The Impact of Infections in Patients Treated with Atezolizumab Plus Bevacizumab for Unresectable Hepatocellular Carcinoma
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analysis and Outcomes
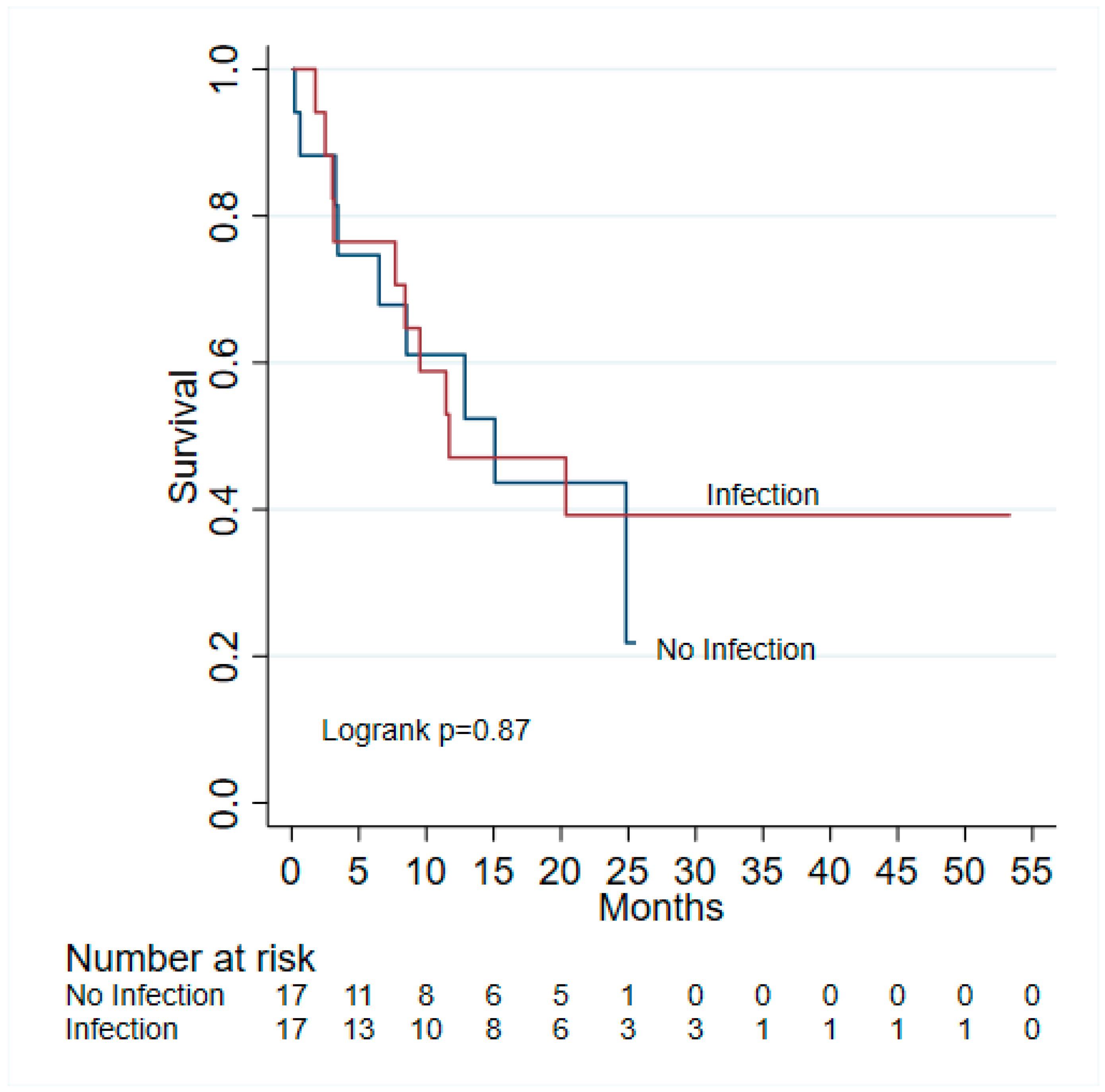
3. Results
3.1. Baseline Factors
3.2. Infections
3.3. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Zhang, J.F.; Shu, Z.J.; Xie, C.Y.; Li, Q.; Jin, X.H.; Gu, W.; Jiang, F.J.; Ling, C.Q. Prognosis of unresectable hepatocellular carcinoma: Comparison of seven staging systems (TNM, Okuda, BCLC, CLIP, CUPI, JIS, CIS) in a Chinese cohort. PLoS ONE 2014, 9, e88182. [Google Scholar] [CrossRef] [PubMed]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Reddy, K.R.; McLerran, D.; Marsh, T.; Parikh, N.; Roberts, L.R.; Schwartz, M.; Nguyen, M.H.; Befeler, A.; Page-Lester, S.; Tang, R.; et al. Incidence and Risk Factors for Hepatocellular Carcinoma in Cirrhosis: The Multicenter Hepatocellular Carcinoma Early Detection Strategy (HEDS) Study. Gastroenterology 2023, 165, 1053–1063.e6. [Google Scholar] [CrossRef]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Brusnic, O.; Onișor, D.M. Fecal Microbiota Transplantation in Liver Cirrhosis. Biomedicines 2023, 11, 2930. [Google Scholar] [CrossRef] [PubMed]
- Piano, S.; Bunchorntavakul, C.; Marciano, S.; Rajender Reddy, K. Infections in cirrhosis. Lancet Gastroenterol. Hepatol. 2024, 9, 745–757. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Chamroonkul, N.; Chavalitdhamrong, D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J. Hepatol. 2016, 8, 307–321. [Google Scholar] [CrossRef]
- Bruns, T.; Zimmermann, H.W.; Stallmach, A. Risk factors and outcome of bacterial infections in cirrhosis. World J. Gastroenterol. 2014, 20, 2542–2554. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, M.; Yibang, Z.; Raza, F.; Hasnat, M.; Cao, J.; Qi, X.; Hussain, A.; Liu, D. Hollow Mesoporous Silica Nanoparticles for Dual Chemo-starvation Therapy of Hepatocellular Carcinoma. Pharm. Res. 2023, 40, 2215–2228. [Google Scholar] [CrossRef]
- Hegde, P.S.; Wallin, J.J.; Mancao, C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin. Cancer Biol. 2018, 52, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Hurwitz, H. Combinations of Bevacizumab with Cancer Immunotherapy. Cancer J. 2018, 24, 193–204. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.X.; Fu, S.; Zhang, Q.; Guo, X.M. Bevacizumab increases the risk of infections in cancer patients: A systematic review and pooled analysis of 41 randomized controlled trials. Crit. Rev. Oncol. Hematol. 2015, 94, 323–336. [Google Scholar] [CrossRef]
