Sleep Disordered Breathing and Neurocognitive Disorders
Abstract
1. Introduction
2. Materials and Methods
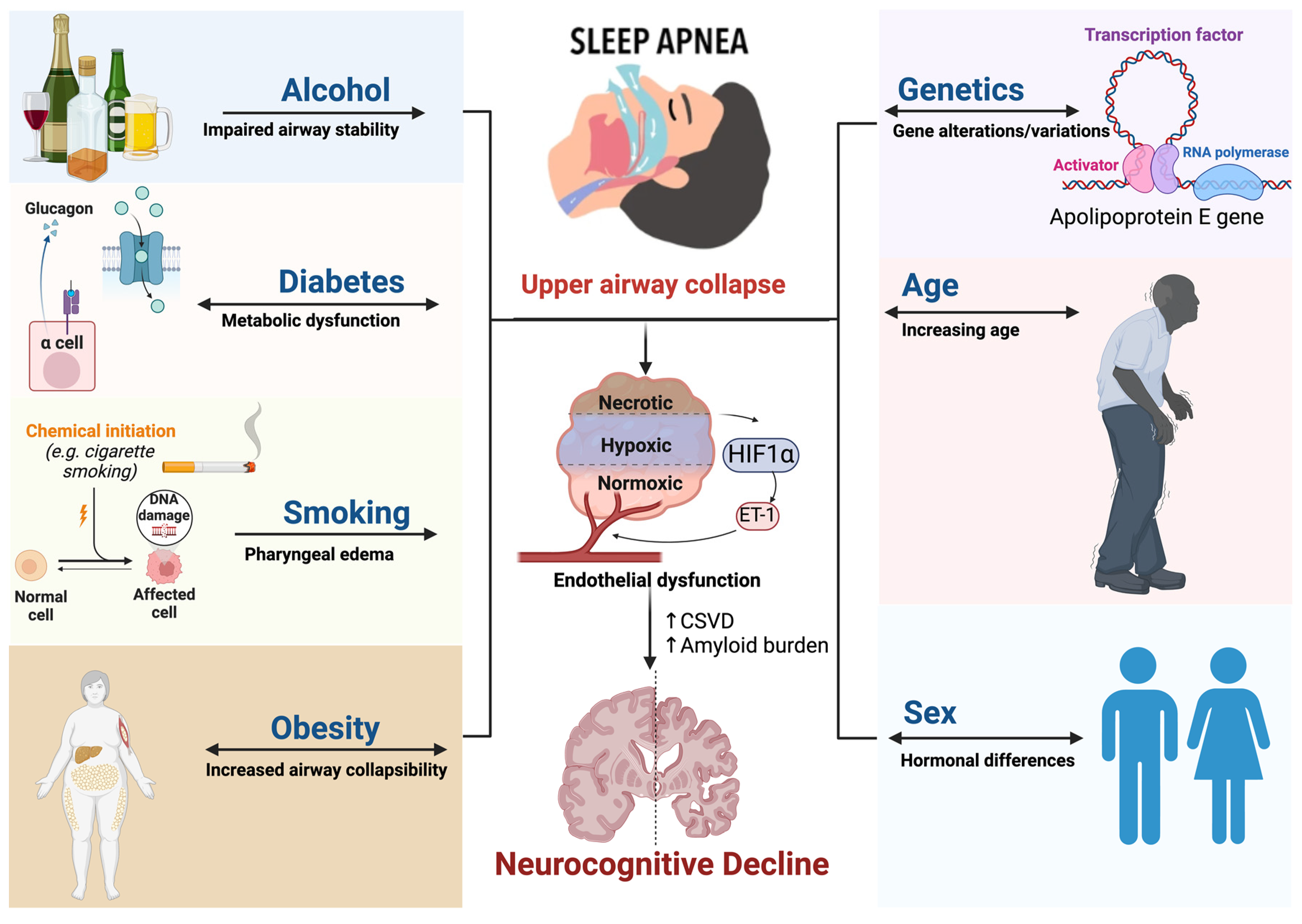
3. Epidemiology and Risk Factors
3.1. Genetics
3.2. Age
3.3. Sex
3.4. Alcohol
3.5. Smoking
3.6. Diabetes and Obesity
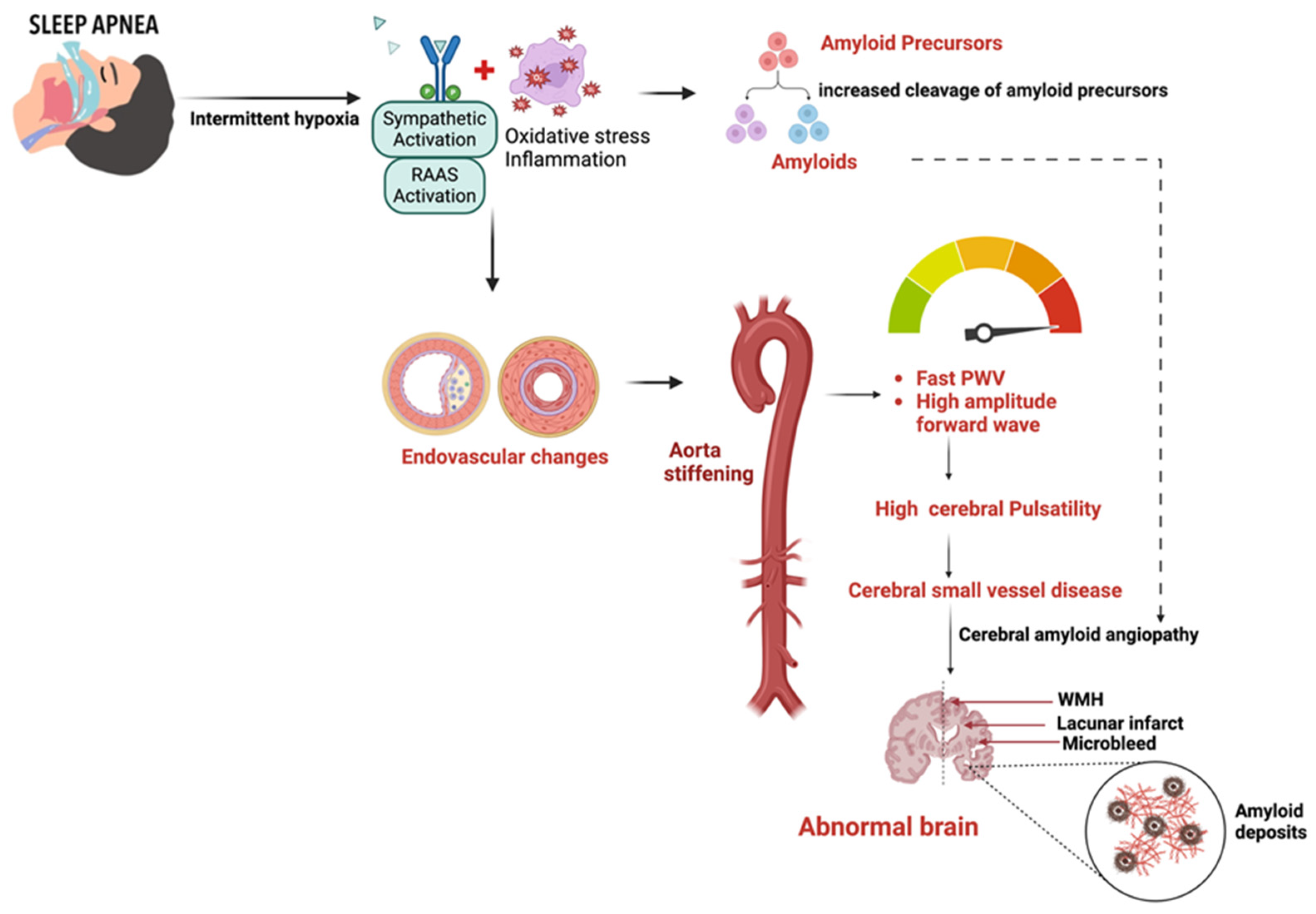
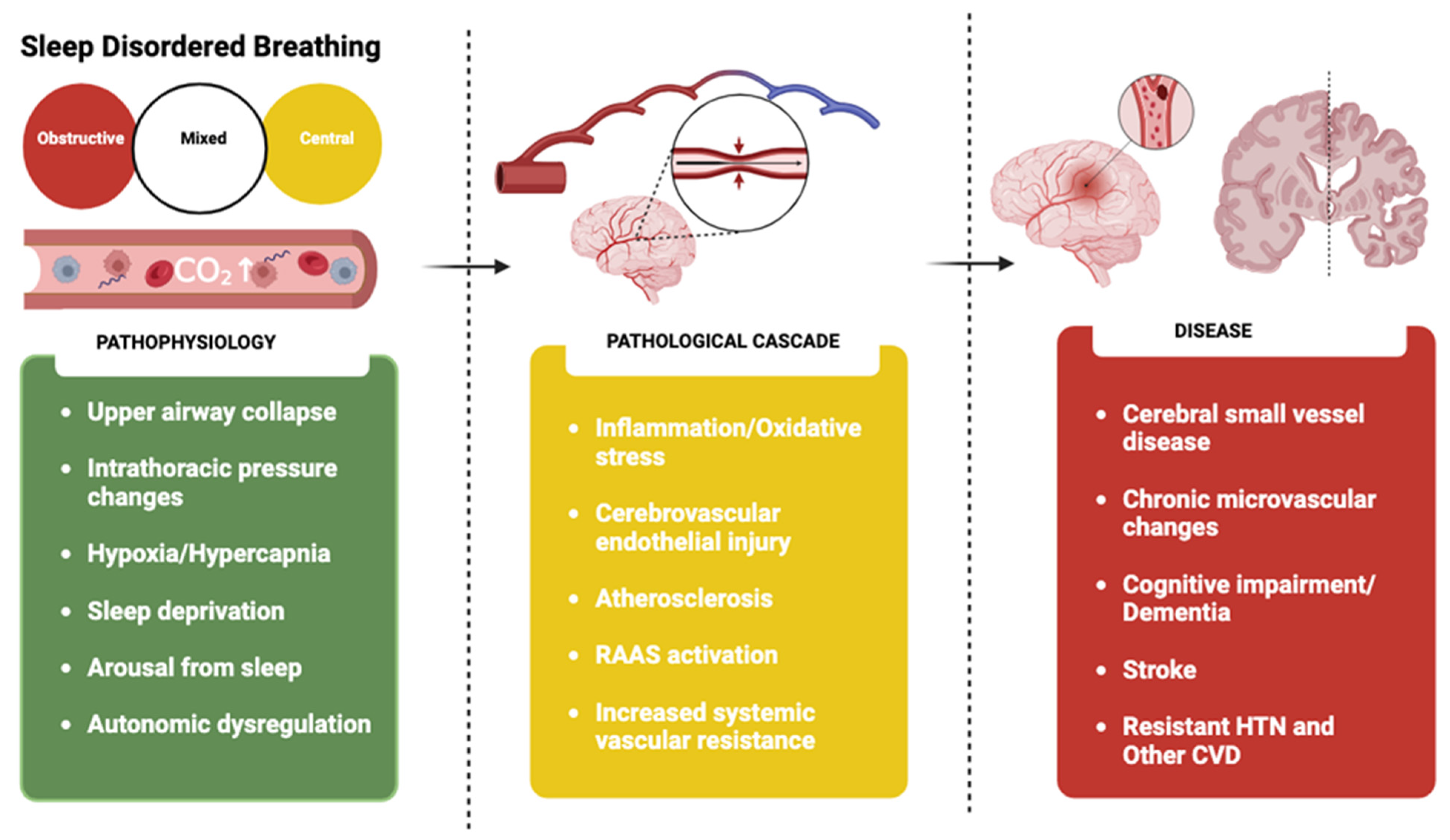
4. Pathophysiology
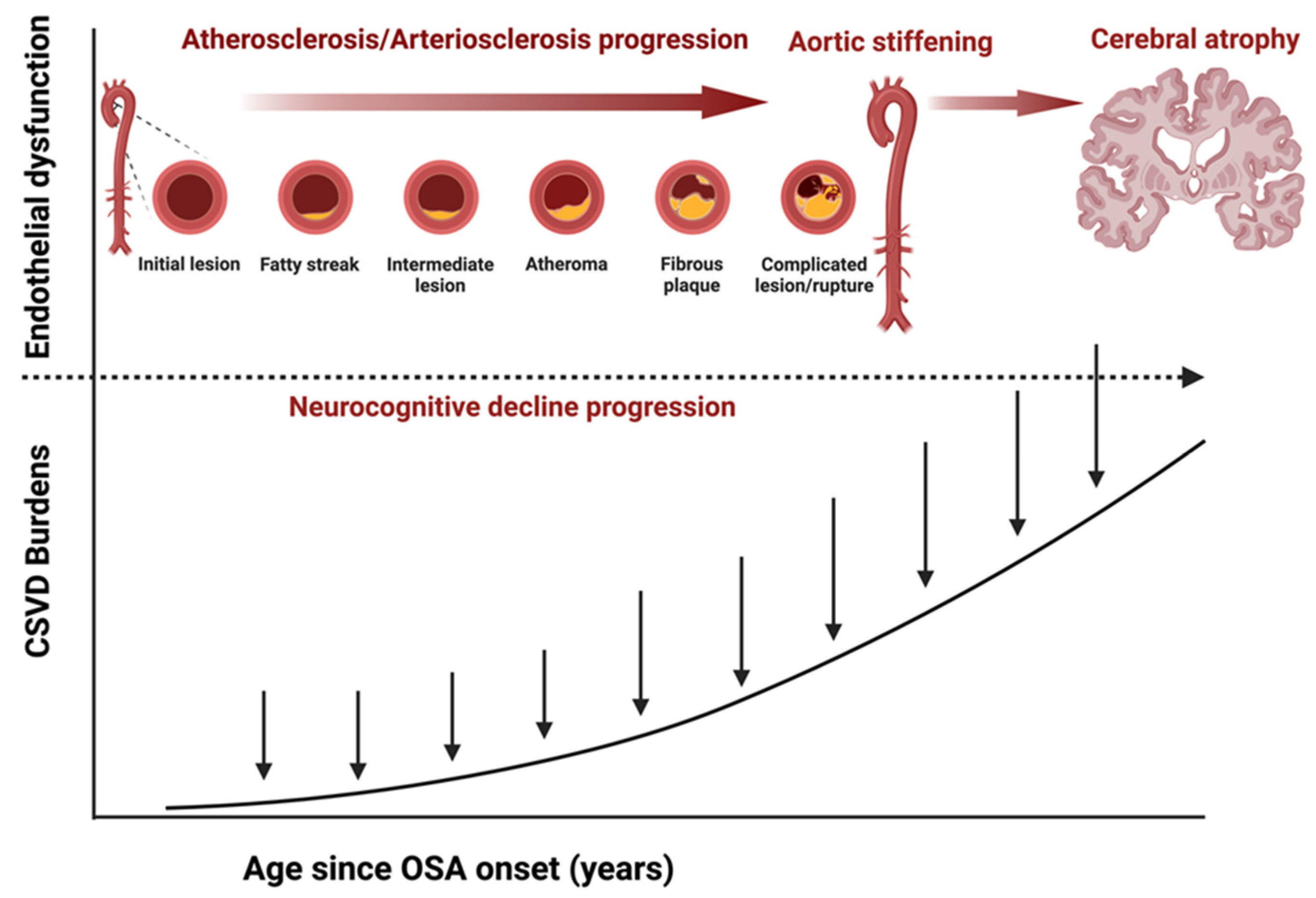
4.1. Role of Hypoxia and Endothelial Dysfunction
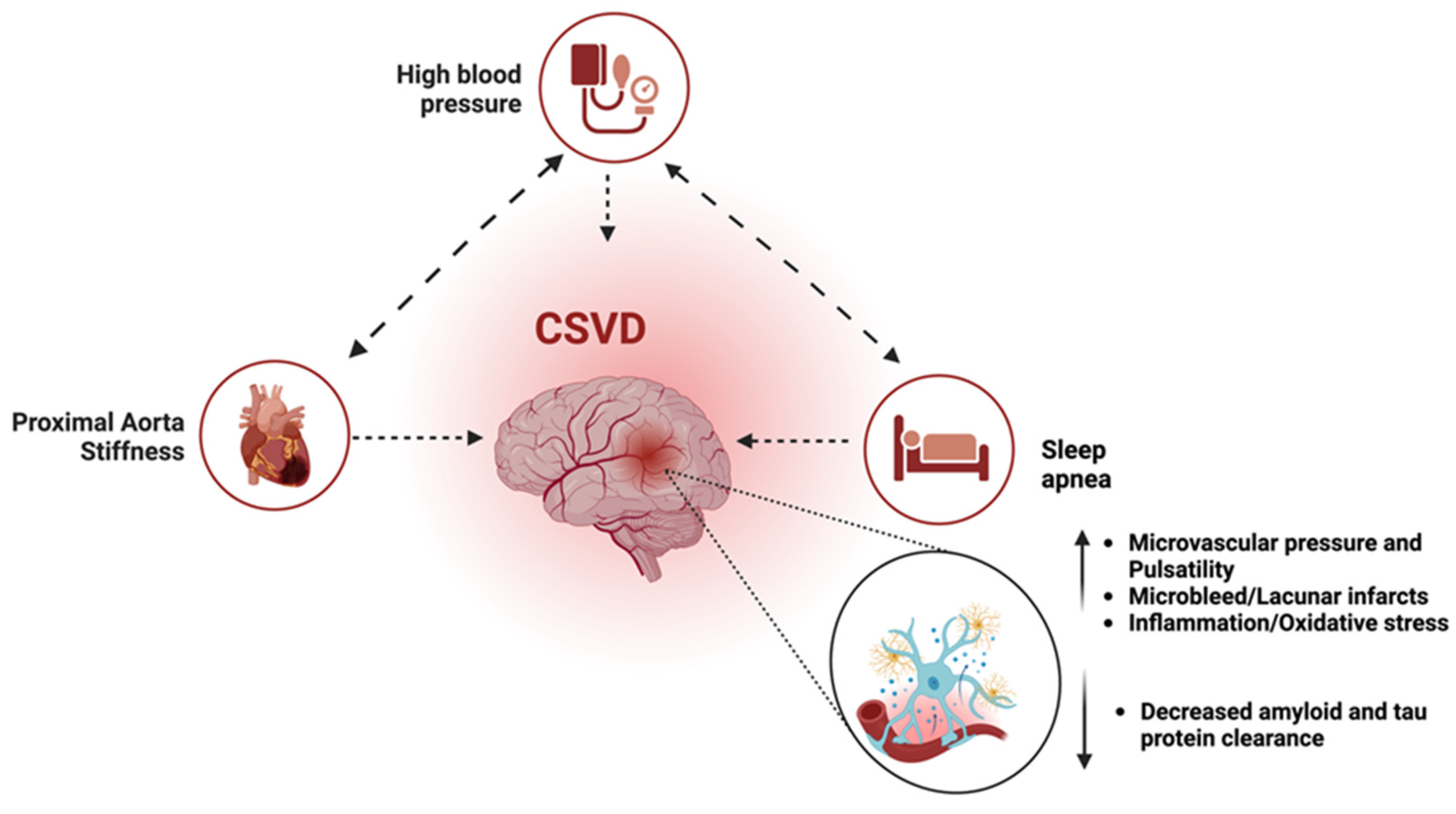
4.2. Role of Pulse Wave Velocity and Cerebral Pulsatility
4.3. Role of Cerebral Small Vessel Disease
| Authors | Year | Number of Studies | MCI/Dementia |
|---|---|---|---|
| Bubu [23] | 2017 | 27 | RR 1.68 (1.51–1.87) |
| Leng [37] | 2017 | 14 | RR 1.35 (1.11–1.65) |
| Shi [46] | 2018 | 18 | RR 1.19 (1.11–1.29) |
| Tian [70] | 2023 | 15 | HR 1.52 (1.32–1.74) |
| Guay-Gagnon [146] | 2022 | 11 | HR 1.43 (1.26–1.62) |
4.4. Role of Cerebrovascular Hemodynamics
4.5. Role of Cerebral Amyloid Microangiopathy
5. Imaging Marker of Early Cardiocerebrovascular Disease
6. Treatment
7. Future Directions
8. Conclusions
- OSA is an independent risk factor for neurocognitive abnormalities.
- OSA, hypertension, and arterial stiffness can create a triple-hit effect that contributes to the development of neurocognitive abnormalities.
- A heart-brain imaging protocol could identify markers of early signs of pathology in both organ systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Riha, R.L.; Gislasson, T.; Diefenbach, K. The Phenotype and Genotype of Adult Obstructive Sleep Apnoea/Hypopnoea Syndrome. Eur. Respir. J. 2009, 33, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Redline, S.; Kirchner, H.L.; Quan, S.F.; Gottlieb, D.J.; Kapur, V.; Newman, A. The Effects of Age, Sex, Ethnicity, and Sleep-Disordered Breathing on Sleep Architecture. Arch. Intern. Med. 2004, 164, 406–418. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, W.T.; Korkalainen, H. Translation of Obstructive Sleep Apnea Pathophysiology and Phenotypes to Personalized Treatment: A Narrative Review. Front. Neurol. 2023, 14, 1239016. [Google Scholar] [CrossRef]
- Bruyneel, M. Obstructive Sleep Apnea Phenotypes Eligible for Pharmacological Treatment. Front. Sleep 2023, 2, 1261276. [Google Scholar] [CrossRef]
- Cowie, M.R.; Linz, D.; Redline, S.; Somers, V.K.; Simonds, A.K. Sleep Disordered Breathing and Cardiovascular Disease. J. Am. Coll. Cardiol. 2021, 78, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.L.; Goldberg, A.N.; Alt, J.A.; Alzoubaidi, M.; Ashbrook, L.; Auckley, D.; Ayappa, I.; Bakhtiar, H.; Barrera, J.E.; Bartley, B.L.; et al. International Consensus Statement on Obstructive Sleep Apnea. Int. Forum Allergy Rhinol. 2023, 13, 1061–1482. [Google Scholar] [CrossRef]
- Feltner, C.; Wallace, I.F.; Aymes, S.; Cook Middleton, J.; Hicks, K.L.; Schwimmer, M.; Baker, C.; Balio, C.P.; Moore, D.; Voisin, C.E.; et al. Screening for Obstructive Sleep Apnea in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 328, 1951–1971. [Google Scholar] [CrossRef]
- Redline, S.; Foody, J. Sleep Disturbances: Time to Join the Top 10 Potentially Modifiable Cardiovascular Risk Factors? Circulation 2011, 124, 2049–2051. [Google Scholar] [CrossRef]
- André, C.; Rehel, S.; Kuhn, E.; Landeau, B.; Moulinet, I.; Touron, E.; Ourry, V.; Le Du, G.; Mézenge, F.; Tomadesso, C.; et al. Association of Sleep-Disordered Breathing with Alzheimer Disease Biomarkers in Community-Dwelling Older Adults: A Secondary Analysis of a Randomized Clinical Trial. JAMA Neurol. 2020, 77, 716–724. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of Obstructive Sleep Apnea in the General Population: A Systematic Review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Osorio, R.S.; Gumb, T.; Pirraglia, E.; Varga, A.W.; Lu, S.; Lim, J.; Wohlleber, M.E.; Ducca, E.L.; Koushyk, V.; Glodzik, L.; et al. Sleep-Disordered Breathing Advances Cognitive Decline in the Elderly. Neurology 2015, 84, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.; Pepin, J.-L.; Arnaud, C.; Tamisier, R.; Borel, J.-C.; Dematteis, M.; Godin-Ribuot, D.; Ribuot, C. Intermittent Hypoxia and Sleep-Disordered Breathing: Current Concepts and Perspectives. Eur. Respir. J. 2008, 32, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L. Intermittent Hypoxia and Obstructive Sleep Apnea: Mechanisms, Interindividual Responses and Clinical Insights. In Atherosclerosis, Arteriosclerosis and Arteriolosclerosis; Gianturco, L., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-83880-303-2. [Google Scholar]
- Durgan, D.J.; Bryan, R.M. Cerebrovascular Consequences of Obstructive Sleep Apnea. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2012, 1, e000091. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhao, Z.; Qin, S.; Cheng, M.; Wang, Y.; Li, M.; Jia, P.; Li, J.; Yu, H. Elucidating the Association of Obstructive Sleep Apnea with Brain Structure and Cognitive Performance. BMC Psychiatry 2024, 24, 338. [Google Scholar] [CrossRef]
