Circulating Endothelin 1 but Not Transforming Growth Factor-β Levels Are Reduced after Pulmonary Endarterectomy in Subjects Affected by Chronic Thromboembolic Pulmonary Hypertension: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
4.1. Overall Operative Data
4.2. Correlations between Preoperative ET-1 and TGF-β Levels and Hemodynamic and Clinical Parameters
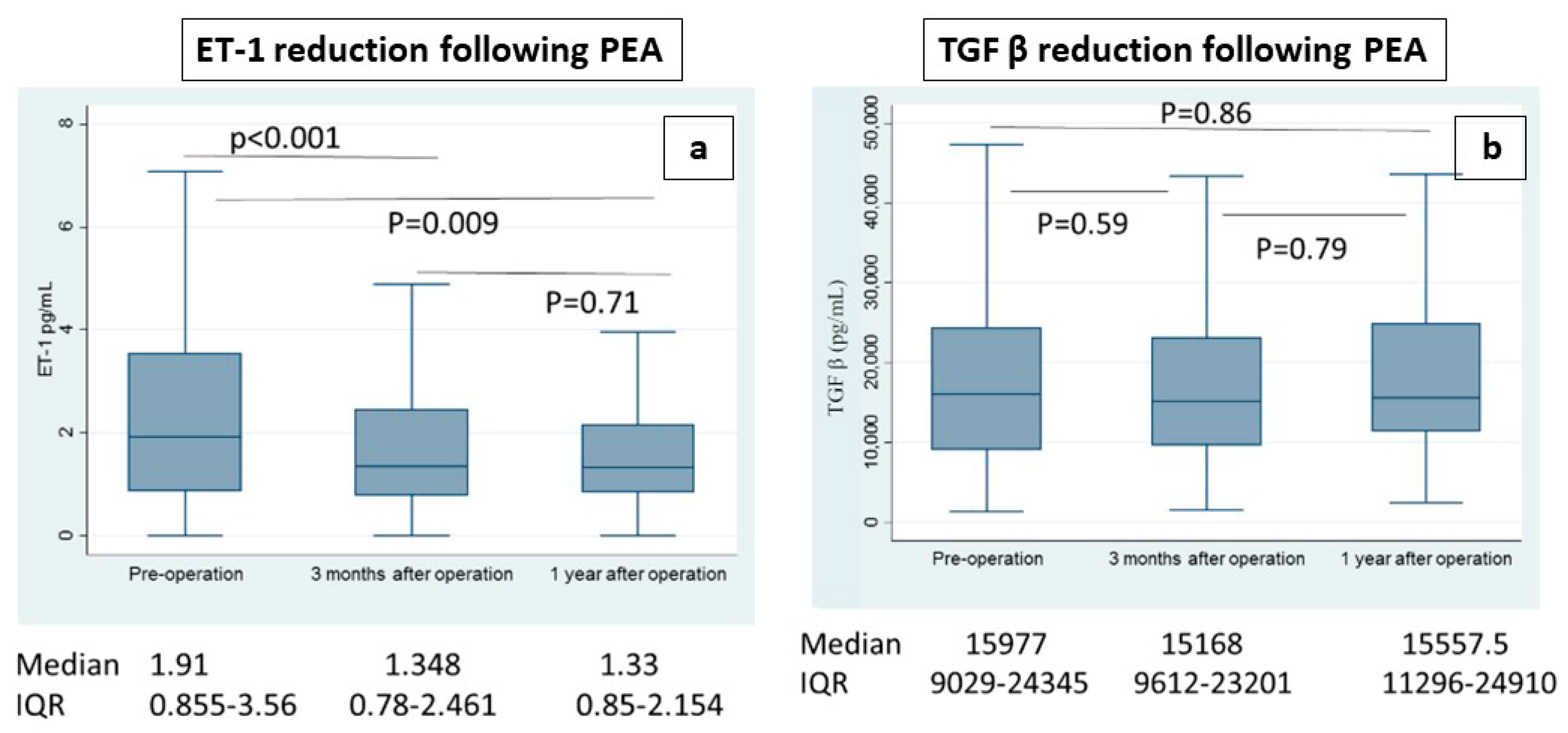
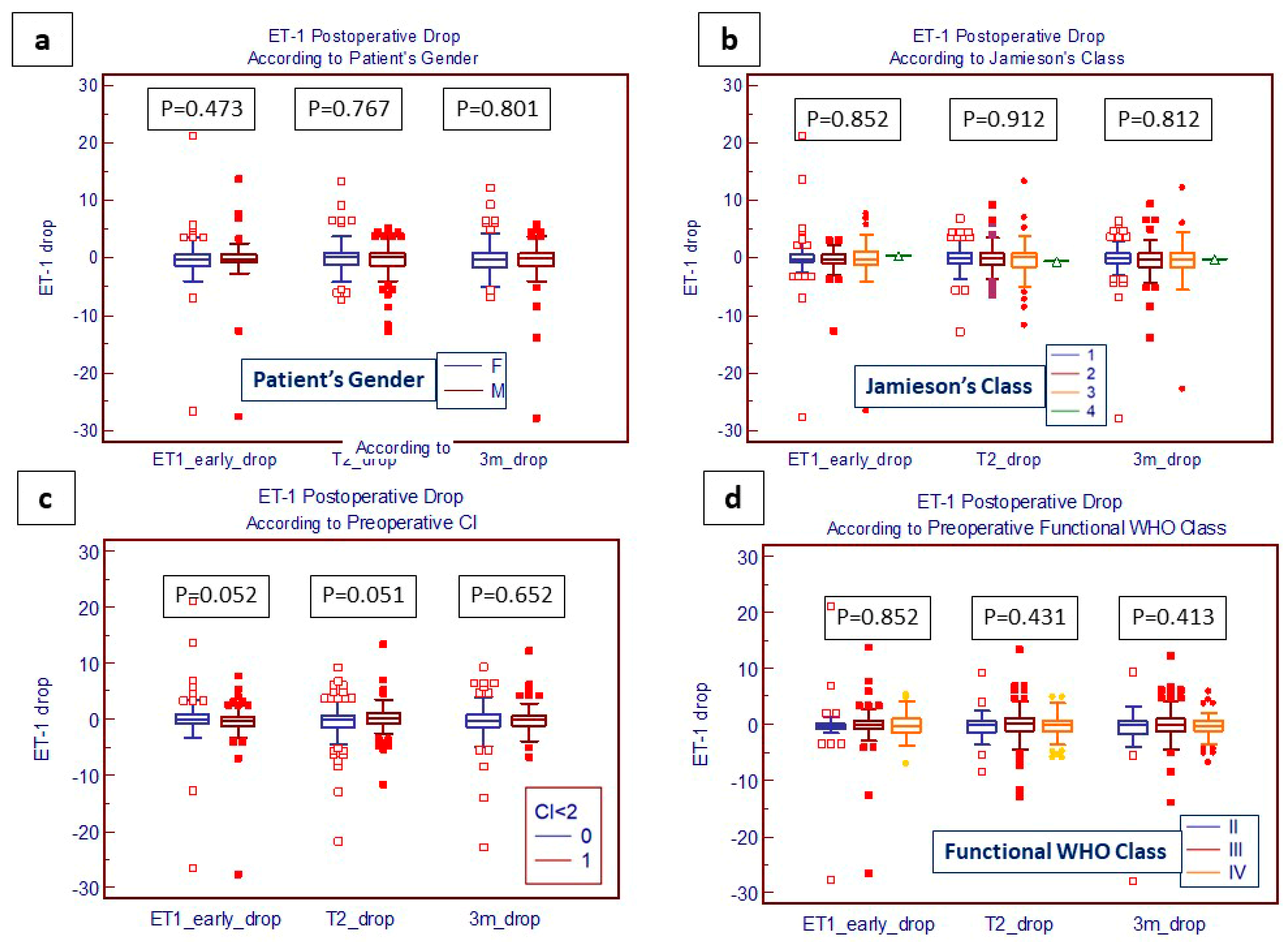
4.3. Evaluation of Post-PEA Levels of ET-1 and TGF-β
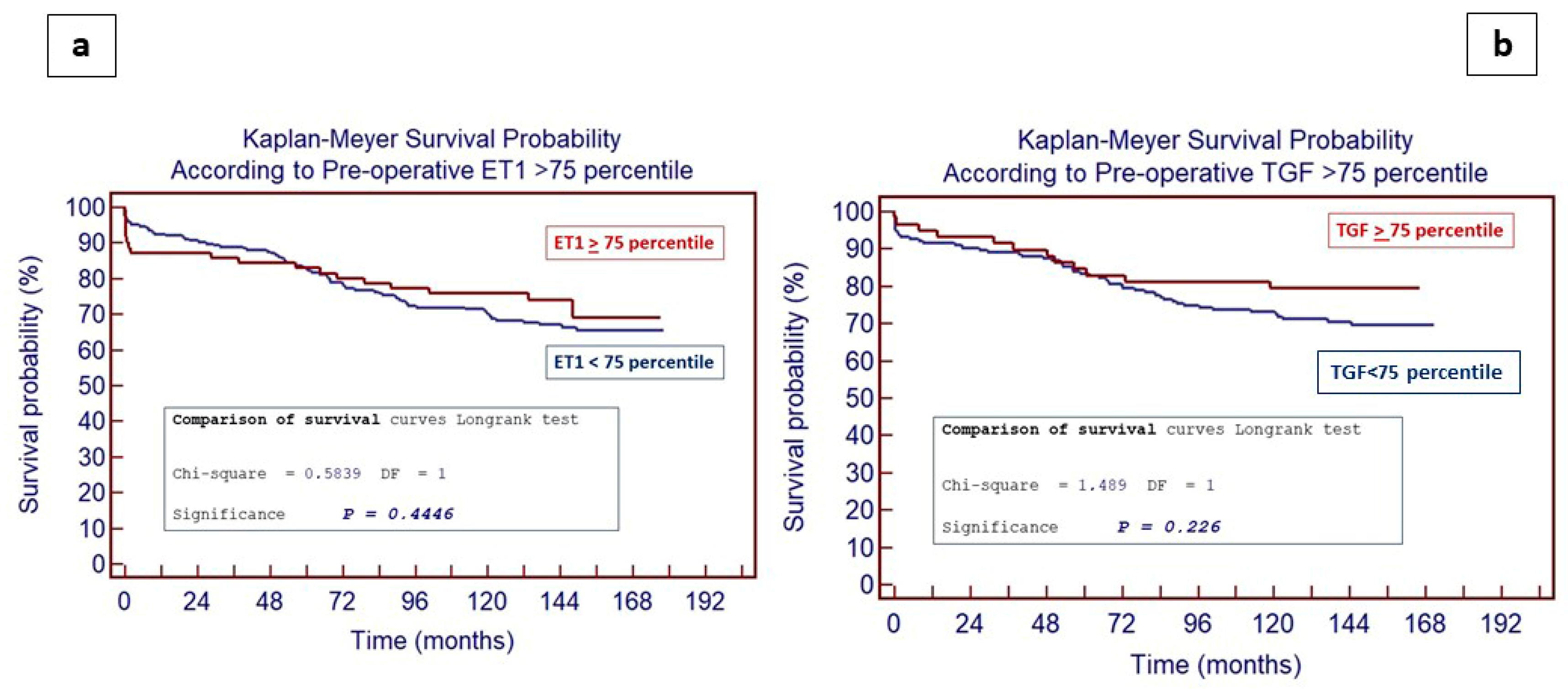
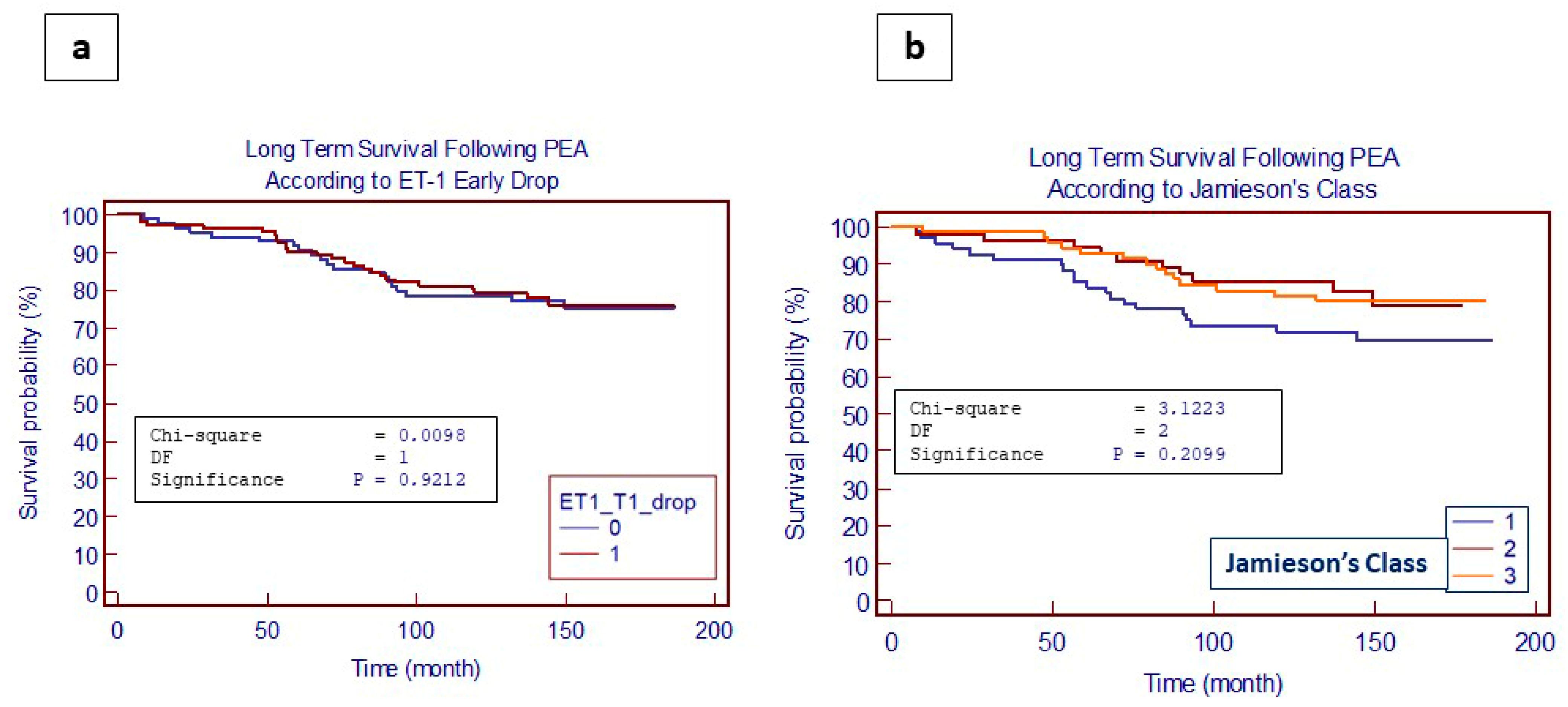
4.4. Impact of ET-1 and TGF-β Levels on Long-Term Outcomes Following PEA
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrell, N.W.; Yang, X.; Upton, P.D.; Jourdan, K.B.; Morgan, N.; Sheares, K.K.; Trembath, R.C. Altered Growth Responses of Pulmonary Artery Smooth Muscle Cells From Pa-tients With Primary Pulmonary Hypertension to Transforming Growth Factor-1 and Bone Morphogenetic Proteins. Circulation 2001, 104, 790–795. [Google Scholar] [CrossRef]
- Cannon, J.E.; Jenkins, D.P.; Hoole, S.P. Chronic thromboembolic pulmonary hypertension: A review of risk factors, management and current challenges. Expert. Rev. Cardiovasc. Ther. 2022, 20, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Campean, I.A.; Sadushi-Kolici, R.; Badr-Eslam, R.; Gerges, C.; Skoro-Sajer, N. Chronic Thromboembolic Disease and Chronic Thromboembolic Pulmonary Hypertension. Clin. Chest Med. 2021, 42, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, S.W.; Kapelanski, D.P. Pulmonary endarterectomy. Curr. Probl. Surg. 2000, 37, 165–252. [Google Scholar] [CrossRef] [PubMed]
- Madani, M.M.; Jamieson, S.W. Pulmonary endarterectomy for chronic thromboembolic disease. Oper. Tech. Thorac. Cardiovasc. Surg. 2006, 11, 264–274. [Google Scholar] [CrossRef]
