CSF Neopterin Levels Are Elevated in Various Neurological Diseases and Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurement of Neopterin
2.3. Statistical Analysis
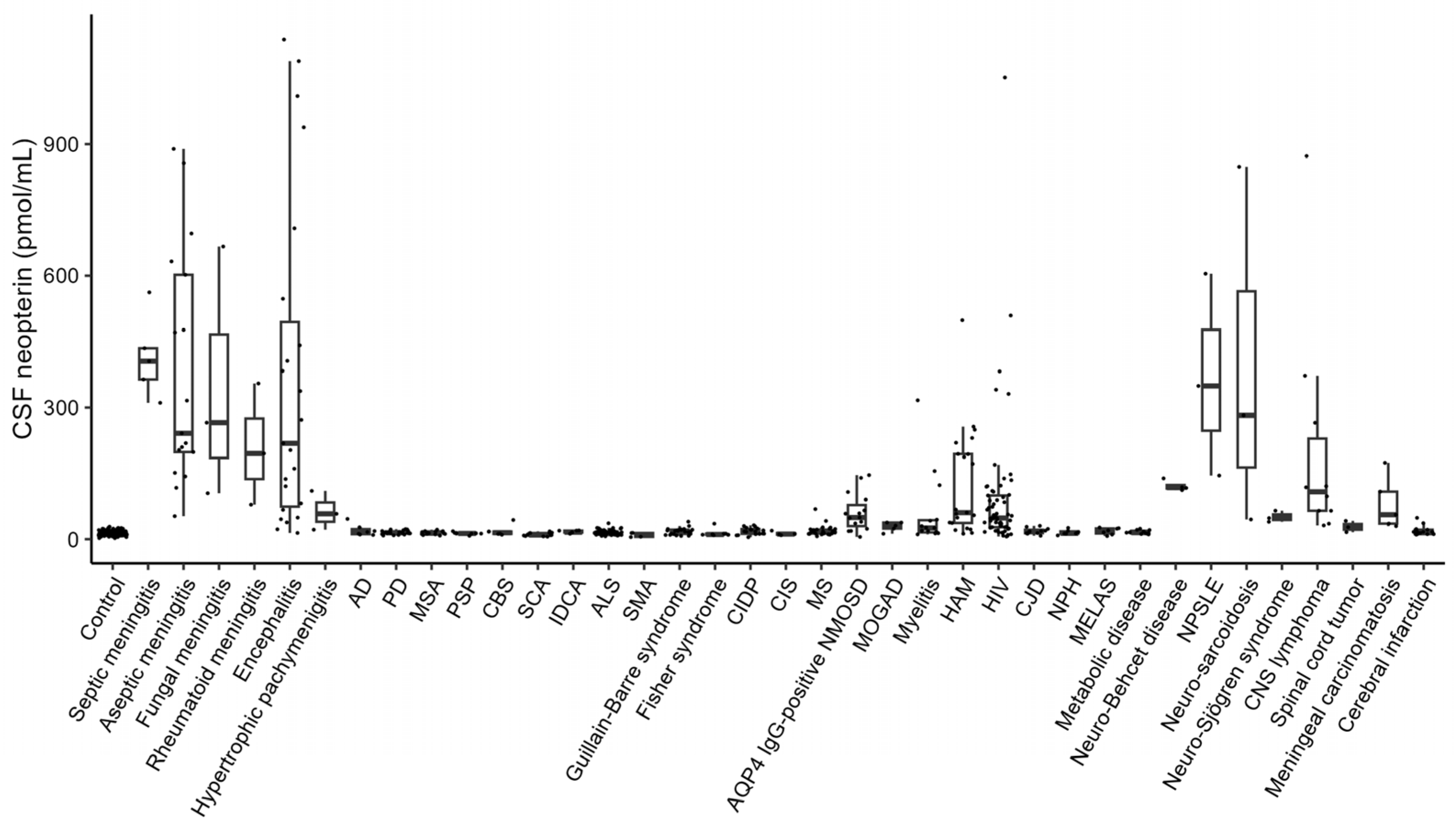
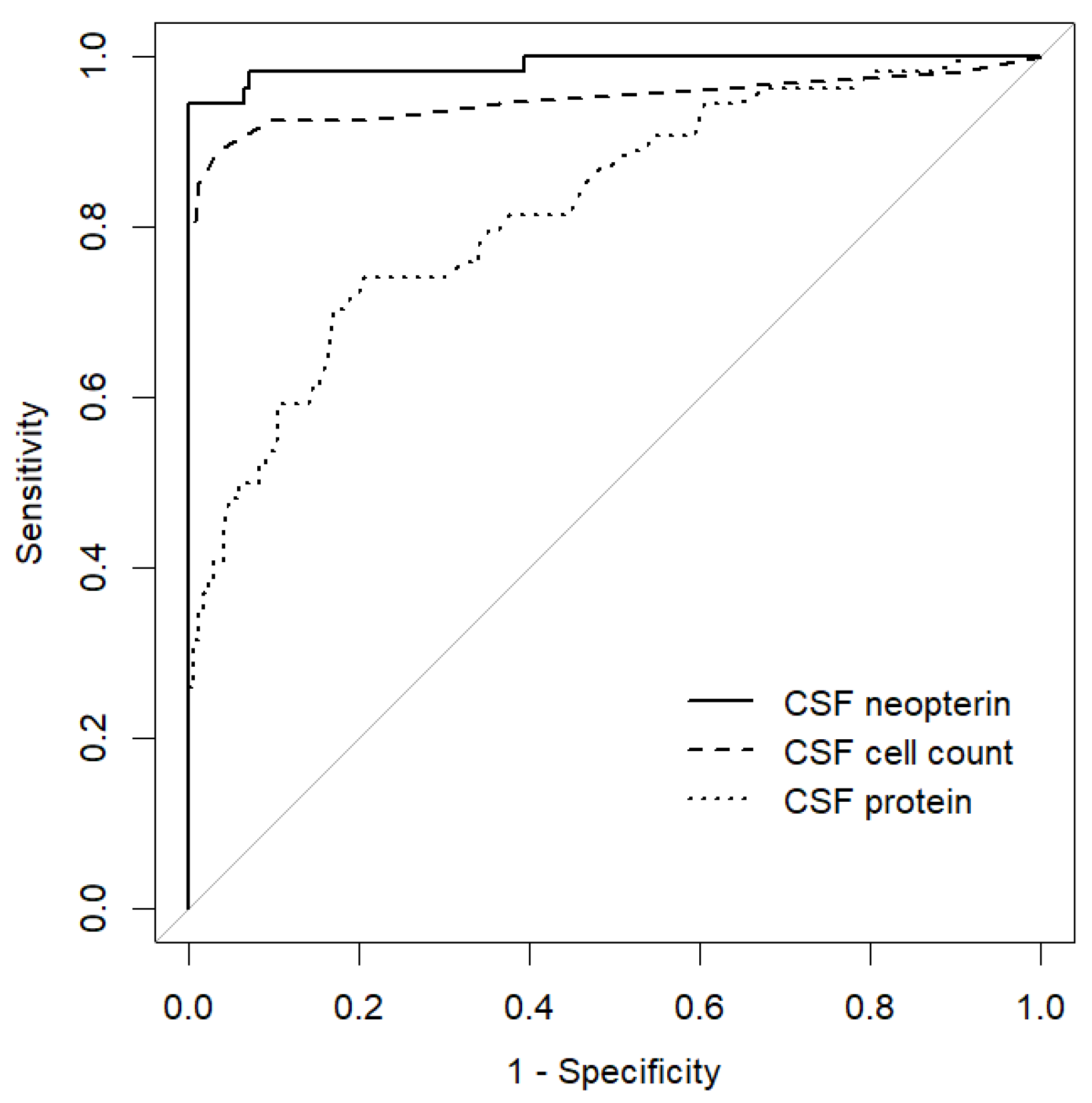
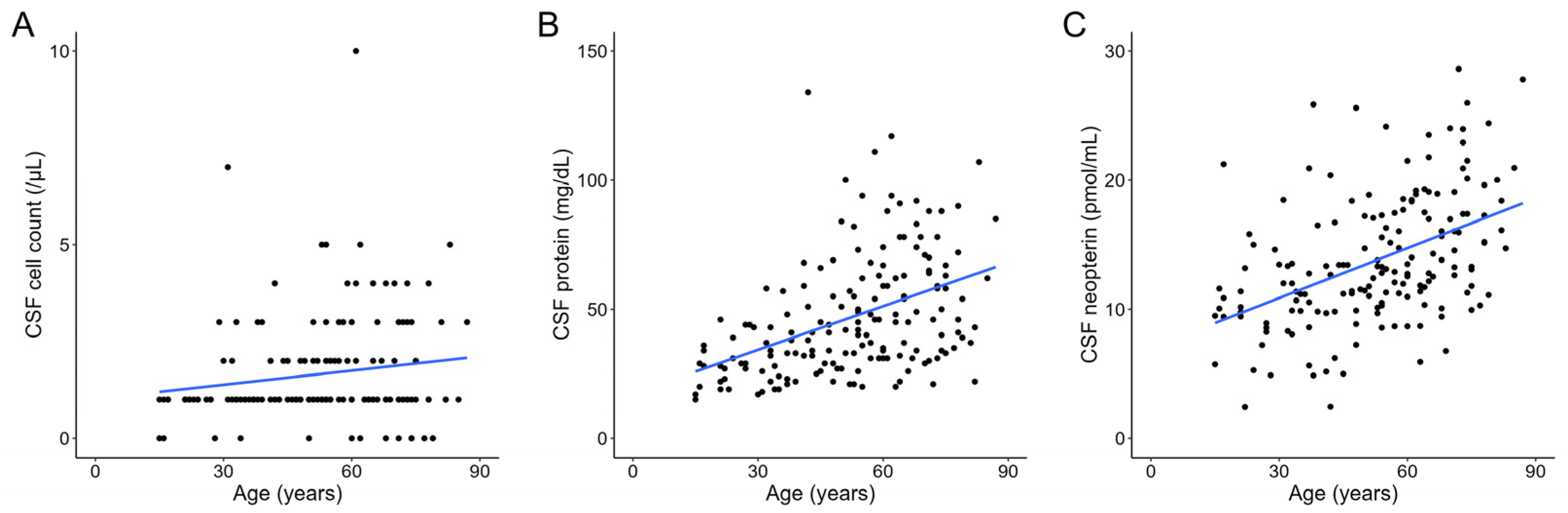
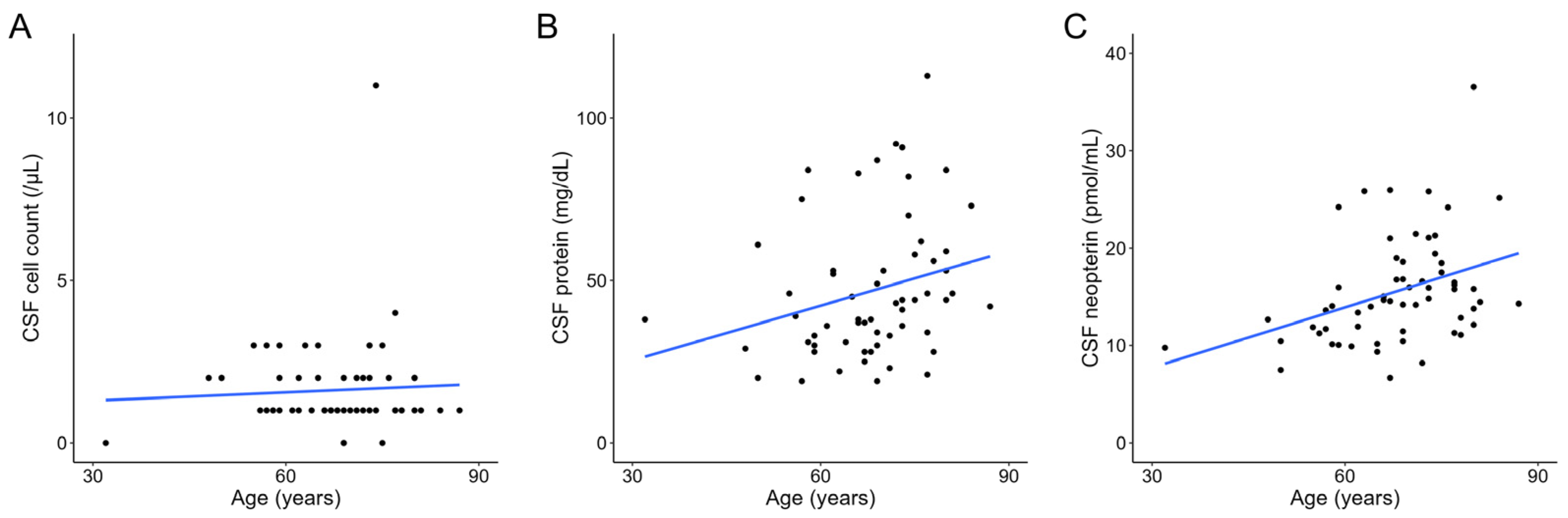
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millner, M.M.; Franthal, W.; Thalhammer, G.H.; Berghold, A.; Aigner, R.M.; Füger, G.F.; Reibnegger, G. Neopterin concentrations in cerebrospinal fluid and serum as an aid in differentiating central nervous system and peripheral infections in children. Clin. Chem. 1998, 44, 161–167. [Google Scholar] [CrossRef]
- Di Stefano, A.; Alcantarini, C.; Atzori, C.; Lipani, F.; Imperiale, D.; Burdino, E.; Audagnotto, S.; Mighetto, L.; Milia, M.G.; Di Perri, G.; et al. Cerebrospinal fluid biomarkers in patients with central nervous system infections: A retrospective study. CNS Spectr. 2020, 25, 402–408. [Google Scholar] [CrossRef]
- Fominykh, V.; Brylev, L.; Gaskin, V.; Luzin, R.; Yakovlev, A.; Komoltsev, I.; Belousova, I.; Rosliakova, A.; Guekht, A.; Gulyaeva, N. Neuronal damage and neuroinflammation markers in patients with autoimmune encephalitis and multiple sclerosis. Metab. Brain Dis. 2019, 34, 1473–1485. [Google Scholar] [CrossRef]