- Dong, J.; Yu, X.; Wang, Y.; Zhang, H.; Guo, R. Obstructive Sleep Apnea and Cognition: Insights Gleaned from Bibliometric Analysis. Front. Psychiatry 2023, 14, 1259251. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, D.; Liu, K.; Weng, J.; Guan, Y.; Chan, K.C.C.; Chu, W.C.W.; Shi, L. Brain Structure Network Analysis in Patients with Obstructive Sleep Apnea. PLoS ONE 2015, 10, e0139055. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K.; et al. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, 3. [Google Scholar] [CrossRef]
- Ihara, M.; Yamamoto, Y. Emerging Evidence for Pathogenesis of Sporadic Cerebral Small Vessel Disease. Stroke 2016, 47, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Handa, A. Consequences of Obstructive Sleep Apnea-Hypopnea Syndrome. In Medicine Update; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2007; Volume 17, p. 768. ISBN 978-81-8448-038-2. [Google Scholar]
- Ferini-Strambi, L. Sleep Disorders and Increased Risk of Dementia. Eur. J. Neurol. 2022, 29, 3484–3485. [Google Scholar] [CrossRef]
- Liguori, C.; Mercuri, N.B.; Izzi, F.; Romigi, A.; Cordella, A.; Sancesario, G.; Placidi, F. Obstructive Sleep Apnea Is Associated with Early but Possibly Modifiable Alzheimer’s Disease Biomarkers Changes. Sleep 2017, 40, zsx011. [Google Scholar] [CrossRef]
- Bubu, O.M.; Brannick, M.; Mortimer, J.; Umasabor-Bubu, O.; Sebastião, Y.V.; Wen, Y.; Schwartz, S.; Borenstein, A.R.; Wu, Y.; Morgan, D.; et al. Sleep, Cognitive Impairment, and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Sleep 2017, 40, zsw032. [Google Scholar] [CrossRef]
- Payne, R.A.; Wilkinson, I.B.; Webb, D.J. Arterial Stiffness and Hypertension: Emerging Concepts. Hypertension 2010, 55, 9–14. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Safar, M.E. Relationship between Aortic Stiffening and Microvascular Disease in Brain and Kidney. Hypertension 2005, 46, 200–204. Available online: https://www.ahajournals.org/doi/epub/10.1161/01.HYP.0000168052.00426.65 (accessed on 14 June 2024). [CrossRef] [PubMed]
- Drager, L.F.; Bortolotto, L.A.; Figueiredo, A.C.; Silva, B.C.; Krieger, E.M.; Lorenzi-Filho, G. Obstructive Sleep Apnea, Hypertension, and Their Interaction on Arterial Stiffness and Heart Remodeling. CHEST 2007, 131, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Sachdev, P.S. Extent and Distribution of White Matter Hyperintensities in Stroke Patients. Stroke 2004, 35, 2813–2819. Available online: https://www.ahajournals.org/doi/epub/10.1161/01.STR.0000147034.25760.3d (accessed on 14 June 2024). [CrossRef]
- Birnefeld, J.; Wåhlin, A.; Eklund, A.; Malm, J. Cerebral Arterial Pulsatility Is Associated with Features of Small Vessel Disease in Patients with Acute Stroke and TIA: A 4D Flow MRI Study. J. Neurol. 2020, 267, 721–730. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small Vessel Disease: Mechanisms and Clinical Implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef]
- Zacharias, H.U.; Weihs, A.; Habes, M.; Wittfeld, K.; Frenzel, S.; Rashid, T.; Stubbe, B.; Obst, A.; Szentkirályi, A.; Bülow, R.; et al. Association between Obstructive Sleep Apnea and Brain White Matter Hyperintensities in a Population-Based Cohort in Germany. JAMA Netw. Open 2021, 4, e2128225. [Google Scholar] [CrossRef]
- Staals, J.; Makin, S.D.J.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke Subtype, Vascular Risk Factors, and Total MRI Brain Small-Vessel Disease Burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- DiCaro, M.V.; Lei, K.; Yee, B.; Tak, T. The Effects of Obstructive Sleep Apnea on the Cardiovascular System: A Comprehensive Review. J. Clin. Med. 2024, 13, 3223. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; McEvoy, R.D.; Barbe, F.; Lorenzi-Filho, G.; Redline, S. Sleep Apnea and Cardiovascular Disease: Lessons from Recent Trials and Need for Team Science. Circulation 2017, 136, 1840–1850. [Google Scholar] [CrossRef]
- WHO. Available online: https://www.Who.Int/News-Room/Fact-Sheets/Detail/Dementia (accessed on 1 July 2024).
- Aini, N.; Chu, H.; Banda, K.J.; Chen, R.; Lee, T.-Y.; Pien, L.-C.; Liu, D.; Lai, Y.-J.; Kang, X.L.; Chou, K.-R. Prevalence of Sleep-Related Breathing Disorders and Associated Risk Factors among People with Dementia: A Meta-Analysis. Sleep Med. 2023, 103, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; McEvoy, C.T.; Allen, I.E.; Yaffe, K. Association of Sleep-Disordered Breathing with Cognitive Function and Risk of Cognitive Impairment: A Systematic Review and Meta-Analysis. JAMA Neurol. 2017, 74, 1237–1245. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Y. Sleep-Disordered Breathing and the Risk of Cognitive Decline: A Meta-Analysis of 19,940 Participants. Sleep Breath. 2018, 22, 165–173. [Google Scholar] [CrossRef]
- Stranks, E.K.; Crowe, S.F. The Cognitive Effects of Obstructive Sleep Apnea: An Updated Meta-Analysis. Arch. Clin. Neuropsychol. 2016, 31, 186–193. [Google Scholar] [CrossRef]
