Precision Medicine in Acute Coronary Syndromes
Abstract
1. Introduction
2. Precision Medicine in MI-CAD
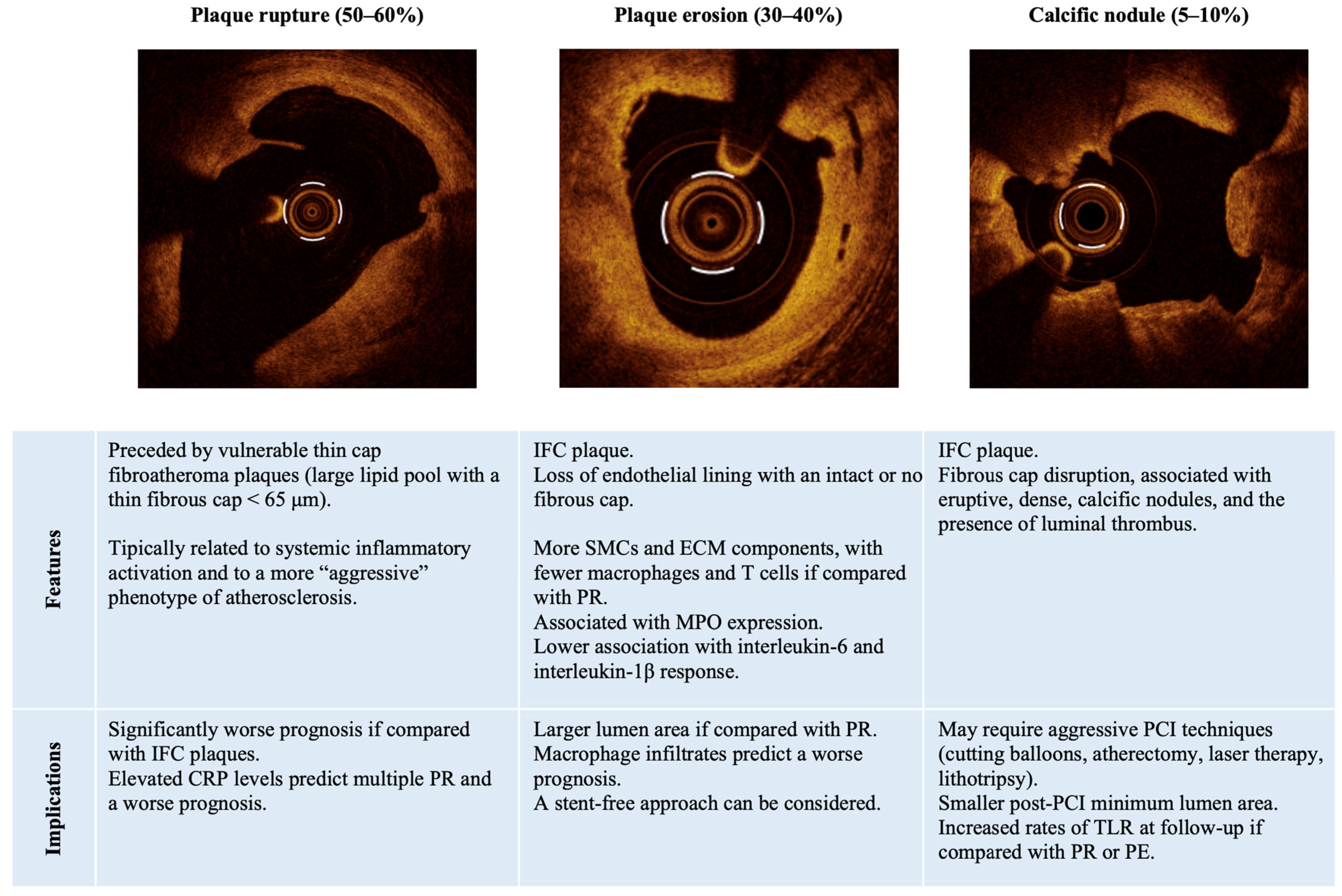
2.1. Culprit Plaque Assessment
| Study | Population | Design | Results/Objectives |
|---|---|---|---|
| PE and stent free management | |||
| EROSION (Jia et al., 2016) [37] | 60 ACS patients (58 STEMI, 2 NSTE-ACS) with an OCT diagnosis of PE and <70% residual angiographic DS after thrombus aspiration, TIMI flow grade 3, and no progressive chest pain. | Single-arm, uncontrolled, prospective study investigating the feasibility of DAPT with ticagrelor without stenting in patients with ACS due to PE. | At 1-month follow-up: thrombus volume significantly decreased (94.2%) in the 55 patients who completed their 1-month follow-up. 78.3% had >50% reduction of thrombus volume. 2 patients experienced MACE. |
| EROSION 1-Year Follow-Up (Xing et al., 2017) [22] | 53 ACS patients (51 STEMI, 2 NSTE-ACS) with an OCT diagnosis of PE and <70% residual angiographic DS after thrombus aspiration, TIMI flow grade 3, and no progressive chest pain. | Single-arm, uncontrolled, prospective study investigating the feasibility of DAPT with ticagrelor without stenting in patients with ACS due to PE. | At 1-year follow-up: 92.5% of patients remained free of MACE for up to 1 year. Thrombus volume further decreased between 1 month and 1 year. |
| EROSION 4-Year Outcomes (He et al., 2021) [38] | 52 ACS patients (50 STEMI, 2 NSTE-ACS) with an OCT diagnosis of PE and <70% residual angiographic DS after thrombus aspiration, TIMI flow grade 3, and no progressive chest pain. | Single-arm, uncontrolled, prospective study investigating the feasibility of DAPT with ticagrelor without stenting in patients with ACS due to PE. | At 4-year follow-up: 21% of cumulative rate of TLR (not associated with ACS). More effective thrombus dissolution during the first month predicted better long-term follow-up in terms of TLR. |
| EROSION III (Jia et al., 2022) [23] | 226 STEMI patients with <70% residual angiographic DS after thrombus aspiration, and TIMI flow grade 3. | Open-label, prospective, multicenter, randomized, controlled study of OCT vs. angiographic guidance in STEMI. | Significantly lower rate of stent implantation in the OCT guidance guided group, compared to the angiographic guided group (43.8% vs. 58.8%; p = 0.024). |
| DANAMI-3-DEFER trial post hoc analysis (Madsen et al., 2022) [24] | 674 STEMI patient from the DANAMI-3-DEFER study (84 randomized to deferred stenting treated with no subsequent stenting; 590 randomized to standard PCI treated with immediate stenting). | Post hoc analysis comparing patients with TIMI flow grade 2–3 after initial PCI and no significant residual stenosis in whom stenting was omitted vs. patients undergoing standard PCI and immediate stenting. | Comparable event rate between stent-free group and standard PCI group (composite of all-cause mortality, recurrent MI, and TVR) (HR 0.87, 95% CI: 0.48–1.60; p = 0.66). |
| PEPSii (NCT04701385) (Wardley et al., ongoing) [32] | 80 NSTEMI patients, 40 stable CAD patients (control group). | Prospective observational pilot study to evaluate the feasibility of studying the differences in endothelial cells and neutrophils between NSTEMI patients presenting with PE or PR as assessed by OCT. | Primary outcome measure: apoptotic circulating endothelial cells. Secondary outcome measures: neutrophils, endothelial progenitor cells, biomarker analysis. |
| MVO assessment | |||
| OxAMI cohort (Fahrni et al., 2017) [39] | 261 patients with STEMI undergoing pPCI. | Prospective study evaluating IMR at the time of pPCI can identify patients at low risk of early major cardiac complications after STEMI. | IMR ≤ 40 identified all patients who were free of major cardiac complications. |
| OxAMI PICSO study (De Maria et al., 2018) [40] | 105 patients with STEMI undergoing pPCI. | Prospective study evaluating PICSO effectiveness in reducing infarct size and IMR in STEMI patients with pre-stenting IMR > 40 units. | Compared to controls, the 25 patients treated with PICSO had a lower IMR at 24–48 h (24.8 [18.5–35.9] vs. 45.0 [32.0–51.3], p < 0.001) and lower infarct size at six months (26.0% [20.2–30.0] vs. 33.0% [28.0–37.0], p = 0.006). |
| PiCSO-AMI-I Trial (De Maria et al., 2024) [41] | 145 patients with STEMI undergoing pPCI. | Prospective, randomized trial evaluating PICSO effectiveness in reducing infarct size, MVO occurrence, and intramyocardial hemorrhage in STEMI patients. | No differences were observed in infarct size, nor in terms of the occurrence of MVO or intramyocardial hemorrhage. |
| Guided antiplatelet therapy de-escalation | |||
