0.5 | 3.2 ± 0.4 | NS | 1.8–7.7 |
| LYMP (103/µL) | 1.4 ± 0.2 | 1.1 ± 0.1 | 1.0 ± 0.1 | NS | 1–4 |
| MONO (103/µL) | 0.5 ± 0.0 | 0.5 ± 0.1 | 0.5 ± 0.1 | NS | 0.0–0.8 |
| EOSI (103/µL) | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | NS | 0.0–0.5 |
| BASO (103/µL) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | NS | 0.0–0.2 |
| FIBRI (mg/dL) | 383.5 ± 24.3 | 294.5 ± 30.3 | 338.1 ± 35.5 | NS | 276–471 |
| PTT (s) | 12.9 ± 0.3 | 17.5 ± 1.6 | 17.6 ± 0.8 | 0.069 | 9.4–12.5 |
| APTT (s) | 83.9 ± 2.9 a | 65.5 ± 4.2 b | 61.9 ± 4.6 b | 0.020 | 25.1–36.5 |
| INR | 1.2 ± 0.0 | 1.5 ± 0.1 | 1.6 ± 0.2 | 0.073 | 0.9–1.2 |
| TTP (s) | 34.4 ± 1.3 | 38.5 ± 1.8 | 37.6 ± 1.4 | NS | 20–40 |
| APTTR (s) | 1.2 ± 0.0 | 1.4 ± 0.1 | 1.2 ± 0.0 | 0.081 | <1.3 |
| Parameter | Encephalopathy | p-Value | |
|---|---|---|---|
| NO | YES | ||
| RBC (106/µL) | 4.07 ± 0.16 | 3.57 ± 0.11 | 0.014 |
| Hb (mg/dL) | 12.15 ± 0.43 | 10.74 ± 0.43 | 0.03 |
| Hto (%) | 35.95 ± 1.26 | 31.9 ± 1.12 | 0.028 |
| PLT (103/µL) | 115.85 ± 9.36 | 85.11 ± 9.4 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Alarcón, L.; Martínez-Nicolás, A.; Jover-Aguilar, M.; López-López, V.; Alconchel-Gago, F.; Ríos, A.; Madrid, J.A.; de los Ángeles Rol, M.; Ramírez, P.; Ramis, G. Relationship between Circadian System Status, Child–Pugh Score, and Clinical Outcome in Cirrhotic Patients on Waiting Lists for Liver Transplantation. J. Clin. Med. 2024, 13, 4529. https://doi.org/10.3390/jcm13154529
Martínez-Alarcón L, Martínez-Nicolás A, Jover-Aguilar M, López-López V, Alconchel-Gago F, Ríos A, Madrid JA, de los Ángeles Rol M, Ramírez P, Ramis G. Relationship between Circadian System Status, Child–Pugh Score, and Clinical Outcome in Cirrhotic Patients on Waiting Lists for Liver Transplantation. Journal of Clinical Medicine. 2024; 13(15):4529. https://doi.org/10.3390/jcm13154529
Chicago/Turabian StyleMartínez-Alarcón, Laura, Antonio Martínez-Nicolás, Marta Jover-Aguilar, Víctor López-López, Felipe Alconchel-Gago, Antonio Ríos, Juan Antonio Madrid, María de los Ángeles Rol, Pablo Ramírez, and Guillermo Ramis. 2024. "Relationship between Circadian System Status, Child–Pugh Score, and Clinical Outcome in Cirrhotic Patients on Waiting Lists for Liver Transplantation" Journal of Clinical Medicine 13, no. 15: 4529. https://doi.org/10.3390/jcm13154529