- Ma, J.; Qiu, X.; Sun, L.; Cong, N.; Wei, Y.; Wei, C.; Huang, J. Utility of the Psychomotor Vigilance Task in Screening for Obstructive Sleep Apnoea. Eur. Arch. Otorhinolaryngol. 2024, 281, 3115–3123. [Google Scholar] [CrossRef]
- Kushida, C.A. (Ed.) Obstructive Sleep Apnea: Pathophysiology, Comorbidities and Consequences: Pathophysiology, Comorbidities, and Consequences; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-429-11561-5. [Google Scholar]
- Gottesman, R.F.; Lutsey, P.L.; Benveniste, H.; Brown, D.L.; Full, K.M.; Lee, J.-M.; Osorio, R.S.; Pase, M.P.; Redeker, N.S.; Redline, S.; et al. Impact of Sleep Disorders and Disturbed Sleep on Brain Health: A Scientific Statement from the American Heart Association. Stroke 2024, 55, 3. Available online: https://www.ahajournals.org/doi/epub/10.1161/STR.0000000000000453 (accessed on 8 June 2024). [CrossRef] [PubMed]
- Lajoie, A.C.; Lafontaine, A.-L.; Kimoff, R.J.; Kaminska, M. Obstructive Sleep Apnea in Neurodegenerative Disorders: Current Evidence in Support of Benefit from Sleep Apnea Treatment. J. Clin. Med. 2020, 9, 297. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Castillo, P.R.; Del Brutto, O.H.; De La Luz Andrade, M.; Zambrano, M.; Nader, J.A. The Association of Sleep-Disordered Breathing with High Cerebral Pulsatility Might Not Be Related to Diffuse Small Vessel Disease. A Pilot Study. BMC Res. Notes 2015, 8, 500. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, S.-J.; Ma, M.-Y.; Bao, Y.-P.; Han, Y.; Wang, Y.-M.; Shi, J.; Vitiello, M.V.; Lu, L. Sleep Disturbances Increase the Risk of Dementia: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2018, 40, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.R.; Cabral, D.; Lee, D.J.; Sacco, R.L.; Rundek, T. Cerebrovascular Pulsatility in Patients with Sleep-Disordered Breathing. Sleep Breath. 2013, 17, 723–726. [Google Scholar] [CrossRef][Green Version]
- Weihs, A.; Frenzel, S.; Grabe, H.J. The Link between Obstructive Sleep Apnoea and Neurodegeneration and Cognition. Curr. Sleep Med. Rep. 2021, 7, 87–96. [Google Scholar] [CrossRef]
- Han, S.-L.; Liu, D.-C.; Tan, C.-C.; Tan, L.; Xu, W. Male- and Female-Specific Reproductive Risk Factors across the Lifespan for Dementia or Cognitive Decline: A Systematic Review and Meta-Analysis. BMC Med. 2023, 21, 457. [Google Scholar] [CrossRef]
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-Israel, S.; Stone, K.L. Sleep Disordered Breathing, Hypoxia, and Risk of Mild Cognitive Impairment and Dementia in Older Women. JAMA J. Am. Med. Assoc. 2011, 306, 613–619. [Google Scholar] [CrossRef]
- Strenth, C.; Wani, A.; Alla, R.; Khan, S.; Schneider, F.D.; Thakur, B. Obstructive Sleep Apnea and Its Cardiac Implications in the United States: An Age-Stratified Analysis between Young and Older Adults. J. Am. Heart Assoc. 2024, 13, e033810. [Google Scholar] [CrossRef]
- Cao, X.; Wang, M.; Zhou, M.; Mi, Y.; Fazekas-Pongor, V.; Major, D.; Lehoczki, A.; Guo, Y. Trends in Prevalence, Mortality, and Risk Factors of Dementia among the Oldest-Old Adults in the United States: The Role of the Obesity Epidemic. GeroScience 2024, 44, 1–18. [Google Scholar] [CrossRef]
- Machado-Fragua, M.D.; Sabia, S.; Fayosse, A.; Hassen, C.B.; Van Der Heide, F.; Kivimaki, M.; Singh-Manoux, A. Is Metabolic-Healthy Obesity Associated with Risk of Dementia? An Age-Stratified Analysis of the Whitehall II Cohort Study. BMC Med. 2023, 21, 436. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum Inflammatory Markers in Obstructive Sleep Apnea: A Meta-Analysis. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Hennessy, E. Co-Morbidity and Systemic Inflammation as Drivers of Cognitive Decline: New Experimental Models Adopting a Broader Paradigm in Dementia Research. Alzheimer’s Res. Ther. 2015, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Seicean, S.; Kirchner, H.L.; Gottlieb, D.J.; Punjabi, N.M.; Resnick, H.; Sanders, M.; Budhiraja, R.; Singer, M.; Redline, S. Sleep-Disordered Breathing and Impaired Glucose Metabolism in Normal-Weight and Overweight/Obese Individuals. Diabetes Care 2008, 31, 1001–1006. [Google Scholar] [CrossRef]
- Subramanian, A.; Adderley, N.J.; Tracy, A.; Taverner, T.; Hanif, W.; Toulis, K.A.; Thomas, G.N.; Tahrani, A.A.; Nirantharakumar, K. Risk of Incident Obstructive Sleep Apnea among Patients with Type 2 Diabetes. Diabetes Care 2019, 42, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Rashtchian, A.; Etemadi, M.H.; Asadi, E.; Binaei, S.; Abbasi, M.; Bayani, M.; Izadi, E.; Sadat-Madani, S.-F.; Naziri, M.; Khoshravesh, S.; et al. Diabetes Mellitus and Risk of Incident Dementia in APOE ε4 Carriers: An Updated Meta-Analysis. BMC Neurosci. 2024, 25, 28. [Google Scholar] [CrossRef]
- Barbiellini Amidei, C.; Fayosse, A.; Dumurgier, J.; Machado-Fragua, M.D.; Tabak, A.G.; Van Sloten, T.; Kivimäki, M.; Dugravot, A.; Sabia, S.; Singh-Manoux, A. Association between Age at Diabetes Onset and Subsequent Risk of Dementia. JAMA 2021, 325, 1640. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.; Gao, M.; Zhang, F.; Gu, C.; Yu, Y.; Wei, Y. Impact of Obstructive Sleep Apnea Syndrome on Endothelial Function, Arterial Stiffening, and Serum Inflammatory Markers: An Updated Meta-analysis and Metaregression of 18 Studies. J. Am. Heart Assoc. 2015, 4, e002454. [Google Scholar] [CrossRef]