| ANTARCTIC (Cayla et al., 2016) [42] | 877 patients 75 years or older who had undergone coronary stent implantation for ACS. | Multicenter, open-label, blinded-endpoint, randomized controlled superiority trial of prasugrel 5 mg qd with dose or drug adjustment in case of inadequate response assessed by PFT (monitoring group) vs. prasugrel 5 mg qd with no monitoring (conventional group). | PFT did not improve clinical outcomes (composite endpoint of CV death, MI, stroke, stent thrombosis, urgent revascularisation) in the monitoring group, compared with the conventional group (HR 1.003, 95% CI 0.78–1.29; p = 0.98) The rate of bleeding events did not significantly differ between groups. |
| TROPICAL-ACS (Sibbing et al., 2017) [43] | 2610 ACS patients treated with PCI with a planned DAPT duration of 12 months. | Randomized, open-label, assessor-blinded, multicenter trial comparing prasugrel for 12 months (control group) with a PFT-guided step-down regimen to clopidogrel maintenance therapy after 1 week of prasugrel (guided de-escalation group). | At 1 year after PCI, guided de-escalation of antiplatelet treatment was non-inferior to standard treatment with prasugrel in terms of CV death, MI, or stroke (pnon-inferiority = 0.0004; HR 0.81, 95% CI 0.62–1.06, psuperiority = 0.12). No significant difference in bleeding events was observed (HR 0.82, 95% CI 0.59–1.13; p = 0.23). |
| POPular Genetics (Claassens et al., 2019) [44] | 2488 patients undergoing primary PCI with stenting implantation. | Randomized, open-label, assessor-blinded trial comparing the administration of a P2Y12 inhibitor based on early CYP2C19 genetic testing (genotype-guided group) with a standard treatment with either ticagrelor or prasugrel (standard-treatment group) for 12 months. | CYP2C19 genotype-guided strategy (with clopidogrel being assigned to noncarriers of CYP2C19*2 or CYP2C19*3 loss-of-function) was associated with a lower risk of bleeding (HR 0.78, 95% CI 0.61–0.98; p = 0.04) and was non-inferior to standard treatment with ticagrelor or prasugrel at 12 months in terms of death from any cause, MI, definite stent thrombosis, or stroke (absolute difference, −0.7%; 95% CI, −2.0 to 0.7; pnon-inferiority < 0.001). |
| Guided antiplatelet therapy escalation | |||
| PHARMCLO (Notarangelo et al., 2018) [45] | 888 ACS patients (prematurely stopped: 24.6% of the pre-specified sample size). | Randomization to standard of care or pharmacogenomic approach (including genotyping of ABCB1, CYP2C19*2, and CYP2C19*17). | Significantly lower risk of primary composite endpoint of CV death, MI, stroke, and major bleeding in the pharmacogenomic arm (HR 0.58; 95% CI 0.43–0.78; p < 0.001). Ticagrelor use was significantly higher in the pharmacogenomic arm (42.6% vs. 32.7%; p = 0.02). |
| Al-Rubaish et al. (2019) [46] | 755 STEMI patients. | Randomization to a genotype-guided approach or to standard treatment. | 31 patients carrying a loss-of-function allele in the genotype-guided arm were treated with ticagrelor. All other patients received clopidogrel. Significantly lower risk of CV death, MI, stroke, and major bleeding (OR 0.34; 95% CI 0.20–0.59; p = 0.0001). Non-significant difference in stent thrombosis (OR 0.85; 95% CI 0.43–1.71; p = 0.65). |
| TAILOR-PCI (Pereira et al., 2020) [47] | 5302 patients undergoing PCI for ACS or stable CAD. | Open-label, randomization to a genotype-guided group (undergoing point-of-care genotyping, CYP2C19 LOF carriers being prescribed ticagrelor, while noncarriers clopidogrel) or to a conventional group (receiving clopidogrel). | At 12 months, no significant difference was observed in a composite endpoint of CV death, MI, stroke, stent thrombosis, and severe recurrent ischemia (HR 0.84; 95% CI 0.65–1.07; p = 0.16), nor minor or major bleeding (HR 1.22; 95% CI 0.60–2.51; p = 0.58). |
| MINOCA | |||
| PROMISE (NCT05122780) (Montone et al., ongoing) [48] | 145 MINOCA patients. | Randomized, multicenter, prospective, open-label, superiority trial comparing a “precision medicine approach” versus “standard of care approach” in MINOCA patients. | Primary endpoint: angina status evaluated by SAQSS at 12-month follow-up. Secondary endpoints and exploratory analysis: MACE, healthcare cost analysis, CMR characteristics, circulating biomarkers, diagnostic utility of the “precision medicine approach”. |
| StratMed-MINOCA (NCT05198791) (Berry et al., ongoing) [49] | 300 MINOCA patients. | Prospective, randomized, open-label, endpoint blinded trial comparing eplerone 25–50 mg vs. standard of care in patients with IMR ≥ 25 without heart failure or LVEF ≤ 40%. | Change in NT-proBNP levels at six months. Prospective measurement of coronary physiology parameters including CFR, RRR, and LVEDP. |
| Inflammation | |||
| CANTOS Trial (Ridker et al., 2017) [50] | 10,061 patients with previous MI and a hs-CRP level of <2 mg/L. | Double-blinded, randomized to canakinumab (50 mg, 150 mg, and 300 mg s.c. every 3 months) or placebo. | Canakinumab, at a dose of 150 mg every three months, resulted in a significantly lower rate of recurrent cardiovascular events than placebo (HR 0.83; 95% CI 0.73–0.95; p = 0.005). |