- D’armini, A.M.; Celentano, A.; Alloni, A.; Silvaggio, G.; Monterosso, C.; Pellegrini, C.; Ghio, S. Surgical management of pulmonary endarterectomy avoiding deep hypo-thermia: The Pavia experience. Ann. Cardiothorac. Surg. 2022, 11, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Madani, M.; Fadel, E.; D’Armini, A.M.; Mayer, E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160111. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef]
- Saito, Y.; Nakao, K.; Mukoyama, M.; Imura, H. Increased plasma endothelin level in patients with essential hypertension. N. Engl. J. Med. 1990, 322, 205. [Google Scholar] [PubMed]
- Stewart, D.J.; Levy, R.D.; Cernacek, P.; Langleben, D. Increased plasma endothelin-1 in pulmonary hypertension: Marker or mediator of disease? Ann. Intern. Med. 1991, 114, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Lerman, A.; Edwards, B.S.; Hallett, J.W.; Heublein, D.M.; Sandberg, S.M.; Burnett, J.C. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N. Engl. J. Med. 1991, 325, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Jankowich, M.; Choudhary, G. Endothelin-1 levels and cardiovascular events. Trends Cardiovasc. Med. 2020, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reesink, H.J.; Meijer, R.C.; Lutter, R.; Boomsma, F.; Jansen, H.M.; Kloek, J.J.; Bresser, P. Hemodynamic and clinical correlates of endothelin-1 in chronic thromboembolic pulmonary hypertension. Circ. J. 2006, 70, 1058–1063. [Google Scholar] [CrossRef]
- Düger, M.; Karakurt, G.; Seyhan, E.C.; Bolatkale, M. Endothelin-1 Gene Polymorphism in Chronic Obstructive Pulmonary Disease: A Case-Control Study. New Trend Med. Sci. 2023, 4, 143–148. [Google Scholar] [CrossRef]