- Sato, T.; Coler-Reilly, A.; Utsunomiya, A.; Araya, N.; Yagishita, N.; Ando, H.; Yamauchi, J.; Inoue, E.; Ueno, T.; Hasegawa, Y.; et al. CSF CXCL10, CXCL9, and neopterin as candidate prognostic biomarkers for HTLV-1-associated myelopathy/tropical spastic paraparesis. PLoS Negl. Trop. Dis. 2013, 7, e2479. [Google Scholar] [CrossRef]
- Geng, M.; Xiao, H.; Liu, J.; Song, Y.; Fu, P.; Cheng, X.; Zhang, J.; Wang, G. The diagnostic role and dynamic changes in cerebrospinal fluid neopterin during treatment of patients with primary central nervous system lymphoma. Cancer Med. 2018, 7, 3889–3898. [Google Scholar] [CrossRef]
- Miyaue, N.; Yabe, H.; Yamanishi, Y.; Tada, S.; Ando, R.; Nagai, M. Three cases of rheumatoid meningitis with elevated CSF neopterin levels. J. Neuroimmunol. 2020, 348, 577396. [Google Scholar] [CrossRef]
- Miyaue, N.; Hosokawa, Y.; Yamanishi, Y.; Tada, S.; Ando, R.; Nagai, M. Clinical use of CSF neopterin levels in CNS demyelinating diseases. J. Neurol. Sci. 2022, 441, 120385. [Google Scholar] [CrossRef]
- Molero-Luis, M.; Fernández-Ureña, S.; Jordán, I.; Serrano, M.; Ormazábal, A.; Garcia-Cazorla, À.; Artuch, R.; Neopterin Working Group. Cerebrospinal fluid neopterin analysis in neuropediatric patients: Establishment of a new cut off-value for the identification of inflammatory-immune mediated processes. PLoS ONE 2013, 8, e83237. [Google Scholar] [CrossRef]
- Molero-Luis, M.; Casas-Alba, D.; Orellana, G.; Ormazabal, A.; Sierra, C.; Oliva, C.; Valls, A.; Velasco, J.; Launes, C.; Cuadras, D.; et al. Cerebrospinal fluid neopterin as a biomarker of neuroinflammatory diseases. Sci. Rep. 2020, 10, 18291. [Google Scholar] [CrossRef]