| MRC-ILA Heart Study (Morton et al., 2015) [51] | 182 patients with NSTE-ACS within 48 h from symptoms onset. | Double-blinded, randomized to daily IL-1ra s.c. or placebo for 14 days. | Significant reduction of hs-CRP and IL-6 at 14 days in the IL-1ra group. Significant MACE excess at 1 year in the IL-1ra group, driven by a non-significant increase in recurrent MI. |
| VCUART3 (Abbate et al., 2020) [52] | 99 patients with STEMI. | Randomized, double-blind, clinical trial evaluating IL-1ra vs. placebo. | Significant reduction of hs-CRP and improvement of heart failure outcomes in the IL1-ra group. |
| ASSAIL-MI (Broch et al., 2021) [53] | 199 patients with STEMI within 6 h from symptoms onset. | Randomized, double-blind trial evaluating tocilizumab vs. placebo. | Increased myocardial salvage, reduced MVO. No significant difference in infarct size. |
| LILACS (Zhao et al., 2022) [54] | 26 patients with stable CAD. 18 patients with NSTE-ACS. | Randomized, double-blind, dose-escalation trial, testing low-dose s.c. aldesleukin (recombinant IL-2) vs. placebo. | Recombinant low-dose IL-2 expandend regulatory T cells. One serious adverse event after drug administration. |
| IVORY (NCT04241601) (Sriranjan et al., ongoing) [55] | 60 patients with ACS and hs-CRP > 2 mg/L. | Double-blind, randomized, phase II clinical trial testing low-dose s.c. aldesleukin vs. placebo. | Change in mean maximum target to background ratios (TBRmax) in the index vessel as assessed by 18F-FDG PET-CT at follow-up. Changes in circulating T-cell subtypes and safety endpoints. |
| COLCOT (Tardif et al., 2019) [56] | 4746 patients with recent MI (within 30 days) | Randomized, double-blinded trial of colchicine vs. placebo. | Significant reduction in the combined endpoint of death from CV causes, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina leading to coronary revascularization (HR 0.77; 95% CI 0.61–0.96; p = 0.02). Significantly higher pneumonia incidence in the treated arm (p = 0.03). |
| CLEAR-SYNERGY (NCT03048825) (Sanjit et al., ongoing) [57] | Patients with MI who have undergone PCI. >7000 enrolled patients (primary completion in June 2024). | Randomized, blinded, double-dummy, 2 × 2 factorial design trial of colchicine 0.5 mg vs. placebo and spironolactone 25 mg vs. placebo. | MACE at follow-up. |
| FLAVOUR (Prescott et al., 2022) [58] | 128 MI patients with <50% left anterior descending coronary artery stenosis and TIMI flow grade ≥ 2 after PCI. | Parallel-group trial with 2:1:2 randomization to receive once-daily 5-lipoxygenase-activating protein inhibitor AZD5718 200 mg, AZD5718 50 mg or placebo. | Urine leukotriene E4 levels were significantly reduced (>80%) in the treated arms. No significant changes in non-invasive CFR. No treatment-related serious adverse events. |
2.2. Approach to Non-Culprit Lesions
2.3. Microvascular Obstruction Assessment
2.4. Antithrombotic Therapy
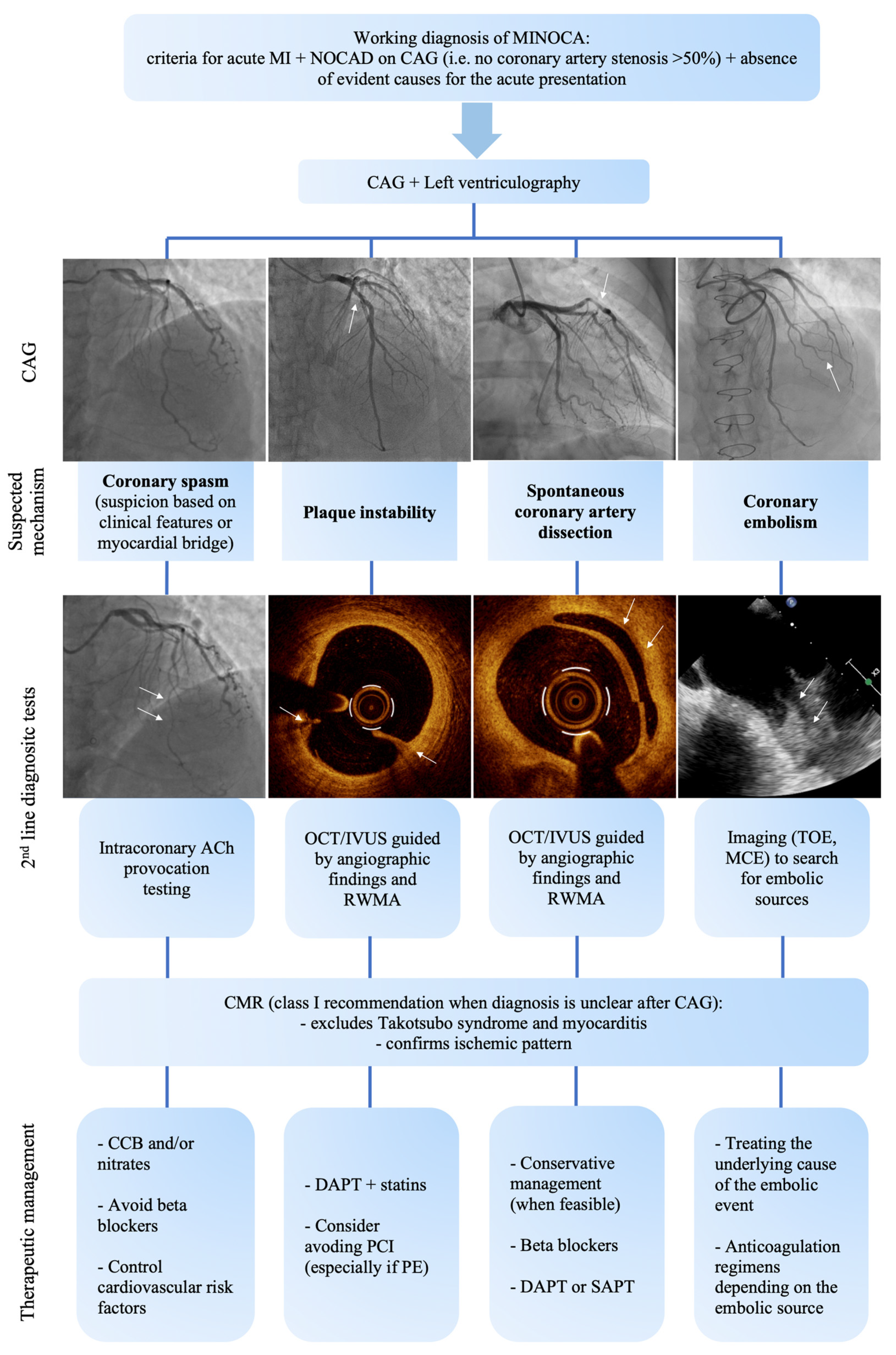
3. Precision Medicine in MINOCA
3.1. Diagnostic Process
3.2. Tailored Therapy in MINOCA
3.3. Future Perspectives in MINOCA-Tailored Therapy
4. Tailored Prevention Approaches
4.1. Inflammatory Markers and Anti-Inflammatory Therapies
4.2. Beyond Traditional Risk Factors: Considering the Role of the Exposome
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Bueno, H. Epidemiology of acute coronary syndromes. In ESC CardioMed; James, S., Ed.; Oxford University Press: Oxford, UK, 2018; pp. 1213–1218. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Lüderitz, B. Augustus Desiré Waller (1856–1922)—The first to record the electrical activity of the human heart. J. Interv. Card. Electrophysiol. 2003, 9, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.P.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Montone, R.A.; Iannaccone, G.; Meucci, M.C.; Rinaldi, R.; D’amario, D.; Niccoli, G. Diagnostic work-up and therapeutic implications in MINOCA: Need for a personalized approach. Future Cardiol. 2021, 17, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.L.; Longo, D.L. Precision medicine—Personalized, problematic, and promising. N. Engl. J. Med. 2015, 372, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Ford, T.J.; Galli, M.; Rinaldi, R.; Bland, A.; Morrow, A.; Angiolillo, D.J.; Berry, C.; Kaski, J.C.; Crea, F. Stratified medicine for acute and chronic coronary syndromes: A patient-tailored approach. Prog. Cardiovasc. Dis. 2024, 25, S0033-0620. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Libby, P. Acute Coronary Syndromes: The Way Forward From Mechanisms to Precision Treatment. Circulation 2017, 136, 1155–1166. [Google Scholar] [CrossRef]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef]