- Tirelli, C.; Mira, S.; Belmonte, L.A.; De Filippi, F.; De Grassi, M.; Italia, M.; Maggioni, S.; Guido, G.; Mondoni, M.; Canonica, G.W.; et al. Exploring the Potential Role of Metabolomics in COPD: A Concise Review. Cells 2024, 13, 475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tirelli, C.; Arbustini, E.; Meloni, F. Bilateral Cystic Bronchiectasis as Novel Phenotype of Niemann-Pick Disease Type B Successfully Treated With Double Lung Transplantation. Chest 2021, 159, e293–e297. [Google Scholar] [CrossRef] [PubMed]
- Tirelli, C.; Rondinone, O.; Italia, M.; Mira, S.; Belmonte, L.A.; De Grassi, M.; Guido, G.; Maggioni, S.; Mondoni, M.; Miozzo, M.R.; et al. The Genetic Basis, Lung Involvement, and Therapeutic Options in Niemann-Pick Disease: A Comprehensive Review. Biomolecules 2024, 14, 211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grosjean, F.; De Amici, M.; Klersy, C.; Marchi, G.; Sciortino, A.; Spaltini, F.; Pin, M.; Grazioli, V.; Celentano, A.; Vanini, B.; et al. High preoperative plasma endothelin-1 levels are associated with increased acute kidney injury risk after pulmonary endarterectomy. J. Nephrol. 2018, 31, 881–888. [Google Scholar] [CrossRef]
- Bochenek, M.L.; Leidinger, C.; Rosinus, N.S.; Gogiraju, R.; Guth, S.; Hobohm, L.; Jurk, K.; Mayer, E.; Münzel, T.; Lankeit, M.; et al. Activated Endothelial TGFβ1 Signaling Promotes Venous Thrombus Nonresolution in Mice Via Endothelin-1: Potential Role for Chronic Thromboembolic Pulmonary Hypertension. Circ. Res. 2020, 126, 162–181. [Google Scholar] [CrossRef]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tirelli, C.; Pesenti, C.; Miozzo, M.; Mondoni, M.; Fontana, L.; Centanni, S. The Genetic and Epigenetic Footprint in Idiopathic Pulmonary Fibrosis and Familial Pulmonary Fibrosis: A State-of-the-Art Review. Diagnostics 2022, 12, 