- de Paula Martins, R.; Ghisoni, K.; Lim, C.K.; Aguiar, A.S., Jr.; Guillemin, G.J.; Latini, A. Neopterin preconditioning prevents inflammasome activation in mammalian astrocytes. Free Radic. Biol. Med. 2018, 115, 371–382. [Google Scholar] [CrossRef]
- Fautsch, K.J.; Block, D.R.; Graff-Radford, J.; Wang, F.; Craver, E.C.; Hodge, D.O.; Cutsforth-Gregory, J.K.; Kilgore, K.P.; Petersen, R.C.; Knopman, D.S.; et al. Population-based evaluation of total protein in cerebrospinal fluid. Mayo Clin. Proc. 2023, 98, 239–251. [Google Scholar] [CrossRef]
- Guest, J.; Grant, R.; Mori, T.A.; Croft, K.D. Changes in oxidative damage, inflammation and [NAD(H)] with age in cerebrospinal fluid. PLoS ONE 2014, 9, e85335. [Google Scholar] [CrossRef]
- Viaccoz, A.; Ducray, F.; Tholance, Y.; Barcelos, G.K.; Thomas-Maisonneuve, L.; Ghesquières, H.; Meyronet, D.; Quadrio, I.; Cartalat-Carel, S.; Louis-Tisserand, G.; et al. CSF neopterin level as a diagnostic marker in primary central nervous system lymphoma. Neuro Oncol. 2015, 17, 1497–1503. [Google Scholar] [CrossRef]
- Hagberg, L.; Cinque, P.; Gisslen, M.; Brew, B.J.; Spudich, S.; Bestetti, A.; Price, R.W.; Fuchs, D. Cerebrospinal fluid neopterin: An informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res. Ther. 2010, 7, 15. [Google Scholar] [CrossRef]
- Furukawa, Y.; Nishi, K.; Kondo, T.; Tanabe, K.; Mizuno, Y. Significance of CSF total neopterin and biopterin in inflammatory neurological diseases. J. Neurol. Sci. 1992, 111, 65–72. [Google Scholar] [CrossRef]