- Johnson, T.W.; Räber, L.; di Mario, C.; Bourantas, C.; Jia, H.; Mattesini, A.; Gonzalo, N.; Hernandez, J.M.d.l.T.; Prati, F.; Koskinas, K.; et al. Clinical use of intracoronary imaging. Part 2: Acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2019, 40, 2566–2584. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Harrington, R.A. Management of Antithrombotic Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2021, 384, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Mintz, G.S.; Matsumura, M.; Ali, Z.; Maehara, A. Clinical Utility of Intravascular Imaging: Past, Present, and Future. JACC Cardiovasc. Imaging 2022, 15, 1799–1820. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.P.; Farb, A.; Malcom, G.T.; Liang, Y.H.; Smialek, J.; Virmani, R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N. Engl. J. Med. 1997, 336, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Dauerman, H.; Toma, C.; Samady, H.; Itoh, T.; Kuramitsu, S.; Domei, T.; Jia, H.; Vergallo, R.; Soeda, T.; et al. Prevalence and characteristics of TCFA and degree of coronary artery stenosis: An OCT, IVUS, and angiographic study. J. Am. Coll. Cardiol. 2014, 64, 672–680. [Google Scholar] [CrossRef]
- Niccoli, G.; Montone, R.A.; Di Vito, L.; Gramegna, M.; Refaat, H.; Scalone, G.; Leone, A.M.; Trani, C.; Burzotta, F.; Porto, I.; et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur. Heart J. 2015, 36, 1377–1384. [Google Scholar] [CrossRef]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Yonetsu, T.; Ye, J.C.; Libby, P.; Fuster, V.; Jang, I.K. Optical Coherence Tomography of Plaque Erosion: JACC Focus Seminar Part 2/3. J. Am. Coll. Cardiol. 2021, 78, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Lu, J.; Zhang, S.; Wang, J.; Wang, Y.; Li, L.; Wang, Y.; Jiang, S.; Yin, Y.; Guo, J.; et al. Morphological Characteristics of Eroded Plaques with Noncritical Coronary Stenosis: An Optical Coherence Tomography Study. J. Atheroscler. Thromb. 2022, 29, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Yonetsu, T.; Usui, E.; Kanaji, Y.; Ohya, H.; Sumino, Y.; Yamaguchi, M.; Hada, M.; Hamaya, R.; Kanno, Y.; et al. Clinical Significance of the Presence or Absence of Lipid-Rich Plaque Underneath Intact Fibrous Cap Plaque in Acute Coronary Syndrome. J. Am. Heart Assoc. 2019, 8, e011820. [Google Scholar] [CrossRef]
- Xing, L.; Yamamoto, E.; Sugiyama, T.; Jia, H.; Ma, L.; Hu, S.; Wang, C.; Zhu, Y.; Li, L.; Xu, M.; et al. EROSION Study (Effective Anti-Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography-Based Management in Plaque Erosion): A 1-Year Follow-Up Report. Circ. Cardiovasc. Interv. 2017, 38, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Dai, J.; He, L.; Xu, Y.; Shi, Y.; Zhao, L.; Sun, Z.; Liu, Y.; Weng, Z.; Feng, X.; et al. EROSION III: A Multicenter RCT of OCT-Guided Reperfusion in STEMI with Early Infarct Artery Patency. JACC Cardiovasc. Interv. 2022, 15, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.M.; Kelbæk, H.; Nepper-Christensen, L.; Jacobsen, M.J.; Ahtarovski, K.A.; Høfsten, D.H.; Holmvang, L.; Pedersen, F.; Tilsted, H.-H.; Aarøe, J.; et al. Clinical outcomes of no stenting in patients with ST-segment elevation myocardial infarction undergoing deferred primary percutaneous coronary intervention. EuroIntervention 2022, 18, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Finn, A.V.; Virmani, R. Calcified nodule: A rare but important cause of acute coronary syndrome with worse clinical outcomes. Atherosclerosis 2021, 318, 40–42. [Google Scholar] [CrossRef]
- Lee, T.; Mintz, G.S.; Matsumura, M.; Zhang, W.; Cao, Y.; Usui, E.; Kanaji, Y.; Murai, T.; Yonetsu, T.; Kakuta, T.; et al. Prevalence, Predictors, and Clinical Presentation of a Calcified Nodule as Assessed by Optical Coherence Tomography. JACC Cardiovasc. Imaging 2017, 10, 883–891. [Google Scholar] [CrossRef]
- Kobayashi, N.; Takano, M.; Tsurumi, M.; Shibata, Y.; Nishigoori, S.; Uchiyama, S.; Okazaki, H.; Shirakabe, A.; Seino, Y.; Hata, N.; et al. Features and Outcomes of Patients with Calcified Nodules at Culprit Lesions of Acute Coronary Syndrome: An Optical Coherence Tomography Study. Cardiology 2018, 139, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Montone, R.A.; Cataneo, L.; Cosentino, N.; Gramegna, M.; Refaat, H.; Porto, I.; Burzotta, F.; Trani, C.; Leone, A.M.; et al. Morphological-biohumoral correlations in acute coronary syndromes: Pathogenetic implications. Int. J. Cardiol. 2014, 171, 463–466. [Google Scholar] [CrossRef]
- Liuzzo, G.; Montone, R.A.; Gabriele, M.; Pedicino, D.; Giglio, A.F.; Trotta, F.; Galiffa, V.A.; Previtero, M.; Severino, A.; Biasucci, L.M.; et al. Identification of unique adaptive immune system signature in acute coronary syndromes. Int. J. Cardiol. 2013, 168, 564–567. [Google Scholar] [CrossRef]
- Liuzzo, G.; Biasucci, L.M.; Gallimore, J.R.; Grillo, R.L.; Rebuzzi, A.G.; Pepys, M.B.; Maseri, A. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N. Engl. J. Med. 1994, 331, 417–424. [Google Scholar] [CrossRef]
- Tanaka, A.; Shimada, K.; Sano, T.; Namba, M.; Sakamoto, T.; Nishida, Y.; Kawarabayashi, T.; Fukuda, D.; Yoshikawa, J. Multiple plaque rupture and C-reactive protein in acute myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 1594–1599. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Plaque Erosion Prospective Study ii (PEPSii). NCT04701385. Available online: https://clinicaltrials.gov/study/NCT04701385 (accessed on 20 April 2024).