3107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tirelli, C.; De Amici, M.; Albrici, C.; Mira, S.; Nalesso, G.; Re, B.; Corsico, A.G.; Mondoni, M.; Centanni, S. Exploring the Role of Immune System and Inflammatory Cytokines in SARS-CoV-2 Induced Lung Disease: A Narrative Review. Biology 2023, 12, 177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melazzini, F.; Colaneri, M.; Fumoso, F.; Freddi, G.; Lenti, M.V.; Pieri, T.C.; Piloni, D.; Noris, P.; Pieresca, C.; Preti, P.S.; et al. Venous thromboembolism and COVID-19: A single center experience from an academic tertiary referral hospital of Northern Italy. Intern. Emerg. Med. 2021, 16, 1141–1152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valerio, L.; Mavromanoli, A.C.; Barco, S.; Abele, C.; Becker, D.; Bruch, L.; Ewert, R.; Faehling, M.; Fistera, D.; Gerhardt, F.; et al. FOCUS Investigators. Chronic thromboembolic pulmonary hypertension and impairment after pulmonary embolism: The FOCUS study. Eur. Heart J. 2022, 43, 3387–3398. [Google Scholar] [CrossRef]
- Hyder, S.N.; Chatterjee, S.; Aggarwal, V. Percutaneous treatments for pulmonary hypertension: Reviewing the growing procedural role for interventional cardiology. Interv. Cardiol. Clin. 2022, 11, 293–305. [Google Scholar] [CrossRef]
- Kelava, M.; Koprivanac, M.; Smedira, N.; Mihaljevic, T.; Alfirevic, A. Extracorporeal Membrane Oxygenation in Pulmonary Endarterectomy Patients. J. Cardiothorac. Vasc. Anesth. 2019, 33, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Magoń, W.; Stępniewski, J.; Waligóra, M.; Jonas, K.; Przybylski, R.; Podolec, P.; Kopeć, G. Changes in Inflammatory Markers in Patients with Chronic Thromboem-bolic Pulmonary Hypertension Treated with Balloon Pulmonary Angioplasty. Cells 2022, 11, 1491. [Google Scholar] [CrossRef]