- Petrelli, F.; Morelli, A.M.; Luciani, A.; Ghidini, A.; Solinas, C. Risk of Infection with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. Target. Oncol. 2021, 16, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Kanjanapan, Y.; Yip, D. Characteristics and risk factors for microbial infections during cancer immune checkpoint therapy. Cancer Med. 2020, 9, 9027–9035. [Google Scholar] [CrossRef]
- Sharma, R.; Pillai, A.; Marron, T.U.; Fessas, P.; Saeed, A.; Jun, T.; Dharmapuri, S.; Szafron, D.; Naqash Abdul, R.; Gampa, A.; et al. Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC. Hepatol. Commun. 2022, 6, 1776–1785. [Google Scholar] [CrossRef]
- Scheiner, B.; Roessler, D.; Phen, S.; Lim, M.; Pomej, K.; Pressiani, T.; Cammarota, A.; Fründt, T.W.; von Felden, J.; Schulze, K.; et al. Efficacy and safety of immune checkpoint inhibitor rechallenge in individuals with hepatocellular carcinoma. JHEP Rep. 2023, 5, 100620. [Google Scholar] [CrossRef]
- Roessler, D.; Öcal, O.; Philipp, A.B.; Markwardt, D.; Munker, S.; Mayerle, J.; Jochheim, L.S.; Hammer, K.; Lange, C.M.; Geier, A.; et al. Ipilimumab and nivolumab in advanced hepatocellular carcinoma after failure of prior immune checkpoint inhibitor-based combination therapies: A multicenter retrospective study. J. Cancer Res. Clin. Oncol. 2022, 149, 3065–3073. [Google Scholar] [CrossRef]
- Dobosz, P.; Stępień, M.; Golke, A.; Dzieciątkowski, T. Challenges of the Immunotherapy: Perspectives and Limitations of the Immune Checkpoint Inhibitor Treatment. Int. J. Mol. Sci. 2022, 23, 2847. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Onuma, A.E.; Zhang, H.; Huang, H.; Williams, T.M.; Noonan, A.; Tsung, A. Immune Checkpoint Inhibitors in Hepatocellular Cancer: Current Understanding on Mechanisms of Resistance and Biomarkers of Response to Treatment. Gene Expr. 2020, 20, 53–65. [Google Scholar] [CrossRef]
- Mohr, R.; Jost-Brinkmann, F.; Özdirik, B.; Lambrecht, J.; Hammerich, L.; Loosen, S.H.; Luedde, T.; Demir, M.; Tacke, F.; Roderburg, C. Lessons from Immune Checkpoint Inhibitor Trials in Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 652172. [Google Scholar] [CrossRef]
- Yusuf, K.; Sampath, V.; Umar, S. Bacterial infections and cancer: Exploring this association and its implications for cancer patients. Int. J. Mol. Sci. 2023, 24, 3110. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, Y.; Yu, K.; Yang, Y.; Wang, X.; Yang, X.; Qian, J.; Liu, Z.-X.; Wu, B. Fatal infections among cancer patients: A population-based study in the United States. Infect. Dis. Ther. 2021, 10, 871–895. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Galle, P.R.; Zhu, A.X.; Ducreux, M.; Cheng, A.L.; Ikeda, M.; Tsuchiya, K.; Aoki, K.I.; Jia, J.; et al. IMbrave150: Efficacy and Safety of Atezolizumab plus Bevacizumab versus Sorafenib in Patients with Barcelona Clinic Liver Cancer Stage B Unresectable Hepatocellular Carcinoma: An Exploratory Analysis of the Phase III Study. Liver Cancer 2023, 12, 238–250. [Google Scholar] [CrossRef]