- Labouret, M.; Costi, S.; Bondet, V.; Trebossen, V.; Le Roux, E.; Ntorkou, A.; Bartoli, S.; Auvin, S.; Bader-Meunier, B.; Baudouin, V.; et al. Juvenile neuropsychiatric systemic lupus erythematosus: Identification of novel central neuroinflammation biomarkers. J. Clin. Immunol. 2023, 43, 615–624. [Google Scholar] [CrossRef]
- Sfriso, P.; Ostuni, P.; Botsios, C.; Andretta, M.; Oliviero, F.; Punzi, L.; Todesco, S. Serum and salivary neopterin and interferon-gamma in primary Sjögren’s syndrome. Correlation with clinical, laboratory and histopathologic features. Scand. J. Rheumatol. 2003, 32, 74–78. [Google Scholar] [CrossRef]
- Ong, L.T.C.; Galambos, G.; Brown, D.A. Primary Sjogren’s syndrome associated with treatment-resistant obsessive-compulsive disorder. Front. Psychiatry 2017, 8, 124. [Google Scholar] [CrossRef]
- Dale, R.C.; Brilot, F.; Fagan, E.; Earl, J. Cerebrospinal fluid neopterin in paediatric neurology: A marker of active central nervous system inflammation. Dev. Med. Child. Neurol. 2009, 51, 317–323. [Google Scholar] [CrossRef]
- Boyd, R.J.; Avramopoulos, D.; Jantzie, L.L.; McCallion, A.S. Neuroinflammation represents a common theme amongst genetic and environmental risk factors for Alzheimer and Parkinson diseases. J. Neuroinflamm. 2022, 19, 223. [Google Scholar] [CrossRef]