- Holmes, C.; Cunningham, C.; Zotova, E.; Woolford, J.; Dean, C.; Kerr, S.; Culliford, D.; Perry, V.H. Systemic Inflammation and Disease Progression in Alzheimer Disease. Neurology 2009, 73, 768–774. [Google Scholar] [CrossRef]
- Peppard, P.E. Longitudinal Study of Moderate Weight Change and Sleep-Disordered Breathing. JAMA 2000, 284, 3015. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Taheri, S. Excess Weight and Sleep-Disordered Breathing. J. Appl. Physiol. 2005, 99, 1592–1599. [Google Scholar] [CrossRef]
- Lee, M.; Whitsel, E.; Avery, C.; Hughes, T.M.; Griswold, M.E.; Sedaghat, S.; Gottesman, R.F.; Mosley, T.H.; Heiss, G.; Lutsey, P.L. Variation in Population Attributable Fraction of Dementia Associated with Potentially Modifiable Risk Factors by Race and Ethnicity in the US. JAMA Netw. Open 2022, 5, e2219672. [Google Scholar] [CrossRef] [PubMed]
- Varvarigou, V.; Dahabreh, I.J.; Malhotra, A.; Kales, S.N. A Review of Genetic Association Studies of Obstructive Sleep Apnea: Field Synopsis and Meta-Analysis. Sleep 2011, 34, 1461–1468. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; DeStefano, A.L.; Foley, D.J.; Mignot, E.; Redline, S.; Givelber, R.J.; Young, T. APOE ε4 Is Associated with Obstructive Sleep Apnea/Hypopnea. Neurology 2004, 63, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Belloy, M.E.; Andrews, S.J.; Le Guen, Y.; Cuccaro, M.; Farrer, L.A.; Napolioni, V.; Greicius, M.D. APOE Genotype and Alzheimer Disease Risk Across Age, Sex, and Population Ancestry. JAMA Neurol. 2023, 80, 1284. [Google Scholar] [CrossRef] [PubMed]
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Gläser, S.; Glos, M.; Schmidt, C.O.; Stubbe, B.; et al. Prevalence and Association Analysis of Obstructive Sleep Apnea with Sex and Age Differences—Results of SHIP-Trend. J. Sleep Res. 2019, 28, e12770. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Sun, J.; Li, X.; Liu, J.; Zhou, H.; Deng, J.; Li, J. Association between Sleep Apnoea and Risk of Cognitive Impairment and Alzheimer’s Disease: A Meta-Analysis of Cohort-Based Studies. Sleep Breath. 2024, 28, 585–595. [Google Scholar] [CrossRef]
- Spira, A.P.; Blackwell, T.; Stone, K.L.; Redline, S.; Cauley, J.A.; Ancoli-Israel, S.; Yaffe, K. Sleep-Disordered Breathing and Cognition in Older Women. J. Am. Geriatr. Soc. 2008, 56, 45–50. [Google Scholar] [CrossRef]
- Gong, J.; Harris, K.; Lipnicki, D.M.; Castro-Costa, E.; Lima-Costa, M.F.; Diniz, B.S.; Xiao, S.; Lipton, R.B.; Katz, M.J.; Wang, C.; et al. Sex Differences in Dementia Risk and Risk Factors: Individual-participant Data Analysis Using 21 Cohorts across Six Continents from the COSMIC Consortium. Alzheimer’s Dement. 2023, 19, 3365–3378. [Google Scholar] [CrossRef]
- Yang, S.; Guo, X.; Liu, W.; Li, Y.; Liu, Y. Alcohol as an Independent Risk Factor for Obstructive Sleep Apnea. Ir. J. Med. Sci. 1971 2022, 191, 1325–1330. [Google Scholar] [CrossRef]
- Kashyap, R.; Bowman, T.J. Higher Prevalence of Smoking in Patients Diagnosed as Having Obstructive Sleep Apnea. Sleep Breath. 2001, 5, 167–172. [Google Scholar] [CrossRef]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; Dugravot, A.; Akbaraly, T.; Britton, A.; Kivimäki, M.; Singh-Manoux, A. Alcohol Consumption and Risk of Dementia: 23 Year Follow-up of Whitehall II Cohort Study. BMJ 2018, 362, k2927. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Fitzpatrick, A.L.; Rapp, S.R.; Nahin, R.L.; Williamson, J.D.; Lopez, O.L.; DeKosky, S.T.; Kuller, L.H.; Mackey, R.H.; Mukamal, K.J.; et al. Alcohol Consumption and Risk of Dementia and Cognitive Decline among Older Adults with or without Mild Cognitive Impairment. JAMA Netw. Open 2019, 2, e1910319. [Google Scholar] [CrossRef] [PubMed]
- Rusanen, M.; Kivipelto, M.; Quesenberry, C.P.; Zhou, J.; Whitmer, R.A. Heavy Smoking in Midlife and Long-Term Risk of Alzheimer Disease and Vascular Dementia. Arch. Intern. Med. 2011, 171, 333. [Google Scholar] [CrossRef]
- Taveira, K.V.M.; Kuntze, M.M.; Berretta, F.; De Souza, B.D.M.; Godolfim, L.R.; Demathe, T.; De Luca Canto, G.; Porporatti, A.L. Association between Obstructive Sleep Apnea and Alcohol, Caffeine and Tobacco: A Meta-analysis. J. Oral Rehabil. 2018, 45, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.; Strange, C.; Bachman, D. Neurocognitive Impairment in Obstructive Sleep Apnea. Chest 2012, 141, 1601–1610. [Google Scholar] [CrossRef]
- Lambert, G.W.; Straznicky, N.E.; Lambert, E.A.; Dixon, J.B.; Schlaich, M.P. Sympathetic Nervous Activation in Obesity and the Metabolic Syndrome—Causes, Consequences and Therapeutic Implications. Pharmacol. Ther. 2010, 126, 159–172. [Google Scholar] [CrossRef]