- Sang, Y.B.; Lee, C.; Kim, S.G.; Lee, B.; Kang, B.; Kim, C.; Chon, H.J. Impact of Coronavirus Disease 2019 on Unresectable Hepatocellular Carcinoma Treated with Atezolizumab/Bevacizumab. J. Clin. Med. 2024, 13, 1335. [Google Scholar] [CrossRef]
- Yeo, W.; Lam, K.C.; Zee, B.; Chan, P.S.K.; Mo, F.K.F.; Ho, W.M.; Wong, W.L.; Leung, T.W.T.; Chan, A.T.C.; Ma, B.; et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann. Oncol. 2004, 15, 1661–1666. [Google Scholar] [CrossRef]
- Ruli, T.M.; Pollack, E.D.; Lodh, A.; Evers, C.D.; Price, C.A.; Shoreibah, M. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma and Their Hepatic-Related Side Effects: A Review. Cancers 2024, 16, 2042. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, J.; Chen, W.; Zhou, H.; Du, D.; Zhu, K.; Chen, H.; Meng, J.; Yang, J. Hepatitis B reactivation in cancer patients receiving immune checkpoint inhibitors: A systematic review and meta-analysis. Infect. Dis. Poverty 2023, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Ntellas, P.; Chau, I. Updates on Systemic Therapy for Hepatocellular Carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e430028. [Google Scholar] [CrossRef]
- Wu, L.; Bagiella, E.; Rudshteyn, M.; Cohen, D.J. Effect of immune checkpoint inhibitor (ICI) treatment in hepatocellular carcinoma (HCC) based on underlying liver disease. J. Clin. Oncol. 2022, 40, 396. [Google Scholar] [CrossRef]
- Donisi, C.; Puzzoni, M.; Ziranu, P.; Lai, E.; Mariani, S.; Saba, G.; Impera, V.; Dubois, M.; Persano, M.; Migliari, M.; et al. Immune Checkpoint Inhibitors in the Treatment of HCC. Front. Oncol. 2021, 10, 1240. [Google Scholar] [CrossRef] [PubMed]
- Sae-Tia, S.; Naidoo, J.; Mehta, S. Infections in patients receiving immune checkpoint inhibitors. J. Clin. Oncol. 2019, 37, 155. [Google Scholar] [CrossRef]
- Redelman-Sidi, G.; Michielin, O.; Cervera, C.; Ribi, C.; Aguado, J.M.; Fernandez-Ruiz, M.; Manuel, O. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: An infectious diseases perspective (Immune checkpoint inhibitors, cell adhesion inhibitors, sphingosine-1-phosphate receptor modulators and proteasome inhibitors). Clin. Microbiol. Infect. 2018, 24, S95–S107. [Google Scholar] [CrossRef]
- Morelli, T.; Fujita, K.; Redelman-Sidi, G.; Elkington, P.T. Infections due to dysregulated immunity: An emerging complication of cancer immunotherapy. Thorax 2022, 77, 304–311. [Google Scholar] [CrossRef]