- Hu, W.T.; Howell, J.C.; Ozturk, T.; Gangishetti, U.; Kollhoff, A.L.; Hatcher-Martin, J.M.; Anderson, A.M.; Tyor, W.R. CSF cytokines in aging, multiple sclerosis, and dementia. Front. Immunol. 2019, 10, 480. [Google Scholar] [CrossRef]
- Piehl, N.; van Olst, L.; Ramakrishnan, A.; Teregulova, V.; Simonton, B.; Zhang, Z.; Tapp, E.; Channappa, D.; Oh, H.; Losada, P.M.; et al. Cerebrospinal fluid immune dysregulation during healthy brain aging and cognitive impairment. Cell 2022, 185, 5028–5039.e13. [Google Scholar] [CrossRef]
- de Bie, J.; Guest, J.; Guillemin, G.J.; Grant, R. Central kynurenine pathway shift with age in women. J. Neurochem. 2016, 136, 995–1003. [Google Scholar] [CrossRef]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a marker for immune system activation. Curr. Drug Metab. 2002, 3, 175–187. [Google Scholar] [CrossRef]
- Yoshida, Y.; Une, F.; Utatsu, Y.; Nomoto, M.; Furukawa, Y.; Maruyama, Y.; Machigashira, N.; Matsuzaki, T.; Osame, M. Adenosine and neopterin levels in cerebrospinal fluid of patients with neurological disorders. Intern. Med. 1999, 38, 133–139. [Google Scholar] [CrossRef]
- Hall, R.J.; Watne, L.O.; Idland, A.V.; Raeder, J.; Frihagen, F.; MacLullich, A.M.J.; Staff, A.C.; Wyller, T.B.; Fekkes, D. Cerebrospinal fluid levels of neopterin are elevated in delirium after hip fracture. J. Neuroinflamm. 2016, 13, 170. [Google Scholar] [CrossRef]
- Shepheard, S.R.; Karnaros, V.; Benyamin, B.; Schultz, D.W.; Dubowsky, M.; Wuu, J.; Chataway, T.; Malaspina, A.; Benatar, M.; Rogers, M.L. Urinary neopterin: A novel biomarker of disease progression in amyotrophic lateral sclerosis. Eur. J. Neurol. 2022, 29, 990–999. [Google Scholar] [CrossRef]
