Patient-Tailored Therapy for Complex Aortic Arch Anatomy: An Evolving Research Field with Custom-Made Solutions
Abstract
1. Introduction
2. Case Presentation
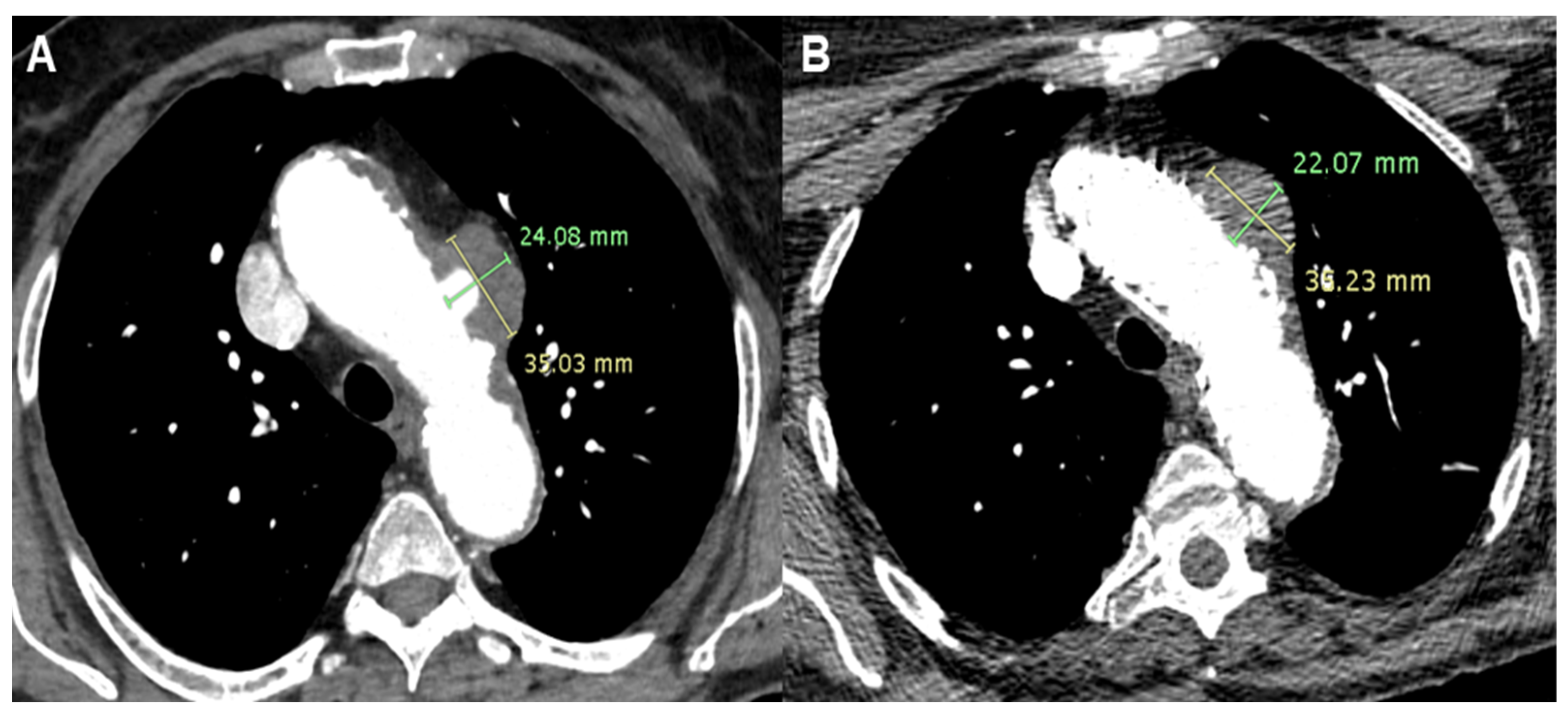
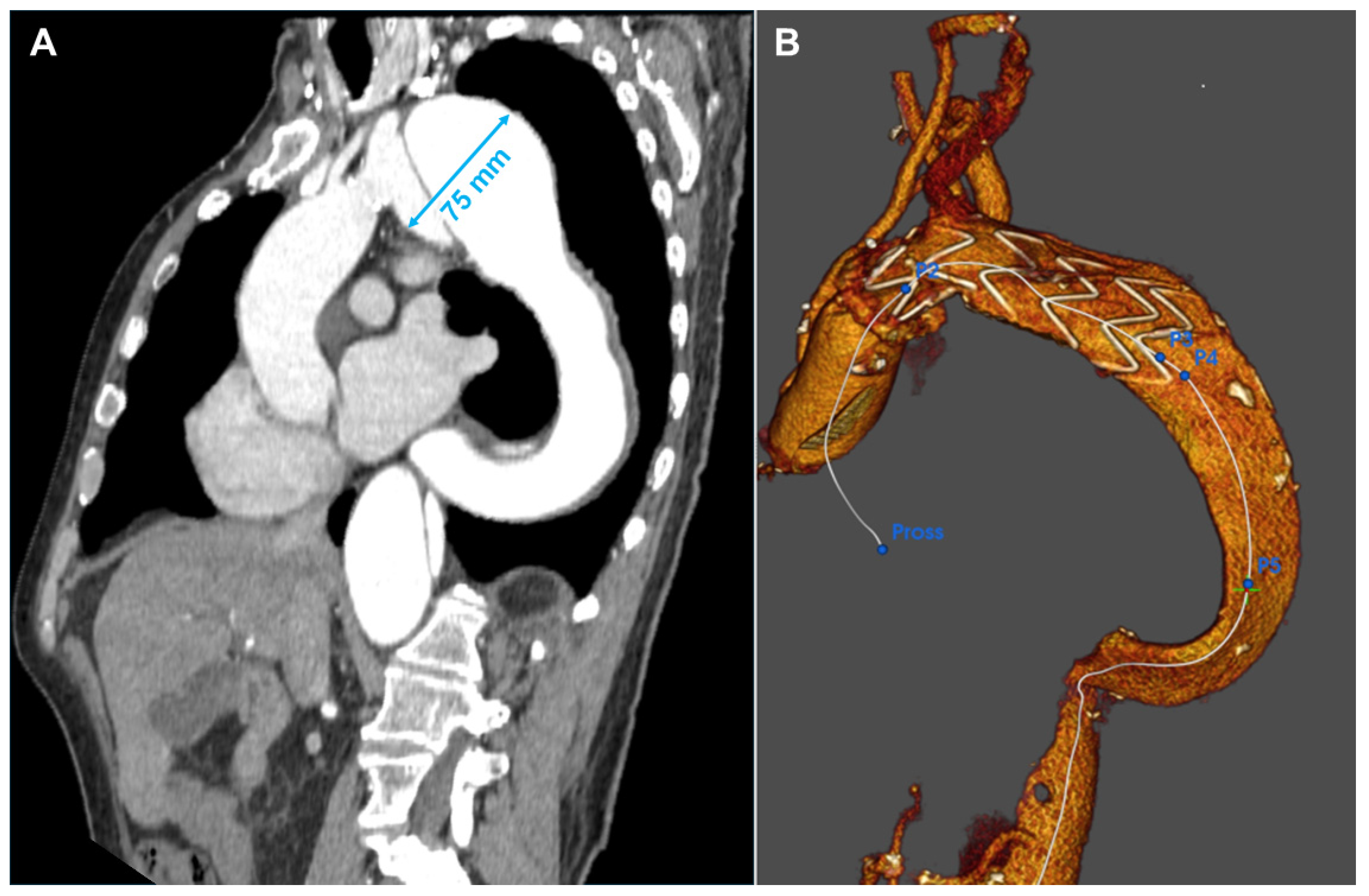
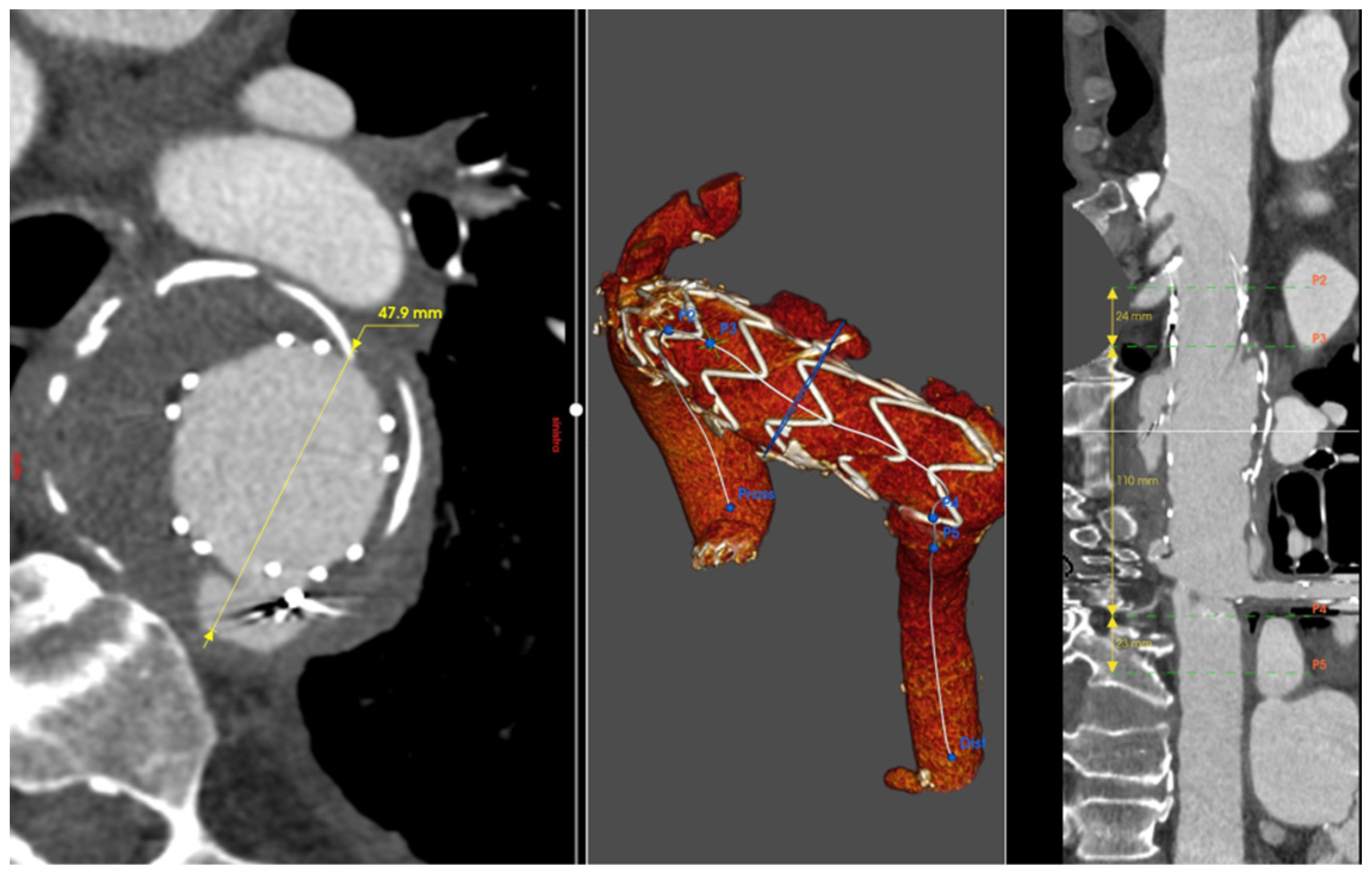
2.1. Patient 1
2.2. Patient 2
2.3. Patient 3
2.4. Patient 4
2.5. Patient 5
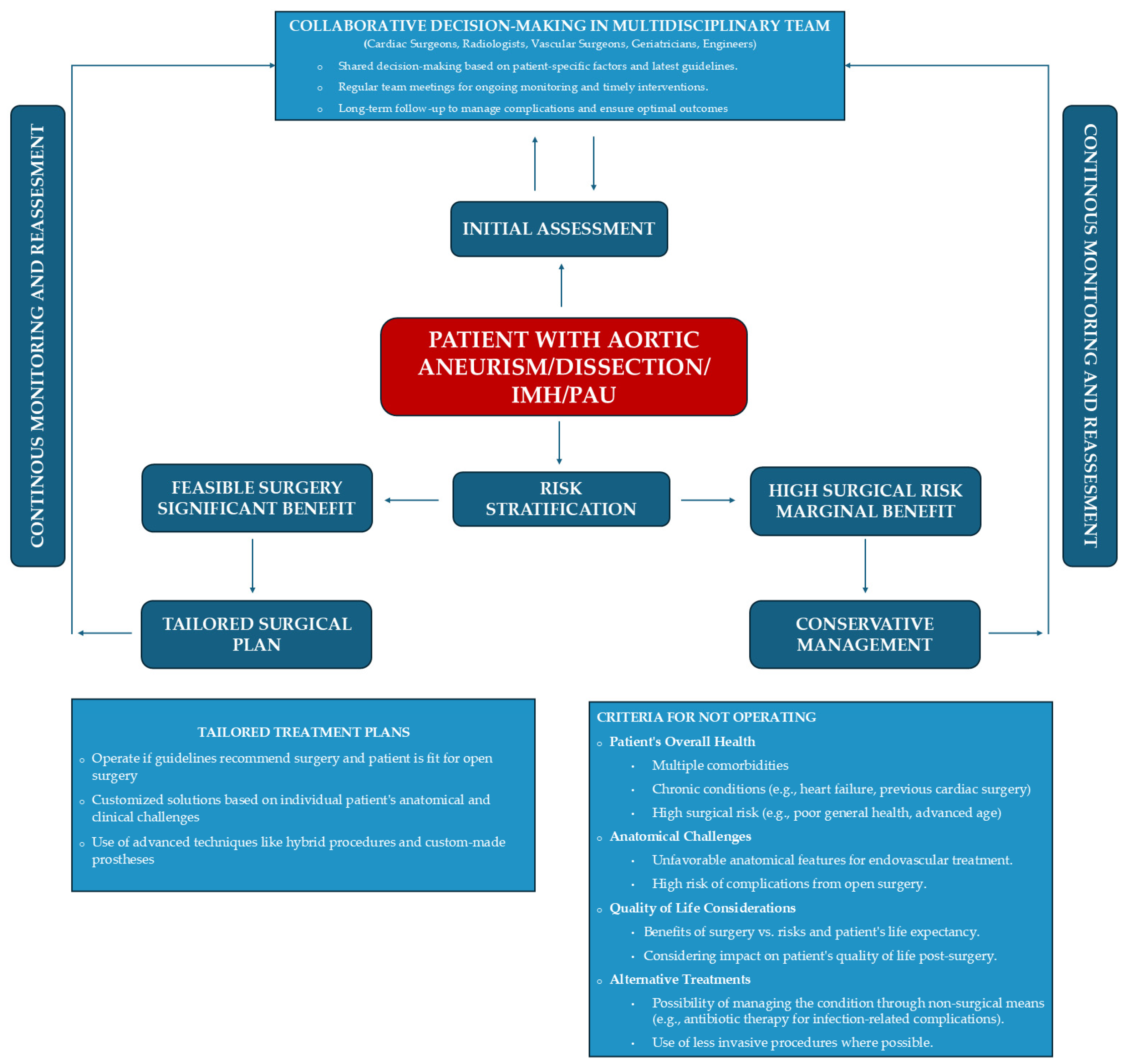
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czerny, M.; Grabenwöger, M.; Berger, T.; Aboyans, V.; Della Corte, A.; Chen, E.P.; Desai, N.D.; Dumfarth, J.; Elefteriades, J.A.; Etz, C.D.; et al. EACTS/STS Guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur. J. Cardio-Thoracic Surg. 2024, 65, ezad426. [Google Scholar] [CrossRef] [PubMed]
- Writing Group Members; Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients with Thoracic Aortic Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010, 121, e266–e369. [Google Scholar] [CrossRef]
- Malina, M.; Sonesson, B.; Ivancev, K. Endografting of thoracic aortic aneurysms and dissections. J. Cardiovasc. Surg. 2005, 46, 333–348. [Google Scholar]