- Ferrante, G.; Nakano, M.; Prati, F.; Niccoli, G.; Mallus, M.T.; Ramazzotti, V.; Montone, R.A.; Kolodgie, F.D.; Virmani, R.; Crea, F. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: A clinicopathological study. Circulation 2010, 122, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, T.; Seppelt, C.; Abdelwahed, Y.S.; Meteva, D.; Wolfram, C.; Stapmanns, P.; Erbay, A.; Zanders, L.; Nelles, G.; Musfeld, J.; et al. Culprit plaque morphology determines inflammatory risk and clinical outcomes in acute coronary syndrome. Eur. Heart J. 2023, 44, 3911–3925. [Google Scholar] [CrossRef]
- Montone, R.A.; Vetrugno, V.; Camilli, M.; Russo, M.; Fracassi, F.; Khan, S.Q.; Doshi, S.N.; Townend, J.N.; Ludman, P.F.; Trani, C.; et al. Macrophage infiltrates in coronary plaque erosion and cardiovascular outcome in patients with acute coronary syndrome. Atherosclerosis 2020, 311, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Watkins, J.; Abdul-Aziz, A.; Shafat, M.; Calvert, P.A.; Bowles, K.M.; Flather, M.D.; Rushworth, S.A.; Ryding, A.D. Inflammatory Differences in Plaque Erosion and Rupture in Patients with ST-Segment Elevation Myocardial Infarction. J. Am. Heart Assoc. 2017, 6, e005868. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Dai, J.; Hou, J.; Xing, L.; Ma, L.; Liu, H.; Xu, M.; Yao, Y.; Hu, S.; Yamamoto, E.; et al. Effective anti-thrombotic therapy without stenting: Intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur. Heart J. 2017, 38, 792–800. [Google Scholar] [CrossRef]
- He, L.; Qin, Y.; Xu, Y.; Hu, S.; Wang, Y.; Zeng, M.; Feng, X.; Liu, Q.; Syed, I.; Demuyakor, A.; et al. Predictors of non-stenting strategy for acute coronary syndrome caused by plaque erosion: Four-year outcomes of the EROSION study. EuroIntervention 2021, 17, 497–505. [Google Scholar] [CrossRef]
- Fahrni, G.; Wolfrum, M.; De Maria, G.L.; Cuculi, F.; Dawkins, S.; Alkhalil, M.; Patel, N.; Forfar, J.C.; Prendergast, B.D.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance at the Time of Primary Percutaneous Coronary Intervention Predicts Early Cardiac Complications: Insights from the OxAMI (Oxford Study in Acute Myocardial Infarction) Cohort. J. Am. Heart Assoc. 2017, 6, e005409. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Alkhalil, M.; Borlotti, A.; Wolfrum, M.; Gaughran, L.; Dall’armellina, E.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.K.; et al. Index of microcirculatory resistance-guided therapy with pressure-controlled intermittent coronary sinus occlusion improves coronary microvascular function and reduces infarct size in patients with ST-elevation myocardial infarction: The Oxford Acute Myocardial Infarction—Pressure-controlled Intermittent Coronary Sinus Occlusion study (OxAMI-PICSO study). EuroIntervention 2018, 14, e352–e359. [Google Scholar] [CrossRef]
- De Maria, G.L.; Greenwood, J.P.; Zaman, A.G.; Carrié, D.; Coste, P.; Valgimigli, M.; Behan, M.; Berry, C.; Erglis, A.; Panoulas, V.F.; et al. Pressure-Controlled Intermittent Coronary Sinus Occlusion (PiCSO) in Acute Myocardial Infarction: The PiCSO-AMI-I Trial. Circ. Cardiovasc. Interv. 2024, 17, e013675. [Google Scholar] [CrossRef]
- Cayla, G.; Cuisset, T.; Silvain, J.; Leclercq, F.; Manzo-Silberman, S.; Saint-Etienne, C.; Delarche, N.; Bellemain-Appaix, A.; Range, G.; El Mahmoud, R.; et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): An open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016, 388, 2015–2022. [Google Scholar] [CrossRef]
- Sibbing, D.; Aradi, D.; Jacobshagen, C.; Gross, L.; Trenk, D.; Geisler, T.; Orban, M.; Hadamitzky, M.; Merkely, B.; Kiss, R.G.; et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): A randomised, open-label, multicentre trial. Lancet 2017, 390, 1747–1757. [Google Scholar] [CrossRef]
- Claassens, D.M.F.; Vos, G.J.A.; Bergmeijer, T.O.; Hermanides, R.S.; van ’t Hof, A.W.J.; Van Der Harst, P.; Barbato, E.; Morisco, C.; Gin, R.M.T.J.; Asselbergs, F.W.; et al. A Genotype-Guided Strategy for Oral P2Y12 Inhibitors in Primary PCI. N. Engl. J. Med. 2019, 381, 1621–1631. [Google Scholar] [CrossRef]
- Notarangelo, F.M.; Maglietta, G.; Bevilacqua, P.; Cereda, M.; Merlini, P.A.; Villani, G.Q.; Moruzzi, P.; Patrizi, G.; Tagliazucchi, G.M.; Crocamo, A.; et al. Pharmacogenomic Approach to Selecting Antiplatelet Therapy in Patients with Acute Coronary Syndromes: The PHARMCLO Trial. J. Am. Coll. Cardiol. 2018, 71, 1869–1877. [Google Scholar] [CrossRef]
- Al-Rubaish, A.M.; Al-Muhanna, F.A.; Alshehri, A.M.; Al-Mansori, M.A.; Alali, R.A.; Khalil, R.M.; Al-Faraidy, K.A.; Cyrus, C.; Sulieman, M.M.; Vatte, C.; et al. Bedside testing of CYP2C19 vs. conventional clopidogrel treatment to guide antiplatelet therapy in ST-segment elevation myocardial infarction patients. Int. J. Cardiol. 2021, 343, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.L.; Farkouh, M.E.; So, D.; Lennon, R.; Geller, N.; Mathew, V.; Bell, M.; Bae, J.-H.; Jeong, M.H.; Chavez, I.; et al. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes after Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA 2020, 324, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Cosentino, N.; Graziani, F.; Gorla, R.; Del Buono, M.G.; La Vecchia, G.; Rinaldi, R.; Marenzi, G.; Bartorelli, A.L.; De Marco, F.; et al. Precision medicine versus standard of care for patients with myocardial infarction with non-obstructive coronary arteries (MINOCA): Rationale and design of the multicentre, randomised PROMISE trial. EuroIntervention 2022, 18, e933–e939. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Stratified Medicine of Eplerenone in Acute MI/Injury (StratMed-MINOCA). NCT05198791. Available online: https://clinicaltrials.gov/study/NCT05198791 (accessed on 14 April 2024).