- Leonardi, A.; Abbruzzese, G.; Arata, L.; Cocito, L.; Vische, M. Cerebrospinal fluid (CSF) findings in amyotrophic lateral sclerosis. J. Neurol. 1984, 231, 75–78. [Google Scholar] [CrossRef]
- Czaplinski, A.; Yen, A.A.; Appel, S.H. Forced vital capacity (FVC) as an indicator of survival and disease progression in an ALS Clinic population. J. Neurol. Neurosurg. Psychiatry 2006, 77, 390–392. [Google Scholar] [CrossRef]
| Parameter | Control | Meningitis/Encephalitis | p |
|---|---|---|---|
| n | 170 | 54 | |
| Age, years | 52.56 ± 17.99 | 52.19 ± 21.13 | 0.899 |
| Men (%) | 99 (58.2) | 29 (53.7) | 0.668 |
| CSF cell count, /µL | 1.66 ± 1.37 | 420.85 ± 1925.55 | 0.005 |
| CSF protein, mg/dL | 46.99 ± 23.14 | 136.22 ± 143.59 | <0.001 |
| CSF neopterin, pmol/mL | 13.77 ± 5.08 | 349.04 ± 297.51 | <0.001 |
| Parameter | Estimate | Standard Error | z Value | p |
|---|---|---|---|---|
| (Intercept) | −2.255 | 0.575 | −3.921 | <0.001 |
| Age | 0.016 | 0.011 | 1.549 | 0.121 |
| CSF protein | 0.039 | 0.011 | 3.741 | <0.001 |
| Parameter | Estimate | Standard Error | z Value | p |
|---|---|---|---|---|
| (Intercept) | 5.070 | 0.902 | 5.623 | <0.001 |
| Age | −0.081 | 0.014 | −5.666 | <0.001 |
| CSF protein | 0.019 | 0.008 | 2.348 | 0.019 |
| Parameter | Bulbar Dysfunction − | Bulbar Dysfunction + | p |
|---|---|---|---|