- Aggarwal, V.; Giri, J.; Visovatti, S.H.; Mahmud, E.; Matsubara, H.; Madani, M.; Rogers, F.; Gopalan, D.; Rosenfield, K.; McLaughlin, V.V. American Heart Association Council on Clinical Cardiology; Council on Peripheral Vascular Disease; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; and Council on Cardiovas-cular and Stroke Nursing. Status and Future Directions for Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Disease With and Without Pulmonary Hypertension: A Scientific Statement From the American Heart Associa-tion. Circulation 2024, 149, e1090–e1107. [Google Scholar] [CrossRef] [PubMed]
- Ogo, T.; Chowdhury, H.M.; Yang, J.; Long, L.; Li, X.; Cleuren, Y.N.T.; Morrell, N.W.; Schermuly, R.T.; Trembath, R.C.; Nasim, T. Inhibition of Overactive Transforming Growth Factor-b Signaling by Prostacy-clin Analogs in Pulmonary Arterial Hypertension CLINICAL RELEVANCE. Am. J. Respir. Cell Mol. Biol. 2013, 48, 733–741. [Google Scholar] [CrossRef]
- Markewitz, B.A.; Farrukh, I.S.; Chen, Y.; Li, Y.; Michael, J.R. Regulation of endothelin-1 synthesis in human pulmonary arterial smooth muscle cells: Effects of transforming growth factor-b and hypoxia. Cardiovasc. Res. 2001, 49, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Castañares, C.; Redondo-Horcajo, M.; Magán-Marchal, N.; Dijke, P.T.; Lamas, S.; Rodríguez-Pascual, F. Signaling by ALK5 mediates TGF-beta-induced ET-1 ex-pression in endothelial cells: A role for migration and proliferation. J. Cell Sci. 2007, 120, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Lambers, C.; Roth, M.; Zhong, J.; Campregher, C.; Binder, P.; Burian, B.; Petkov, V.; Block, L.-H. The Interaction of Endothelin-1 and TGF-β1 Mediates Vascular Cell Remodeling. PLoS ONE 2013, 8, e73399. [Google Scholar] [CrossRef]