- Chu, Y.-C.; Fang, K.-C.; Chen, H.-C.; Yeh, Y.-C.; Tseng, C.-E.; Chou, T.-Y.; Lai, C.-L. Pericardial Tamponade Caused by a Hypersensitivity Response to Tuberculosis Reactivation after Anti–PD-1 Treatment in a Patient with Advanced Pulmonary Adenocarcinoma. J. Thorac. Oncol. 2017, 12, e111–e114. [Google Scholar] [CrossRef]
- Fujita, K.; Terashima, T.; Mio, T. Anti-PD1 Antibody Treatment and the Development of Acute Pulmonary Tuberculosis. J. Thorac. Oncol. 2016, 11, 2238–2240. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Adjei, A.A. Anti–PD-1 Antibody Treatment and the Development of Acute Pulmonary Tuberculosis. J. Thorac. Oncol. 2016, 11, 2048–2050. [Google Scholar] [CrossRef]
- Qin, H.; Yuan, B.; Huang, W.; Wang, Y. Utilizing Gut Microbiota to Improve Hepatobiliary Tumor Treatments: Recent Advances. Front. Oncol. 2022, 12, 924696. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Seo, G.S. Fecal Microbiota Transplantation: Is It Safe? Clin. Endosc. 2021, 54, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wiesnoski, D.H.; Helmink, B.A.; Gopalakrishnan, V.; Choi, K.; DuPont, H.L.; Jiang, Z.D.; Abu-Sbeih, H.; Sanchez, C.A.; Chang, C.C.; et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 2018, 24, 1804–1808. [Google Scholar] [CrossRef] [PubMed]

| Total | No Infection | Infection | p-Value | |
|---|---|---|---|---|
| N = 34 | N = 17 | N = 17 | ||
| Age | 66.29 ± 9.39 | 68.43 ± 10.47 | 64.15 ± 7.89 | 0.19 |
| Sex | 0.66 | |||
| Male | 28 (82.35) | 13 (76.47) | 15 (88.24) | |
| Female | 6 (17.65) | 4 (23.53) | 2 (11.76) | |
| Race | 0.69 | |||
| White | 26 (76.47) | 12 (70.59) | 14 (82.35) | |
| Other | 8 (23.53) | 5 (29.41) | 3 (17.65) | |
| Comorbidities | 20 (58.82) | 10 (58.82) | 10 (58.82) | 1.00 |
| ECOG PS | 1.00 | |||
| 0 | 6 (17.65) | 3 (17.65) | 3 (17.65) | |
| 1 | 11 (32.35) | 6 (35.29) | 5 (29.41) | |
| 2 | 4 (11.76) | 2 (11.76) | 2 (11.76) | |
| Unknown | 13 (38.24) | 6 (35.29) | 7 (41.18) | |
| History of Immunosuppressive Use | 2 (5.88) | 0 (0.00) | 2 (11.76) | 0.48 |
| History of Solid or Hematopoietic Cell Transplant | 3 (8.82) | 1 (5.88) | 2 (11.76) | 1.00 |
| Patients with Chronic Infection | 21 (61.76) | 13 (76.47) | 8 (47.06) | 0.16 |
| Hepatitis B Only | 1 (2.94) | 1 (5.88) | ||
| Hepatitis C Only | 14 (41.17) | 8 (47.06) | 6 (35.29) | |
| Hepatitis B and C | 3 (8.82) | 2 (11.76) | 1 (5.88) | |
| Hepatitis B and Latent TB | 1 (2.94) | 1 (5.88) | ||
| Hepatitis B and C and Latent TB | 1 (2.94) | 1 (5.88) | ||
| HSV | 1 (2.94) | 1 (5.88) | ||
| Number of Cycles of ICIs Given | 7 (3–13) | 4 (3–12) | 12 (5–17) | 0.18 |
| Line of Systemic Therapy | 0.36 | |||
| 1 | 25 (73.53) | 14 (82.35) | 11 (64.71) | |