- McMillan, A.; Morrell, M.J. Sleep Disordered Breathing at the Extremes of Age: The Elderly. Breathe 2016, 12, 50–60. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Yang, D.; Luo, G.; Chen, S.; Le, W. Hypoxia Increases Aβ Generation by Altering β- and γ-Cleavage of APP. Neurobiol. Aging 2009, 30, 1091–1098. [Google Scholar] [CrossRef]
- Raulin, A.-C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.-C. ApoE in Alzheimer’s Disease: Pathophysiology and Therapeutic Strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef]
- Narasimhan, S.; Holtzman, D.M.; Apostolova, L.G.; Cruchaga, C.; Masters, C.L.; Hardy, J.; Villemagne, V.L.; Bell, J.; Cho, M.; Hampel, H. Apolipoprotein E in Alzheimer’s Disease Trajectories and the next-Generation Clinical Care Pathway. Nat. Neurosci. 2024, 27, 1236–1252. [Google Scholar] [CrossRef]
- Himali, J.J.; Baril, A.-A.; Cavuoto, M.G.; Yiallourou, S.; Wiedner, C.D.; Himali, D.; DeCarli, C.; Redline, S.; Beiser, A.S.; Seshadri, S.; et al. Association between Slow-Wave Sleep Loss and Incident Dementia. JAMA Neurol. 2023, 80, 1326–1333. [Google Scholar] [CrossRef]
- Tranah, G.J.; Yaffe, K.; Nievergelt, C.M.; Parimi, N.; Glymour, M.M.; Ensrud, K.E.; Cauley, J.A.; Ancoli-Israel, S.; Mariani, S.; Redline, S.; et al. APOEε4 and Slow Wave Sleep in Older Adults. PLoS ONE 2018, 13, e0191281. [Google Scholar] [CrossRef]
- Legault, J.; Thompson, C.; Martineau-Dussault, M.-È.; André, C.; Baril, A.-A.; Martinez Villar, G.; Carrier, J.; Gosselin, N. Obstructive Sleep Apnea and Cognitive Decline: A Review of Potential Vulnerability and Protective Factors. Brain Sci. 2021, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Blackman, J.; Love, S.; Sinclair, L.; Cain, R.; Coulthard, E. APOE Ε4, Alzheimer’s Disease Neuropathology and Sleep Disturbance, in Individuals with and without Dementia. Alzheimer’s Res. Ther. 2022, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Nikodemova, M.; Finn, L.; Mignot, E.; Salzieder, N.; Peppard, P.E. Association of Sleep Disordered Breathing and Cognitive Deficit in APOE Ε4 Carriers. Sleep 2013, 36, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Won, C.H.J.; Reid, M.; Sofer, T.; Azarbarzin, A.; Purcell, S.; White, D.; Wellman, A.; Sands, S.; Redline, S. Sex Differences in Obstructive Sleep Apnea Phenotypes, the Multi-Ethnic Study of Atherosclerosis. Sleep 2019, 43, zsz274. [Google Scholar] [CrossRef]
- Ludwig, K.; Malatantis-Ewert, S.; Huppertz, T.; Bahr-Hamm, K.; Seifen, C.; Pordzik, J.; Matthias, C.; Simon, P.; Gouveris, H. Central Apneic Event Prevalence in REM and NREM Sleep in OSA Patients: A Retrospective, Exploratory Study. Biology 2023, 12, 298. [Google Scholar] [CrossRef]
- Alotair, H.; BaHammam, A. Sex Differences in Saudi Patients with Obstructive Sleep Apnea. Sleep Breath. 2008, 12, 323–329. [Google Scholar] [CrossRef]
- Wimms, A.; Woehrle, H.; Ketheeswaran, S.; Ramanan, D.; Armitstead, J. Obstructive Sleep Apnea in Women: Specific Issues and Interventions. BioMed Res. Int. 2016, 2016, 1764837. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Mazzuca, E.; Baiamonte, P.; Bouckaert, B.; Verbeke, W.; Pevernagie, D.A. REM Sleep Obstructive Sleep Apnoea. Eur. Respir. Rev. 2024, 33, 230166. [Google Scholar] [CrossRef]
- Zaw, M.; Hein, L.; Martinez, A.C.; Ascher, K.B.; Abreu, A.R.; Chediak, A.D. Sex Differences in Sleep Disordered Breathing—A Review of Literature. Curr. Pulmonol. Rep. 2021, 10, 121–128. [Google Scholar] [CrossRef]
- Guadagni, V.; Pun, M. Untangling Sex Differences in Obstructive Sleep Apnea: A Significant Step toward Precision Medicine. Sleep 2020, 43, zsaa022. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Bhuiyan, A.R.; Jones, E.A. Association and Risk Factors for Obstructive Sleep Apnea and Cardiovascular Diseases: A Systematic Review. Diseases 2021, 9, 88. [Google Scholar] [CrossRef]
- Esen, A.D.; Akpinar, M. Relevance of Obstructive Sleep Apnea and Smoking: Obstructive Sleep Apnea and Smoking. Fam. Pract. 2021, 38, 180–185. [Google Scholar] [CrossRef]
- Pataka, A.; Kotoulas, S.; Kalamaras, G.; Tzinas, A.; Grigoriou, I.; Kasnaki, N.; Argyropoulou, P. Does Smoking Affect OSA? What about Smoking Cessation? J. Clin. Med. 2022, 11, 5164. [Google Scholar] [CrossRef]
- Zeng, X.; Ren, Y.; Wu, K.; Yang, Q.; Zhang, S.; Wang, D.; Luo, Y.; Zhang, N. Association between Smoking Behavior and Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Nicotine Tob. Res. 2023, 25, 364–371. [Google Scholar] [CrossRef]
- Krishnan, V.; Patel, S.R. Sleep Apnea and Obesity. In Sleep Loss and Obesity; Shiromani, P., Horvath, T., Redline, S., Van Cauter, E., Eds.; Springer New York: New York, NY, USA, 2012; pp. 119–131. ISBN 978-1-4614-3491-7. [Google Scholar]
- Doumit, J.; Prasad, B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectr. 2016, 29, 14–19. [Google Scholar] [CrossRef]
- Tasali, E.; Mokhlesi, B.; Van Cauter, E. Obstructive Sleep Apnea and Type 2 Diabetes. Chest 2008, 133, 496–506. [Google Scholar] [CrossRef]