- Marco, L.; Murana, G.; Lovato, L.; Gliozzi, G.; Buia, F.; Attinà, D.; Pacini, D. Endovascular Solutions for Aortic Arch Diseases: Total and Hybrid. Surg. Technol. Online 2021, 38, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Feng, J.; Zhou, J.; Zhao, Z.; Li, H.; Teng, Z.; Jing, Z. Endovascular repair by customized branched stent-graft: A promising treatment for chronic aortic dissection involving the arch branches. J. Thorac. Cardiovasc. Surg. 2015, 150, 1631–1638.e5. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Chen, D.; Zhao, J.; Zhang, Y.; Luo, M.; Fang, K.; Tian, C.; Sun, X.; Guo, H.; Qian, X.; et al. Surgical treatment patterns and clinical outcomes of type B aortic dissection involving the aortic arch. J. Vasc. Surg. 2023, 77, 1016–1027.e9. [Google Scholar] [CrossRef]
- Hughes, G.C.; Vekstein, A. Current state of hybrid solutions for aortic arch aneurysms. Ann. Cardiothorac. Surg. 2021, 10, 731–743. [Google Scholar] [CrossRef]
- Shen, Y.; Qi, Y.; Zhao, J.; Huang, B.; Yuan, D.; Wang, T.; Wang, J. Predictive Factors for Major Adverse Cardiac and Cerebrovascular Events in Octogenarians after Elective Endovascular Aneurysm Repair. Ann. Vasc. Surg. 2023, 88, 363–372. [Google Scholar] [CrossRef]