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Morton, A.C.; Rothman, A.M.K.; Greenwood, J.P.; Gunn, J.; Chase, A.; Clarke, B.; Hall, A.S.; Fox, K.; Foley, C.; Banya, W.; et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: The MRC-ILA Heart Study. Eur. Heart J. 2015, 36, 377–384. [Google Scholar] [CrossRef]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients with ST-Segment–Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef]
- Broch, K.; Anstensrud, A.K.; Woxholt, S.; Sharma, K.; Tøllefsen, I.M.; Bendz, B.; Aakhus, S.; Ueland, T.; Amundsen, B.H.; Damås, J.K.; et al. Randomized Trial of Interleukin-6 Receptor Inhibition in Patients with Acute ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1845–1855. [Google Scholar] [CrossRef]
- Zhao, T.X.; Sriranjan, R.S.; Tuong, Z.K.; Lu, Y.; Sage, A.P.; Nus, M.; Hubsch, A.; Kaloyirou, F.; Vamvaka, E.; Helmy, J.; et al. Regulatory T-Cell Response to Low-Dose Interleukin-2 in Ischemic Heart Disease. NEJM Evid. 2022, 1, EVIDoa2100009. [Google Scholar] [CrossRef]
- Sriranjan, R.; Zhao, T.X.; Tarkin, J.; Hubsch, A.; Helmy, J.; Vamvaka, E.; Jalaludeen, N.; Bond, S.; Hoole, S.P.; Knott, P.; et al. Low-dose interleukin 2 for the reduction of vascular inflammation in acute coronary syndromes (IVORY): Protocol and study rationale for a randomised, double-blind, placebo-controlled, phase II clinical trial. BMJ Open 2022, 12, e062602. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Colchicine and Spironolactone in Patients with MI/SYNERGY Stent Registry (CLEAR SYNERGY). NCT03048825. Available online: https://clinicaltrials.gov/study/NCT03048825 (accessed on 10 May 2024).
- Prescott, E.; Angerås, O.; Erlinge, D.; Grove, E.L.; Hedman, M.; Jensen, L.O.; Pernow, J.; Saraste, A.; Åkerblom, A.; Svedlund, S.; et al. Safety and efficacy of the 5-lipoxygenase-activating protein inhibitor AZD5718 in patients with recent myocardial infarction: The phase 2a FLAVOUR study. Int. J. Cardiol. 2022, 365, 34–40. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Demetriou, D.; Grines, C.L.; Pica, M.; Shoukfeh, M.; O’Neill, W.W. Multiple complex coronary plaques in patients with acute myocardial infarction. N. Engl. J. Med. 2000, 343, 915–922. [Google Scholar] [CrossRef]
- Montone, R.A.; Niccoli, G.; Crea, F.; Jang, I.K. Management of non-culprit coronary plaques in patients with acute coronary syndrome. Eur. Heart J. 2020, 41, 3579–3586. [Google Scholar] [CrossRef]
- Mehta, S.R.; Wood, D.A.; Storey, R.F.; Mehran, R.; Bainey, K.R.; Nguyen, H.; Meeks, B.; Di Pasquale, G.; López-Sendón, J.; Faxon, D.P.; et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N. Engl. J. Med. 2019, 381, 1411–1421. [Google Scholar] [CrossRef]
- Pinilla-Echeverri, N.; Mehta, S.R.; Wang, J.; Lavi, S.; Schampaert, E.; Cantor, W.J.; Bainey, K.R.; Welsh, R.C.; Kassam, S.; Mehran, R.; et al. Nonculprit Lesion Plaque Morphology in Patients with ST-Segment-Elevation Myocardial Infarction: Results from the COMPLETE Trial Optical Coherence Tomography Substudys. Circ. Cardiovasc. Interv. 2020, 13, e008768. [Google Scholar] [CrossRef]
- Erlinge, D.; Maehara, A.; Ben-Yehuda, O.; Bøtker, H.E.; Maeng, M.; Kjøller-Hansen, L.; Engstrøm, T.; Matsumura, M.; Crowley, A.; Dressler, O.; et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): A prospective natural history study. Lancet 2021, 397, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahn, J.M.; Kang, D.Y.; Yun, S.-C.; Ahn, Y.-K.; Kim, W.-J.; Nam, C.-W.; Jeong, J.-O.; Chae, I.-H.; Shiomi, H.; et al. Preventive percutaneous coronary intervention versus optimal medical therapy alone for the treatment of vulnerable atherosclerotic coronary plaques (PREVENT): A multicentre, open-label, randomised controlled trial. Lancet 2024, 403, 1753–1765. [Google Scholar] [CrossRef]
- Bujak, K.; Rinaldi, R.; Vidal-Cales, P.; Montone, R.A.; Diletti, R.; Gąsior, M.; Crea, F.; Sabaté, M.; Brugaletta, S. Immediate versus staged complete revascularization in acute coronary syndrome: A meta-analysis of randomized controlled trials. Int. J. Cardiol. 2023, 393, 131397. [Google Scholar] [CrossRef]
- Diletti, R.; den Dekker, W.K.; Bennett, J.; Schotborgh, C.E.; van der Schaaf, R.; Sabaté, M.; Moreno, R.; Ameloot, K.; van Bommel, R.; Forlani, D.; et al. Immediate versus staged complete revascularisation in patients presenting with acute coronary syndrome and multivessel coronary disease (BIOVASC): A prospective, open-label, non-inferiority, randomised trial. Lancet 2023, 401, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Niccoli, G.; De Maria, G.; Brugaletta, S.; Montone, R.A.; Vergallo, R.; Benenati, S.; Magniani, G.; D’amario, D.; Porto, I.; et al. Coronary microvascular obstruction and dysfunction in patients with acute myocardial infarction. Nat. Rev. Cardiol. 2024, 21, 283–298. [Google Scholar] [CrossRef] [PubMed]
- de Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef]
- Patel, N.; Petraco, R.; Dall’Armellina, E.; Kassimis, G.; De Maria, G.L.; Dawkins, S.; Lee, R.; Prendergast, B.D.; Choudhury, R.P.; Forfar, J.C.; et al. Zero-Flow Pressure Measured Immediately After Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction Provides the Best Invasive Index for Predicting the Extent of Myocardial Infarction at 6 Months. JACC Cardiovasc. Interv. 2015, 8, 1410–1421. [Google Scholar] [CrossRef]
- Capodanno, D.; Angiolillo, D.J. Timing, Selection, Modulation, and Duration of P2Y12 Inhibitors for Patients with Acute Coronary Syndromes Undergoing PCI. JACC Cardiovasc. Interv. 2023, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; van Klaveren, D.; James, S.; Heg, D.; Räber, L.; Feres, F.; Pilgrim, T.; Hong, M.-K.; Kim, H.-S.; Colombo, A.; et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017, 389, 1025–1034. [Google Scholar] [CrossRef]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation 2019, 140, 240–261. [Google Scholar] [CrossRef]
- Hwang, D.; Lim, Y.H.; Park, K.W.; Chun, K.J.; Han, J.-K.; Yang, H.-M.; Kang, H.-J.; Koo, B.-K.; Kang, J.; Cho, Y.-K.; et al. Prasugrel Dose De-escalation Therapy after Complex Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome: A Post Hoc Analysis From the HOST-REDUCE-POLYTECH-ACS Trial. JAMA Cardiol. 2022, 7, 418–426. [Google Scholar] [CrossRef]