- Foster, G.D.; Sanders, M.H.; Millman, R.; Zammit, G.; Borradaile, K.E.; Newman, A.B.; Wadden, T.A.; Kelley, D.; Wing, R.R.; Pi Sunyer, F.X.; et al. Obstructive Sleep Apnea among Obese Patients with Type 2 Diabetes. Diabetes Care 2009, 32, 1017–1019. [Google Scholar] [CrossRef]
- Andayeshgar, B.; Janatolmakan, M.; Soroush, A.; Azizi, S.M.; Khatony, A. The Prevalence of Obstructive Sleep Apnea in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Sleep Sci. Pract. 2022, 6, 6. [Google Scholar] [CrossRef]
- Pase, M.P.; Harrison, S.; Misialek, J.R.; Kline, C.E.; Cavuoto, M.; Baril, A.-A.; Yiallourou, S.; Bisson, A.; Himali, D.; Leng, Y.; et al. Sleep Architecture, Obstructive Sleep Apnea, and Cognitive Function in Adults. JAMA Netw. Open 2023, 6, e2325152. [Google Scholar] [CrossRef]
- Seda, G.; Matwiyoff, G.; Parrish, J.S. Effects of Obstructive Sleep Apnea and CPAP on Cognitive Function. Curr. Neurol. Neurosci. Rep. 2021, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Labarca, G.; Schmidt, A.; Dreyse, J.; Jorquera, J.; Enos, D.; Torres, G.; Barbe, F. Efficacy of Continuous Positive Airway Pressure (CPAP) in Patients with Obstructive Sleep Apnea (OSA) and Resistant Hypertension (RH): Systematic Review and Meta-Analysis. Sleep Med. Rev. 2021, 58, 101446. [Google Scholar] [CrossRef] [PubMed]
- Dakterzada, F.; Benítez, I.D.; Targa, A.; Carnes, A.; Pujol, M.; Jové, M.; Mínguez, O.; Vaca, R.; Sánchez-de-la-Torre, M.; Barbé, F.; et al. Blood-Based Lipidomic Signature of Severe Obstructive Sleep Apnoea in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2022, 14, 163. [Google Scholar] [CrossRef]
- Yadav, R.; France, M.; Aghamohammadzadeh, R.; Liu, Y.; Hama, S.; Kwok, S.; Schofield, J.; Turkington, P.; Syed, A.A.; Malik, R.; et al. Impairment of High-Density Lipoprotein Resistance to Lipid Peroxidation and Adipose Tissue Inflammation in Obesity Complicated by Obstructive Sleep Apnea. J. Clin. Endocrinol. Metab. 2014, 99, 3390–3398. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Dong, N.; Wang, X.; Lv, R.; Yu, Q.; Yue, H. Obstructive Sleep Apnea Affects Cognition: Dual Effects of Intermittent Hypoxia on Neurons. Sleep Breath. 2024, 28, 1051–1065. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Ouyang, R.; Zeng, Z.; Zhan, Z.; Lu, H.; Cui, Y.; Dai, Z.; Luo, L.; He, C.; et al. The Relationship between Inflammation and Neurocognitive Dysfunction in Obstructive Sleep Apnea Syndrome. J. Neuroinflamm. 2020, 17, 229. [Google Scholar] [CrossRef]
- Jelic, S.; Padeletti, M.; Kawut, S.M.; Higgins, C.; Canfield, S.M.; Onat, D.; Colombo, P.C.; Basner, R.C.; Factor, P.; LeJemtel, T.H. Inflammation, Oxidative Stress, and Repair Capacity of the Vascular Endothelium in Obstructive Sleep Apnea. Circulation 2008, 117, 2270–2278. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Tang, Y.; Niu, X.; Sun, H.-Y. The Role of Nitric Oxide (NO) Levels in Patients with Obstructive Sleep Apnea-Hypopnea Syndrome: A Meta-Analysis. Sleep Breath. 2021, 25, 9–16. [Google Scholar] [CrossRef]
- Haight, J.S.J.; Djupesland, P.G. Nitric Oxide (NO) and Obstructive Sleep Apnea (OSA). Sleep Breath. 2003, 7, 53–62. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Pathogenesis of Cognitive Dysfunction in Patients with Obstructive Sleep Apnea: A Hypothesis with Emphasis on the Nucleus Tractus Solitarius. Sleep Disord. 2012, 2012, 251096. [Google Scholar] [CrossRef] [PubMed]
- Daulatzai, M.A. Evidence of Neurodegeneration in Obstructive Sleep Apnea: Relationship between Obstructive Sleep Apnea and Cognitive Dysfunction in the Elderly. J. Neurosci. Res. 2015, 93, 1778–1794. [Google Scholar] [CrossRef]
- Harańczyk, M.; Konieczyńska, M.; Płazak, W. Endothelial Dysfunction in Obstructive Sleep Apnea Patients. Sleep Breath. Schlaf. Atm. 2022, 26, 231–242. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Ren, J. Endothelial Dysfunction: Mechanisms and Contribution to Diseases. Acta Pharmacol. Sin. 2024, 1–9. [Google Scholar] [CrossRef]
- Alvarez-Bueno, C.; Cunha, P.G.; Martinez-Vizcaino, V.; Pozuelo-Carrascosa, D.P.; Visier-Alfonso, M.E.; Jimenez-Lopez, E.; Cavero-Redondo, I. Arterial Stiffness and Cognition among Adults: A Systematic Review and Meta-Analysis of Observational and Longitudinal Studies. J. Am. Heart Assoc. 2020, 9, e014621. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.M.; Kuller, L.H.; Barinas-Mitchell, E.J.M.; Mackey, R.H.; McDade, E.M.; Klunk, W.E.; Aizenstein, H.J.; Cohen, A.D.; Snitz, B.E.; Mathis, C.A.; et al. Pulse Wave Velocity Is Associated with β-Amyloid Deposition in the Brains of Very Elderly Adults. Neurology 2013, 81, 1711–1718. [Google Scholar] [CrossRef]
- Saji, N.; Kinjo, Y.; Murotani, K.; Niida, S.; Takeda, A.; Sakurai, T. High Pulse Wave Velocity Is Associated with Enlarged Perivascular Spaces in Dementia with Lewy Bodies. Sci. Rep. 2024, 14, 13911. [Google Scholar] [CrossRef]