- Alnahhal, K.I.; Narayanan, M.K.; Lingutla, R.; Parikh, S.; Iafrati, M.; Kumar, S.; Zhan, Y.; Salehi, P. Outcomes of Thoracic Endovascular Aortic Repair in Octogenarians. Vasc. Endovasc. Surg. 2022, 56, 158–165. [Google Scholar] [CrossRef]
- Li, C.; de Guerre, L.E.; Dansey, K.; Lu, J.; Patel, P.B.; Yao, M.; Malas, M.B.; Jones, D.W.; Schermerhorn, M.L. The impact of completion and follow-up endoleaks on survival, reintervention, and rupture. J. Vasc. Surg. 2023, 77, 1676–1684. [Google Scholar] [CrossRef]
- Chaves, C.E.R.; Rojas, S.; Rosso, J.; Peláez, M.; Sánchez, E.F.; Rodríguez, O.G.H. Aortoesophageal fistulae following TEVAR: Case report and literature review. Int. J. Surg. Case Rep. 2023, 106, 108126. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.; Rocha-Neves, J.; Riambau, V.; Teixeira, J. Hostile Thoracic Aortic Aneurysm Treated by Fenestrated Thoracic Stentgraft with Proximal Sealing in Ishimaru Zone 0. Rev. Port. Cir. Cardio-Torac. E Vasc. 2018, 24, 173. [Google Scholar]
- Andrási, T.B.; Grossmann, M.; Zenker, D.; Danner, B.C.; Schöndube, F.A. Supra-aortic interventions for endovascular exclusion of the entire aortic arch. J. Vasc. Surg. 2017, 66, 281–297.e2. [Google Scholar] [CrossRef] [PubMed]