- Kim, C.J.; Park, M.W.; Kim, M.C.; Choo, E.-H.; Hwang, B.-H.; Lee, K.Y.; Choi, Y.S.; Kim, H.-Y.; Yoo, K.-D.; Jeon, D.-S.; et al. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): An investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet 2021, 398, 1305–1316. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.; Wiviott, S.D. Clopidogrel response variability and future therapies: Clopidogrel: Does one size fit all? Circulation 2006, 114, e600–e606. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Aradi, D.; Alexopoulos, D.; Berg, J.T.; Bhatt, D.L.; Bonello, L.; Collet, J.-P.; Cuisset, T.; Franchi, F.; Gross, L.; et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Tamis-Holland, J.E.; Jneid, H.; Reynolds, H.R.; Agewall, S.; Brilakis, E.S.; Brown, T.M.; Lerman, A.; Cushman, M.; Kumbhani, D.J.; Arslanian-Engoren, C.; et al. Contemporary Diagnosis and Management of Patients with Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e891–e908. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF). Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Montone, R.A.; Rinaldi, R. Pathophysiology of Coronary Microvascular Dysfunction. Circ. J. 2022, 86, 1319–1328. [Google Scholar] [CrossRef]
- Montone, R.A.; Niccoli, G.; Fracassi, F.; Russo, M.; Gurgoglione, F.; Cammà, G.; Lanza, G.A.; Crea, F. Patients with acute myocardial infarction and non-obstructive coronary arteries: Safety and prognostic relevance of invasive coronary provocative tests. Eur. Heart J. 2018, 39, 91–98. [Google Scholar] [CrossRef]
- Takahashi, J.; Onuma, S.; Hao, K.; Godo, S.; Shiroto, T.; Yasuda, S. Pathophysiology and diagnostic pathway of myocardial infarction with non-obstructive coronary arteries. J. Cardiol. 2024, 83, 17–24. [Google Scholar] [CrossRef]
- Gue, Y.X.; Kanji, R.; Gati, S.; Gorog, D.A. MI with Non-obstructive Coronary Artery Presenting with STEMI: A Review of Incidence, Aetiology, Assessment and Treatment. Eur. Cardiol. 2020, 15, e20. [Google Scholar] [CrossRef]
- Raphael, C.E.; Heit, J.A.; Reeder, G.S.; Bois, M.C.; Maleszewski, J.J.; Tilbury, R.T.; Holmes, D.R. Coronary Embolus: An Underappreciated Cause of Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2018, 11, 172–180. [Google Scholar] [CrossRef]
- Hayes, S.N.; Tweet, M.S.; Adlam, D.; Kim, E.S.; Gulati, R.; Price, J.E.; Rose, C.H. Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Jang, I.K.; Beltrame, J.F.; Sicari, R.; Meucci, M.C.; Bode, M.; Gaibazzi, N.; Niccoli, G.; Bucciarelli-Ducci, C.; Crea, F. The evolving role of cardiac imaging in patients with myocardial infarction and non-obstructive coronary arteries. Prog. Cardiovasc. Dis. 2021, 68, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Meucci, M.C.; De Vita, A.; Lanza, G.A.; Niccoli, G. Coronary provocative tests in the catheterization laboratory: Pathophysiological bases, methodological considerations and clinical implications. Atherosclerosis 2021, 318, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Rinaldi, R.; Del Buono, M.G.; Gurgoglione, F.; La Vecchia, G.; Russo, M.; Caffè, A.; Burzotta, F.; Leone, A.M.; Romagnoli, E.; et al. Safety and prognostic relevance of acetylcholine testing in patients with stable myocardial ischaemia or myocardial infarction and non-obstructive coronary arteries. EuroIntervention 2022, 18, e666–e676. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Gurgoglione, F.L.; Del Buono, M.G.; Rinaldi, R.; Meucci, M.C.; Iannaccone, G.; La Vecchia, G.; Camilli, M.; D’amario, D.; Leone, A.M.; et al. Interplay Between Myocardial Bridging and Coronary Spasm in Patients with Myocardial Ischemia and Non-Obstructive Coronary Arteries: Pathogenic and Prognostic Implications. J. Am. Heart Assoc. 2021, 10, e020535. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Colucci, M.; Torre, I.; Ausiello, D.; Bonanni, A.; Basile, M.; Salzillo, C.; Sanna, T.; Liuzzo, G.; Leone, A.M.; et al. Predicting the response to acetylcholine in ischemia or infarction with non-obstructive coronary arteries: The ABCD score. Atherosclerosis 2024, 391, 117503. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Nakou, E.; Del Buono, M.G.; Montone, R.A.; D’Amario, D.; Bucciarelli-Ducci, C. The Role of Cardiac Magnetic Resonance in Myocardial Infarction and Non-obstructive Coronary Arteries. Front. Cardiovasc. Med. 2021, 8, 821067. [Google Scholar] [CrossRef] [PubMed]
- Pristipino, C.; Sievert, H.; D’Ascenzo, F.; Mas, J.L.; Meier, B.; Scacciatella, P.; Hildick-Smith, D.; Gaita, F.; Toni, D.; Kyrle, P.; et al. European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eur. Heart J. 2019, 40, 3182–3195. [Google Scholar] [CrossRef] [PubMed]
- Senior, R.; Becher, H.; Monaghan, M.; Agati, L.; Zamorano, J.; Vanoverschelde, J.L.; Nihoyannopoulos, P.; Edvardsen, T.; Lancellotti, P.; Delgado, V.; et al. Clinical practice of contrast echocardiography: Recommendation by the European Association of Cardiovascular Imaging (EACVI) 2017. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1205–1205af. [Google Scholar] [CrossRef]
- Saric, M.; Armour, A.C.; Arnaout, M.S.; Chaudhry, F.A.; Grimm, R.A.; Kronzon, I.; Landeck, B.F.; Maganti, K.; Michelena, H.I.; Tolstrup, K. Guidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism. J. Am. Soc. Echocardiogr. 2016, 29, 1–42. [Google Scholar] [CrossRef]
- Corban, M.T.; Hung, O.Y.; Eshtehardi, P.; Rasoul-Arzrumly, E.; McDaniel, M.; Mekonnen, G.; Timmins, L.H.; Lutz, J.; Guyton, R.A.; Samady, H. Myocardial bridging: Contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J. Am. Coll. Cardiol. 2014, 63, 2346–2355. [Google Scholar] [CrossRef]
- Adlam, D.; Alfonso, F.; Maas, A.; Vrints, C.; Writing Committee. European Society of Cardiology, acute cardiovascular care association, SCAD study group: A position paper on spontaneous coronary artery dissection. Eur. Heart J. 2018, 39, 3353–3368. [Google Scholar] [CrossRef]
- Cerrato, E.; Giacobbe, F.; Quadri, G.; Macaya, F.; Bianco, M.; Mori, R.; Biolè, C.A.; Boi, A.; Bettari, L.; Rolfo, C.; et al. Antiplatelet therapy in patients with conservatively managed spontaneous coronary artery dissection from the multicentre DISCO registry. Eur. Heart J. 2021, 42, 3161–3171. [Google Scholar] [CrossRef]
- Niccoli, G.; Scalone, G.; Crea, F. Acute myocardial infarction with no obstructive coronary atherosclerosis: Mechanisms and management. Eur. Heart J. 2015, 36, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.; Baron, T.; Erlinge, D.; Hadziosmanovic, N.; Nordenskjöld, A.; Gard, A.; Jernberg, T. Medical Therapy for Secondary Prevention and Long-Term Outcome in Patients with Myocardial Infarction With Nonobstructive Coronary Artery Disease. Circulation 2017, 135, 1481–1489. [Google Scholar] [CrossRef]