| 2 | 6 (17.65) | 3 (17.65) | 3 (17.65) | |
| >2 | 3 (8.82) | 0 (0.00) | 3 (17.65) | |
| Rifaximin at ICI Initiation | 4 (11.76) | 1 (5.88) | 3 (17.65) | 0.60 |
| Patients on Antibiotic | 8 (23.53) | 3 (17.65) | 5 (29.41) | 0.69 |
| IrAE | 10 (29.41) | 4 (23.53) | 6 (35.29) | 0.71 |
| Steroids for irAE | 4 (28.57) | 2 (33.33) | 2 (25.00) | 1.00 |
| Number of ED Visits | 0.64 | |||
| 0 | 24 (70.59) | 13 (76.47) | 11 (64.71) | |
| 1 | 4 (11.76) | 1 (5.88) | 3 (17.65) | |
| 2 | 5 (14.71) | 3 (17.65) | 2 (11.76) | |
| 5 | 1 (2.94) | 0 (0.00) | 1 (5.88) | |
| Number of Inpatient Hospitalizations | 1 (0–2) | 1 (0–1) | 1 (0–4) | 0.098 |
| ICU Admissions | 5 (14.71) | 0 (0.00) | 5 (29.41) | 0.044 |
| Number of Reported Infections | ||||
| 1 | 8 (47.06) | |||
| 2 | 5 (29.41) | |||
| 3 | 2 (11.76) | |||
| >3 | 2 (11.76) | |||
| Bacterial | 12 (70.59) | |||
| Infection Causing Treatment Delays/Interruptions | 11 (64.71) | |||
| Infection Resulting in Treatment Discontinuation | 7 (41.18) |
| OR (95% CI) | p-Value | |
|---|---|---|
| Age | 1.06 (0.97–1.1) | 0.178 |
| Sex | 5 | |
| Male | Ref | |
| Female | 0.43 (0.07, 2.76) | 0.376 |
| Race | ||
| White | Ref | |
| Other | 0.51 (0.10, 2.61) | 0.423 |
| Comorbidities | 1.00 (0.26, 3.92) | 1.000 |
| ECOG PS | ||
| 0 | Ref | |
| 1 | 0.83 (0.11, 6.11) | 0.858 |
| 2 | 1.00 (0.08, 12.56) | 1.000 |
| Unknown | 1.17 (0.17, 8.09) | 0.876 |
| History of Immunosuppressive Use | NA | |
| History of Solid or Hematopoietic Cell Transplant | 2.13 (0.17, 26.03) | 0.553 |
| Chronic Infection | 0.27 (0.06, 1.19) | 0.084 |
| Number of Cycles ICIs Given | 1.06 (0.97–1.15) | 0.178 |
| Line of Systemic Therapy | ||
| 1 | Ref | |
| 2 | 1.27 (0.21, 7.58) | 0.791 |
| >2 | NA | |
| Rifaximin at ICI Initiation | 3.43 (0.32, 36.83) | 0.309 |
| Was the Patient on Antibiotic | 1.94 (0.38, 9.88) | 0.423 |
| IrAE | 1.77 (0.40, 7.93) | 0.454 |
| Steroids for irAE | 0.67 (0.06, 6.87) | 0.733 |
| ED Visits | 1.77 (0.40, 7.93) | 0.454 |
| Inpatient Hospitalizations | 2.13 (0.52, 8.76) | 0.293 |
| ICU Admissions | NA |
| Death | PFS | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Infection | 0.93 (0.38–2.29) | 0.873 | 1.04 (0.46, 2.39) | 0.917 |
| Age | 1.04 (0.99–1.10) | 0.097 | 1.02 (0.97–1.07) | 0.460 |
| Sex | ||||
| Male | Ref | |||
| Female | 0.28 (0.04, 2.10) | 0.215 | 1.11 (0.33, 3.79) | 0.864 |
| Race | 0.69 | |||
| White | Ref | |||
| Other | 0.49 (0.14, 1.70) | 0.261 | 0.29 (0.08, 1.00) | 0.050 |
| Comorbidities | 1.31 (0.51, 3.35) | 0.568 | 1.12 (0.48, 2.59) | 0.795 |
| ECOG PS | 1.00 | |||
| 0 | Ref | Ref | ||
| 1 | 0.79 (0.22–2.86) | 0.725 | 0.39 (0.12, 1.33) | 0.134 |
| 2 | 0.39 (0.04–3.52) | 0.401 | 0.57 (0.11, 3.00) | 0.506 |