- Wermuth, P.J.; Li, Z.; Mendoza, F.A.; Jimenez, S.A. Stimulation of Transforming Growth Factor-β1-Induced Endotheli-al-To-Mesenchymal Transition and Tissue Fibrosis by Endothelin-1 (ET-1): A Novel Profibrotic Effect of ET-1. PLoS ONE 2016, 11, e0161988. [Google Scholar] [CrossRef]
- Ogino, H. Recent advances of pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension in-cluding Japanese experiences. Gen. Thorac. Cardiovasc. Surg. 2014, 62, 9–18. [Google Scholar] [CrossRef]
- Corsico, A.G.; D’armini, A.M.; Conio, V.; Sciortino, A.; Pin, M.; Grazioli, V.; Di Vincenzo, G.; Di Domenica, R.; Celentano, A.; Vanini, B.; et al. Persistent exercise limitation after successful pulmonary endoarterectomy: Fre-quency and determinants. Respir. Res. 2019, 20, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bochenek, M.L.; Saar, K.; Nazari-Jahantigh, M.; Gogiraju, R.; Wiedenroth, C.B.; Münzel, T.; Mayer, E.; Fink, L.; Schober, A.; Hübner, N.; et al. Endothelial Overexpression of TGF-β-Induced Protein Impairs Venous Thrombus Resolution: Possible Role in CTEPH. JACC Basic. Transl. Sci. 2023, 9, 100–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Overall Patients Enrolled n.340 | |||
|---|---|---|---|
| Gender | mPAP(mmHg) | ||
| Male | 145 (42.6%) | Range | 12–81 |
| Female | 195 (57.3%) | Mean ± sd | 44 ± 12 |
| Age (Years) | CO (L/min) | ||
| Range | 18–83 | Range | 1.60–8.50 |
| Mean ± sd | 61 ± 14 | Mean ± sd | 3.90 ± 1.81 |
| Smoking History | PVR (dyn·s/cm5) | ||
| Yes | 26 (7.6%) | PVR [Wood units] | 78–2044 [0.975–25.55] |
| No | 204 (60%) | Range | 851 ± 379 |
| Previous | 107 (31.4%) | Mean ± sd | [10.63 ± 4.73] |
| WHO Class | |||
| Weight (kg) | 73 ± 17 | II | 56 (16.5%) |
| Height (m) | 1.66 ± 10 | III | 175 (51.5%) |
| BSA (m2) | 1.82 ± 0.24 | IV | 109 (32%) |