- Abdu, F.A.; Liu, L.; Mohammed, A.Q.; Xu, B.; Yin, G.; Xu, S.; Xu, Y.; Che, W. Effect of Secondary Prevention Medication on the Prognosis in Patients with Myocardial Infarction with Nonobstructive Coronary Artery Disease. J. Cardiovasc. Pharmacol. 2020, 76, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Kovach, C.P.; Hebbe, A.; O’Donnell, C.I.; Plomondon, M.E.; Hess, P.L.; Rahman, A.; Mulukutla, S.; Waldo, S.W.; Valle, J.A. Comparison of Patients with Nonobstructive Coronary Artery Disease with versus without Myocardial Infarction (from the VA Clinical Assessment Reporting and Tracking [CART] Program). Am. J. Cardiol. 2021, 146, 1–7. [Google Scholar] [CrossRef]
- Lindahl, B.; Baron, T.; Albertucci, M.; Prati, F. Myocardial infarction with non-obstructive coronary artery disease. EuroIntervention 2021, 17, e875–e887. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.J.; Morrow, D.A.; Sabatine, M.S. Inflammatory biomarkers in acute coronary syndromes: Part II: Acute-phase reactants and biomarkers of endothelial cell activation. Circulation 2006, 113, e152–e155. [Google Scholar] [CrossRef][Green Version]
- Adam, C.A.; Șalaru, D.L.; Prisacariu, C.; Marcu, D.T.M.; Sascău, R.A.; Stătescu, C. Novel Biomarkers of Atherosclerotic Vascular Disease-Latest Insights in the Research Field. Int. J. Mol. Sci. 2022, 23, 4998. [Google Scholar] [CrossRef]
- Swastini, D.A.; Wiryanthini, I.A.D.; Ariastuti, N.L.P.; Muliantara, A. Atherosclerosis Prediction with High Sensitivity C-Reactive Protein (hs-CRP) and Related Risk Factor in Patient with Dyslipidemia. Open Access Maced. J. Med. Sci. 2019, 7, 3887–3890. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Pride, Y.B.; Hochberg, C.P.; Sloan, S.; Sabatine, M.S.; Cannon, C.P. Effect of intensive statin therapy on clinical outcomes among patients undergoing percutaneous coronary intervention for acute coronary syndrome. PCI-PROVE IT: A PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) Substudy. J. Am. Coll. Cardiol. 2009, 54, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed]
- Bohula, E.A.; Giugliano, R.P.; Cannon, C.P.; Zhou, J.; Murphy, S.A.; White, J.A.; Tershakovec, A.M.; Blazing, M.A.; Braunwald, E. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation 2015, 132, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Aday, A.W.; Rose, L.M.; Ridker, P.M. Residual Inflammatory Risk on Treatment with PCSK9 Inhibition and Statin Therapy. Circulation 2018, 138, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bohula, E.A.; Giugliano, R.P.; Leiter, L.A.; Verma, S.; Park, J.-G.; Sever, P.S.; Pineda, A.L.; Honarpour, N.; Wang, H.; Murphy, S.A.; et al. Inflammatory and Cholesterol Risk in the FOURIER Trial. Circulation 2018, 138, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, D.; Cappetta, D.; Cappannoli, L.; Princi, G.; Migliaro, S.; Diana, G.; Chouchane, K.; Borovac, J.A.; Restivo, A.; Arcudi, A.; et al. Colchicine in ischemic heart disease: The good, the bad and the ugly. Clin. Res. Cardiol. 2021, 110, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Camilli, M.; Calvieri, C.; Magnani, G.; Bonanni, A.; Bhatt, D.L.; Rajagopalan, S.; Crea, F.; Niccoli, G. Exposome in ischaemic heart disease: Beyond traditional risk factors. Eur. Heart J. 2024, 45, 419–438. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Landrigan, P.J. Pollution and the Heart. N. Engl. J. Med. 2021, 385, 1881–1892. [Google Scholar] [CrossRef]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Russo, M.; Bonanni, A.; Camilli, M.; Caffè, A.; Basile, M.; Salzillo, C.; Animati, F.M.; Trani, C.; Niccoli, G.; et al. Short-term air pollution exposure and mechanisms of plaque instability in acute coronary syndromes: An optical coherence tomography study. Atherosclerosis 2024, 390, 117393. [Google Scholar] [CrossRef]
- Montone, R.A.; Camilli, M.; Russo, M.; Termite, C.; La Vecchia, G.; Iannaccone, G.; Rinaldi, R.; Gurgoglione, F.; Del Buono, M.G.; Sanna, T.; et al. Air Pollution and Coronary Plaque Vulnerability and Instability: An Optical Coherence Tomography Study. JACC Cardiovasc. Imaging 2022, 15, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Camilli, M.; Russo, M.; Rinaldi, R.; Caffè, A.; La Vecchia, G.; Bonanni, A.; Iannaccone, G.; Basile, M.; Vergallo, R.; Aurigemma, C.; et al. Air Pollution and Coronary Vasomotor Disorders in Patients with Myocardial Ischemia and Unobstructed Coronary Arteries. J. Am. Coll. Cardiol. 2022, 80, 1818–1828. [Google Scholar] [CrossRef]
- Russo, M.; Rinaldi, R.; Camilli, M.; Bonanni, A.; Caffè, A.; Basile, M.; Salzillo, C.; Colucci, M.; Torre, I.; Sanna, T.; et al. Air pollution and plaque healing in acute coronary syndromes. Eur. Heart J. 2023, 44, 2403–2405. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Rinaldi, R.; Bonanni, A.; Severino, A.; Pedicino, D.; Crea, F.; Liuzzo, G. Impact of air pollution on ischemic heart disease: Evidence, mechanisms, clinical perspectives. Atherosclerosis 2023, 366, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tonne, C.; Wilkinson, P. Long-term exposure to air pollution is associated with survival following acute coronary syndrome. Eur. Heart J. 2013, 34, 1306–1311. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, A.; Chen, H.; Zhao, Z.; Cai, J.; Wang, C.; Yang, C.; Li, H.; Xu, X.; Ha, S.; et al. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: A randomized, double-blind crossover trial of air purifiers. J. Am. Coll. Cardiol. 2015, 65, 2279–2287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caffè, A.; Animati, F.M.; Iannaccone, G.; Rinaldi, R.; Montone, R.A. Precision Medicine in Acute Coronary Syndromes. J. Clin. Med. 2024, 13, 4569. https://doi.org/10.3390/jcm13154569
Caffè A, Animati FM, Iannaccone G, Rinaldi R, Montone RA. Precision Medicine in Acute Coronary Syndromes. Journal of Clinical Medicine. 2024; 13(15):4569. https://doi.org/10.3390/jcm13154569
Chicago/Turabian StyleCaffè, Andrea, Francesco Maria Animati, Giulia Iannaccone, Riccardo Rinaldi, and Rocco Antonio Montone. 2024. "Precision Medicine in Acute Coronary Syndromes" Journal of Clinical Medicine 13, no. 15: 4569. https://doi.org/10.3390/jcm13154569
APA StyleCaffè, A., Animati, F. M., Iannaccone, G., Rinaldi, R., & Montone, R. A. (2024). Precision Medicine in Acute Coronary Syndromes. Journal of Clinical Medicine, 13(15), 4569. https://doi.org/10.3390/jcm13154569