- Elias, M.F.; Robbins, M.A.; Budge, M.M.; Abhayaratna, W.P.; Dore, G.A.; Elias, P.K. Arterial Pulse Wave Velocity and Cognition with Advancing Age. Hypertension 2009, 53, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, B.C.; Parr, S.K.; Schulze, K.M.; Kunkel, O.N.; Turpin, V.G.; Liang, J.; Ade, C.J. Associations of Cerebrovascular Regulation and Arterial Stiffness with Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2023, 12, e032616. [Google Scholar] [CrossRef]
- Mitchell, G.F. Arterial Stiffness in Aging: Does It Have a Place in Clinical Practice?: Recent Advances in Hypertension. Hypertension 2021, 77, 768–780. [Google Scholar] [CrossRef]
- De Roos, A.; van der Grond, J.; Mitchell, G.; Westenberg, J. Magnetic Resonance Imaging of Cardiovascular Function and the Brain. Circulation 2017, 135, 2178–2195. Available online: https://www.ahajournals.org/doi/epub/10.1161/CIRCULATIONAHA.116.021978 (accessed on 8 June 2024). [CrossRef]
- Amier, R.P.; Marcks, N.; Hooghiemstra, A.M.; Nijveldt, R.; Van Buchem, M.A.; De Roos, A.; Biessels, G.J.; Kappelle, L.J.; Van Oostenbrugge, R.J.; Van Der Geest, R.J.; et al. Hypertensive Exposure Markers by MRI in Relation to Cerebral Small Vessel Disease and Cognitive Impairment. JACC Cardiovasc. Imaging 2021, 14, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.S.; Simoni, M.; Mazzucco, S.; Kuker, W.; Schulz, U.; Rothwell, P.M. Increased Cerebral Arterial Pulsatility in Patients with Leukoaraiosis. Stroke 2012, 43, 2631–2636. Available online: https://www.ahajournals.org/doi/epub/10.1161/STROKEAHA.112.655837 (accessed on 11 June 2024). [CrossRef]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.-H.; Cruickshank, J.K.; et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of Cardiovascular Events and All-Cause Mortality with Arterial Stiffness. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Waldstein, S.R.; Rice, S.C.; Thayer, J.F.; Najjar, S.S.; Scuteri, A.; Zonderman, A.B. Pressure and Pulse Wave Velocity Are Related to Cognitive Decline in the Baltimore Longitudinal Study of Aging. Hypertension 2008, 51, 99–104. Available online: https://www.ahajournals.org/doi/epub/10.1161/HYPERTENSIONAHA.107.093674 (accessed on 14 June 2024). [CrossRef]
- Aribisala, B.S.; Morris, Z.; Eadie, E.; Thomas, A.; Gow, A.; Hernández, M.C.V.; Royle, N.A.; Bastin, M.E.; Starr, J.; Deary, I.J.; et al. Blood Pressure, Internal Carotid Artery Flow Parameters, and Age-Related White Matter Hyperintensities. Hypertension 2014, 63, 1011–1018. Available online: https://www.ahajournals.org/doi/epub/10.1161/HYPERTENSIONAHA.113.02735 (accessed on 14 June 2024). [CrossRef]
- Scuteri, A.; Tesauro, M.; Appolloni, S.; Preziosi, F.; Brancati, A.M.; Volpe, M. Arterial Stiffness as an Independent Predictor of Longitudinal Changes in Cognitive Function in the Older Individual. J. Hypertens. 2007, 25, 1035. [Google Scholar] [CrossRef]
- Hoyos, C.M.; Melehan, K.L.; Liu, P.Y.; Grunstein, R.R.; Phillips, C.L. Does Obstructive Sleep Apnea Cause Endothelial Dysfunction? A Critical Review of the Literature. Sleep Med. Rev. 2015, 20, 15–26. [Google Scholar] [CrossRef]
- Mayer, G.; Frohnhofen, H.; Jokisch, M.; Hermann, D.M.; Gronewold, J. Associations of Sleep Disorders with All-Cause MCI/Dementia and Different Types of Dementia—Clinical Evidence, Potential Pathomechanisms and Treatment Options: A Narrative Review. Front. Neurosci. 2024, 18, 1372326. [Google Scholar] [CrossRef]
- Torres, G.; Sánchez-de-la-Torre, M.; Barbé, F. Relationship between OSA and Hypertension. CHEST 2015, 148, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Doonan, R.J.; Scheffler, P.; Lalli, M.; Kimoff, R.J.; Petridou, E.T.; Daskalopoulos, M.E.; Daskalopoulou, S.S. Increased Arterial Stiffness in Obstructive Sleep Apnea: A Systematic Review. Hypertens. Res. 2011, 34, 23–32. [Google Scholar] [CrossRef]
- Pepine, C.J.; Bairey Merz, C.N. Heart and Brain Interactions: Is Small Vessel Disease a Link? Eur. Heart J. 2023, 44, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Dyken, M.E.; Somers, V.K.; Yamada, T.; Ren, Z.-Y.; Zimmerman, M.B. Investigating the Relationship between Stroke and Obstructive Sleep Apnea. Stroke 1996, 27, 401–407. Available online: https://www.ahajournals.org/doi/epub/10.1161/01.STR.27.3.401 (accessed on 9 June 2024). [CrossRef]
- Dziewas, R.; Humpert, M.; Hopmann, B.; Kloska, S.; Lüdemann, P.; Ritter, M.; Dittrich, R.; Ringelstein, E.B.; Young, P.; Nabavi, D.G. Increased Prevalence of Sleep Apnea in Patients with Recurring Ischemic Stroke Compared with First Stroke Victims. J. Neurol. 2005, 252, 1394–1398. [Google Scholar] [CrossRef]
- Redline, S.; Yenokyan, G.; Gottlieb, D.J.; Shahar, E.; O’Connor, G.T.; Resnick, H.E.; Diener-West, M.; Sanders, M.H.; Wolf, P.A.; Geraghty, E.M.; et al. Obstructive Sleep Apnea–Hypopnea and Incident Stroke. Am. J. Respir. Crit. Care Med. 2010, 182, 269–277. [Google Scholar] [CrossRef]
- Gregori-Pla, C.; Zirak, P.; Cotta, G.; Bramon, P.; Blanco, I.; Serra, I.; Mola, A.; Fortuna, A.; Solà-Soler, J.; Giraldo Giraldo, B.F.; et al. How Does Obstructive Sleep Apnea Alter Cerebral Hemodynamics? SLEEP 2023, 46, zsad122. [Google Scholar] [CrossRef]