| History of DVT | 181 (53.2%) | Hypertension | 126 (37%) |
| O2 Therapy | 158 (46.4%) | PAD | 6 (1.7%) |
| COPD | 54 (15.8%) | CRF | 23 (6.7%) |
| Diabetes | 17 (5%) | AF | 7 (2%) |
| Intraoperative Parameters | Postoperative Parameters | ||
|---|---|---|---|
| ECC Time (min) | In-Hospital Mortality | 25 (7.3%) | |
| Range | 115–721 | ||
| Mean ± sd | 343 ± 77 | ||
| Cross-Clamp Time (pts) | 23 (7%) | ICU Stay (day) | |
| Range (min) | 17–86 | Range | 0–83 |
| Mean ± sd (min) | 33 ± 17 | Mean ± sd | 8 ± 13 |
| NF Temperature (°C) | In-Hospital Stay (day) | ||
| Range | 21–30 | Range | 0–100 |
| Mean ± sd | 24 ± 1 | Mean ± sd | 16 ± 12 |
| CA (number of events) | Postoperative Complications | ||
| Range Mean ± sd | 2–26 12 ± 4 | Arrhythmia ECMO Tracheostomy Neurological | 90 (26.4%) 14 (4.1%) 25 (7.3%) 28 (8.2%) |
| CA Total Time (min) | Caval Filter | 298 (87.6%) | |
| Range | 11–192 | ||
| Mean ± sd | 89 ± 32 | ||
| Combined Procedure | 64 (19%) | ||
| Coefficient | p-Value | 95% CI | |
|---|---|---|---|
| ET-1 | 0.37 | <0.001 | 0.21–0.53 |
| TGF-β | −0.05 | 0.54 | −0.21–0.11 |
| Sex | −0.04 | 0.936 | −0.98–0.91 |
| Value | mPAP ≤ 40 (n.125) | mPAP > 40 (n.215) | p |
| ET-1 pre | 1.35 (1.00–1.72) | 1.87 (1.54–2.24) | 0.0174 |
| ET-1 3 months | 1.22 (0.93–1.59) | 1.29 (1.07–1.69) | 0.5245 |
| ET-1 1 year | 1.40 (1.08–1.83) | 1.28 (1.06–1.52) | 0.5084 |
| Value | mPAP ≤ 40 (n.80) | mPAP > 40 (n.126) | p |
| TGF-β pre | 17,794 (14,408–19,536) | 14,679 (11,061–17,398) | 0.2422 |
| TGF-β 3 months | 16,182 (13,409–18,499) | 15,065 (12,958–18,269) | 0.8212 |
| TGF-β 1 year | 15,736 (13,951–17,721) | 15,529 (13,500–17,394) | 0.7824 |
| ET-1 | Class II (n = 56) | Class III (n = 175) | Class IV (n = 109) | |||
| r | p | r | p | r | p | |
| mPAP | 0.286 | 0.016 | 0.052 | 0.47 | 0.139 | 0.12 |