- Rotar, E.P.; Kron, I.L. Commentary: Getting in the zone: Thoracic endovascular aortic repair safety in Ishimaru zones 0 and 1. JTCVS Technol. 2021, 7, 7–8. [Google Scholar] [CrossRef]
- Nana, P.; Tyrrell, M.R.; Guihaire, J.; Le Houérou, T.; Gaudin, A.; Fabre, D.; Haulon, S.; Nana, P.; Tyrrell, M.R.; Guihaire, J.; et al. A Review: Single and MultiBranch Devices for the Treatment of Aortic Arch Pathologies with Proximal Sealing in Ishimaru Zone 0. Ann. Vasc. Surg. 2023, 94, 45–55. [Google Scholar] [CrossRef]
- Dake, M.D.; Bavaria, J.E.; Singh, M.J.; Oderich, G.; Filinger, M.; Fischbein, M.P.; Matsumura, J.S.; Patel, H.J. Management of arch aneurysms with a single-branch thoracic endograft in zone 0. JTCVS Technol. 2021, 7, 1–6. [Google Scholar] [CrossRef]
- Capoccia, M.; Sherif, M.A.; Nassef, A.; Shaw, D.; Walker, P.; Evans, B.; Kaul, P.; Elmahdy, W. Aortic arch surgery for type B aortic dissection: How far should we go? The value of a hybrid approach. Clin. Case Rep. 2023, 11, e6742. [Google Scholar] [CrossRef]
- Kotelis, D.; Geisbüsch, P.; Attigah, N.; Hinz, U.; Hyhlik-Dürr, A.; Böckler, D. Total vs hemi-aortic arch transposition for hybrid aortic arch repair. J. Vasc. Surg. 2011, 54, 1182–1186.e2. [Google Scholar] [CrossRef]