| n | 28 | 33 | |
| Age, years | 65.96 ± 11.51 | 70.54 ± 7.79 | 0.070 |
| Men (%) | 17 (60.7) | 16 (48.5) | 0.486 |
| Disease duration, months | 21.07 ± 16.76 | 13.89 ± 11.06 | 0.050 |
| %FVC, % (n = 56) | 104.24 ± 20.49 | 73.54 ± 24.09 | <0.001 |
| CSF cell count, /µL | 1.89 ± 2.04 | 1.39 ± 0.79 | 0.200 |
| CSF protein, mg/dL | 46.61 ± 20.87 | 46.79 ± 22.20 | 0.974 |
| CSF neopterin, pmol/mL | 14.49 ± 6.73 | 16.44 ± 4.01 | 0.167 |
| Parameter | Estimate | Standard Error | t Value | p |
|---|---|---|---|---|
| (Intercept) | 4.676 | 6.380 | 0.733 | 0.467 |
| Age | 0.202 | 0.077 | 2.623 | 0.012 |
| Male sex | −0.596 | 1.442 | −0.414 | 0.681 |
| Disease duration | −0.045 | 0.054 | −0.827 | 0.412 |
| %FVC | −0.022 | 0.029 | −0.747 | 0.459 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyaue, N.; Yamanishi, Y.; Ito, Y.; Ando, R.; Nagai, M. CSF Neopterin Levels Are Elevated in Various Neurological Diseases and Aging. J. Clin. Med. 2024, 13, 4542. https://doi.org/10.3390/jcm13154542
Miyaue N, Yamanishi Y, Ito Y, Ando R, Nagai M. CSF Neopterin Levels Are Elevated in Various Neurological Diseases and Aging. Journal of Clinical Medicine. 2024; 13(15):4542. https://doi.org/10.3390/jcm13154542
Chicago/Turabian StyleMiyaue, Noriyuki, Yuki Yamanishi, Yuko Ito, Rino Ando, and Masahiro Nagai. 2024. "CSF Neopterin Levels Are Elevated in Various Neurological Diseases and Aging" Journal of Clinical Medicine 13, no. 15: 4542. https://doi.org/10.3390/jcm13154542