| CO | −0.072 | 0.55 | 0.005 | 0.94 | 0.070 | 0.44 |
| PVR | 0.236 | 0.048 | 0.013 | 0.85 | −0.010 | 0.91 |
| TFG-β | Class II (n = 44) | Class III (n = 103) | Class IV (n = 59) | |||
| r | p | r | p | r | p | |
| mPAP | 0.179 | 0.2298 | 0.160 | 0.0880 | 0.130 | 0.2775 |
| CO | −0.0085 | 0.5668 | 0.0066 | 0.4824 | 0.034 | 0.7765 |
| PVR | 0.197 | 0.1857 | 0.074 | 0.4288 | −0.121 | 0.3117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Totaro, P.; Tirelli, C.; De Amici, M.; Grosjean, F.; Testa, G.; Sacchi, L.; De Silvestri, A.; Alloni, A.; Kushta, E.; Albertini, R.; et al. Circulating Endothelin 1 but Not Transforming Growth Factor-β Levels Are Reduced after Pulmonary Endarterectomy in Subjects Affected by Chronic Thromboembolic Pulmonary Hypertension: A Prospective Cohort Study. J. Clin. Med. 2024, 13, 4977. https://doi.org/10.3390/jcm13174977
Totaro P, Tirelli C, De Amici M, Grosjean F, Testa G, Sacchi L, De Silvestri A, Alloni A, Kushta E, Albertini R, et al. Circulating Endothelin 1 but Not Transforming Growth Factor-β Levels Are Reduced after Pulmonary Endarterectomy in Subjects Affected by Chronic Thromboembolic Pulmonary Hypertension: A Prospective Cohort Study. Journal of Clinical Medicine. 2024; 13(17):4977. https://doi.org/10.3390/jcm13174977
Chicago/Turabian StyleTotaro, Pasquale, Claudio Tirelli, Mara De Amici, Fabrizio Grosjean, Giorgia Testa, Lucia Sacchi, Annalisa De Silvestri, Alessia Alloni, Eraldo Kushta, Riccardo Albertini, and et al. 2024. "Circulating Endothelin 1 but Not Transforming Growth Factor-β Levels Are Reduced after Pulmonary Endarterectomy in Subjects Affected by Chronic Thromboembolic Pulmonary Hypertension: A Prospective Cohort Study" Journal of Clinical Medicine 13, no. 17: 4977. https://doi.org/10.3390/jcm13174977