- Eggebrecht, H.; Mehta, R.H.; Dechene, A.; Tsagakis, K.; Kühl, H.; Huptas, S.; Gerken, G.; Jakob, H.G.; Erbel, R. Aortoesophageal Fistula After Thoracic Aortic Stent-Graft Placement. JACC: Cardiovasc. Interv. 2009, 2, 570–576. [Google Scholar] [CrossRef]
- Ljungquist, O.; Haidl, S.; Dias, N.; Sonesson, B.; Sörelius, K.; Trägårdh, E.; Ahl, J. Conservative Management First Strategy in Aortic Vascular Graft and Endograft Infections. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 896–904. [Google Scholar] [CrossRef]
- Lin, X.-F.; Xie, L.-F.; Zhang, Z.-F.; Wu, Q.-S.; Qiu, Z.-H.; Chen, L.-W. Surgical management of the aortic root in acute type A aortic dissection: A comparative analysis. Int. J. Cardiol. 2024, 410, 132182. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Preventza, O.; Orozco-Sevilla, V.; Coselli, J.S. Perioperative management of patients undergoing thoracic endovascular repair. Ann. Cardiothorac. Surg. 2021, 10, 768–777. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linardi, D.; Gardellini, J.; Boschetti, V.; Di Nicola, V.; Denora, M.; Puntel, G.; Puppini, G.; Luciani, G.B. Patient-Tailored Therapy for Complex Aortic Arch Anatomy: An Evolving Research Field with Custom-Made Solutions. J. Clin. Med. 2024, 13, 4975. https://doi.org/10.3390/jcm13174975
Linardi D, Gardellini J, Boschetti V, Di Nicola V, Denora M, Puntel G, Puppini G, Luciani GB. Patient-Tailored Therapy for Complex Aortic Arch Anatomy: An Evolving Research Field with Custom-Made Solutions. Journal of Clinical Medicine. 2024; 13(17):4975. https://doi.org/10.3390/jcm13174975
Chicago/Turabian StyleLinardi, Daniele, Jacopo Gardellini, Vincenzo Boschetti, Venanzio Di Nicola, Mariateresa Denora, Gino Puntel, Giovanni Puppini, and Giovanni B. Luciani. 2024. "Patient-Tailored Therapy for Complex Aortic Arch Anatomy: An Evolving Research Field with Custom-Made Solutions" Journal of Clinical Medicine 13, no. 17: 4975. https://doi.org/10.3390/jcm13174975
APA StyleLinardi, D., Gardellini, J., Boschetti, V., Di Nicola, V., Denora, M., Puntel, G., Puppini, G., & Luciani, G. B. (2024). Patient-Tailored Therapy for Complex Aortic Arch Anatomy: An Evolving Research Field with Custom-Made Solutions. Journal of Clinical Medicine, 13(17), 4975. https://doi.org/10.3390/jcm13174975