| 5 | 0.90 (0.27–3.03) | 0.868 | 0.77 (0.26, 2.30) | 0.644 |
| History of Immunosuppressive Use | 11.32 (1.87, 68.44) | 0.008 * | 3.64 (0.79, 16.70) | 0.097 |
| History of Solid or Hematopoietic Cell Transplant | 0.97 (0.22, 4.25) | 0.969 | 0.67 (0.16, 2.91) | 0.595 |
| Chronic Infection | 0.54 (0.22, 1.33) | 0.178 | 0.52 (0.23, 1.20) | 0.126 |
| Number of ICI Cycles Given | 0.88 (0.81–0.95) | 0.002 * | 0.85 (0.78–0.93) | <0.001 * |
| Line of Systemic Therapy | ||||
| 1 | Ref | Ref | ||
| 2 | 0.69 (0.20–2.43) | 0.567 | 0.89 (0.30, 2.66) | 0.838 |
| >2 | 1.29 (0.29–5.74) | 0.742 | 1.07 (0.25, 4.71) | 0.924 |
| Rifaximin at ICI Initiation | 2.24 (0.64, 7.80) | 0.204 | 1.32 (0.39, 4.45) | 0.656 |
| Antibiotic Use | 1.13 (0.40, 3.18) | 0.812 | 1.66 (0.68, 4.04) | 0.267 |
| IrAE | 0.66 (0.23, 1.89) | 0.438 | 0.69 (0.27, 1.76) | 0.439 |
| Steroids for irAE | 0.63 (0.13, 3.06) | 0.569 | 0.40 (0.08, 1.92) | 0.253 |
| ED Visits | 0.50 (0.17, 1.53) | 0.226 | 0.30 (0.10, 0.90) | 0.032 * |
| Inpatient Hospitalizations | 1.10 (0.42, 2.85) | 0.852 | 0.54 (0.24, 1.24) | 0.147 |
| ICU Admissions | 3.67 (1.24, 10.86) | 0.019 * | 1.79 (0.65, 4.95) | 0.260 |
| Death | 19 (55.88) | 9 (52.94) | 10 (58.82) | 1.0 |
| Disease Progressed | 10 (29.41) | 5 (29.41) | 5 (29.41) | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmail, A.; Xu, J.; Burns, E.A.; Abboud, K.; Sheikh, A.; Umoru, G.; Gee, K.; Wiechmann, C.; Zhang, Y.; Abdelrahim, M. The Impact of Infections in Patients Treated with Atezolizumab Plus Bevacizumab for Unresectable Hepatocellular Carcinoma. J. Clin. Med. 2024, 13, 4994. https://doi.org/10.3390/jcm13174994
Esmail A, Xu J, Burns EA, Abboud K, Sheikh A, Umoru G, Gee K, Wiechmann C, Zhang Y, Abdelrahim M. The Impact of Infections in Patients Treated with Atezolizumab Plus Bevacizumab for Unresectable Hepatocellular Carcinoma. Journal of Clinical Medicine. 2024; 13(17):4994. https://doi.org/10.3390/jcm13174994
Chicago/Turabian StyleEsmail, Abdullah, Jiaqiong Xu, Ethan A. Burns, Karen Abboud, Ali Sheikh, Godsfavour Umoru, Kelly Gee, Catherine Wiechmann, Yuqi Zhang, and Maen Abdelrahim. 2024. "The Impact of Infections in Patients Treated with Atezolizumab Plus Bevacizumab for Unresectable Hepatocellular Carcinoma" Journal of Clinical Medicine 13, no. 17: 4994. https://doi.org/10.3390/jcm13174994
APA StyleEsmail, A., Xu, J., Burns, E. A., Abboud, K., Sheikh, A., Umoru, G., Gee, K., Wiechmann, C., Zhang, Y., & Abdelrahim, M. (2024). The Impact of Infections in Patients Treated with Atezolizumab Plus Bevacizumab for Unresectable Hepatocellular Carcinoma. Journal of Clinical Medicine, 13(17), 4994. https://doi.org/10.3390/jcm13174994