- Müller, M.B.; Stihl, C.; Schmid, A.; Hirschberger, S.; Mitsigiorgi, R.; Holzer, M.; Patscheider, M.; Weiss, B.G.; Reichel, C.; Hübner, M.; et al. A Novel OSA-Related Model of Intermittent Hypoxia in Endothelial Cells under Flow Reveals Pronounced Inflammatory Pathway Activation. Front. Physiol. 2023, 14, 1108966. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, M.; Faraci, F.M.; BRENDA Network; Betsholtz, C.; Hillman, E.; Joutel, A.; Nelson, M.; Paquet, D. Brain Endothelium: A Nexus for Cerebral Small Vessel Disease. Eur. Heart J. 2023, 44, 4211–4213. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Iadecola, C.; Carnevale, D. Hypertension, Neurovascular Dysfunction, and Cognitive Impairment. Hypertension 2023, 80, 22–34. [Google Scholar] [CrossRef]
- Guay-Gagnon, M.; Vat, S.; Forget, M.; Tremblay-Gravel, M.; Ducharme, S.; Nguyen, Q.D.; Desmarais, P. Sleep Apnea and the Risk of Dementia: A Systematic Review and Meta-analysis. J. Sleep Res. 2022, 31, e13589. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.L. Mechanisms and Subclinical Consequences of Aortic Stiffness. Hypertension 2017, 70, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.R.; Pires, G.N.; Andersen, M.L.; Tufik, S.; Rosa, D.S. Oxygen Saturation as a Predictor of Inflammation in Obstructive Sleep Apnea. Sleep Breath. 2022, 26, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Altay, S.; Fırat, S.; Peker, Y. A Narrative Review of the Association of Obstructive Sleep Apnea with Hypertension: How to Treat Both When They Coexist? J. Clin. Med. 2023, 12, 4144. [Google Scholar] [CrossRef]
- Jiménez-Balado, J.; Riba-Llena, I.; Abril, O.; Garde, E.; Penalba, A.; Ostos, E.; Maisterra, O.; Montaner, J.; Noviembre, M.; Mundet, X.; et al. Cognitive Impact of Cerebral Small Vessel Disease Changes in Patients with Hypertension. Hypertension 2019, 73, 342–349. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Fung, S.H.; Zhang, Y.J.; Verma, A.K. Sleep-Disordered Breathing and Idiopathic Normal-Pressure Hydrocephalus: Recent Pathophysiological Advances. Curr. Neurol. Neurosci. Rep. 2019, 19, 39. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Yuan, R.; Liu, M.; Hao, Z. Association of Obstructive Sleep Apnea and Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis. Sleep 2020, 43, zsz264. [Google Scholar] [CrossRef]
- Lee, W.-J.; Jung, K.-H.; Nam, H.-W.; Lee, Y.-S. Effect of Obstructive Sleep Apnea on Cerebrovascular Compliance and Cerebral Small Vessel Disease. PLoS ONE 2021, 16, e0259469. [Google Scholar] [CrossRef]
- Pallangyo, P.; Mgopa, L.R.; Mkojera, Z.; Komba, M.; Millinga, J.; Misidai, N.; Swai, H.J.; Mayala, H.; Bhalia, S.; Wibonela, S.; et al. Obstructive Sleep Apnea and Associated Factors among Hypertensive Patients Attending a Tertiary Cardiac Center in Tanzania: A Comparative Cross-Sectional Study. Sleep Sci. Pract. 2021, 5, 17. [Google Scholar] [CrossRef]
- Yuan, F.; Zhang, S.; Liu, X.; Liu, Y. Correlation between Obstructive Sleep Apnea Hypopnea Syndrome and Hypertension: A Systematic Review and Meta-Analysis. Ann. Palliat. Med. 2021, 10, 12251–12261. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective Study of the Association between Sleep-Disordered Breathing and Hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep Apnea and Cardiovascular Disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J. Am. Coll. Cardiol. 2008, 52, 686–717. [Google Scholar] [CrossRef]
- Konecny, T.; Kara, T.; Somers, V.K. Obstructive Sleep Apnea and Hypertension: An Update. Hypertension 2014, 63, 203–209. [Google Scholar] [CrossRef]
- Cherubini, J.M.; Cheng, J.L.; Williams, J.S.; MacDonald, M.J. Sleep Deprivation and Endothelial Function: Reconciling Seminal Evidence with Recent Perspectives. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H29–H35. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Van Veluw, S.J. Cerebral Amyloid Angiopathy. Stroke 2024, 55, 1409–1411. [Google Scholar] [CrossRef]
- Simonsen, S.A.; Andersen, A.V.; West, A.S.; Wolfram, F.; Jennum, P.; Iversen, H.K. Sleep-Disordered Breathing and Cerebral Small Vessel Disease—Acute and 6 Months after Ischemic Stroke. Sleep Breath. 2022, 26, 1107–1113. [Google Scholar] [CrossRef]
- Bu, X.-L.; Liu, Y.-H.; Wang, Q.-H.; Jiao, S.-S.; Zeng, F.; Yao, X.-Q.; Gao, D.; Chen, J.-C.; Wang, Y.-J. Serum Amyloid-Beta Levels Are Increased in Patients with Obstructive Sleep Apnea Syndrome. Sci. Rep. 2015, 5, 13917. [Google Scholar] [CrossRef] [PubMed]
- Meliante, P.G.; Zoccali, F.; Cascone, F.; Di Stefano, V.; Greco, A.; De Vincentiis, M.; Petrella, C.; Fiore, M.; Minni, A.; Barbato, C. Molecular Pathology, Oxidative Stress, and Biomarkers in Obstructive Sleep Apnea. Int. J. Mol. Sci. 2023, 24, 5478. [Google Scholar] [CrossRef]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ. Res. 2021, 128, 864–886. Available online: https://www.ahajournals.org/doi/epub/10.1161/CIRCRESAHA.121.318061 (accessed on 12 June 2024). [CrossRef]
- Lau, K.K.; Li, L.; Schulz, U.; Simoni, M.; Chan, K.H.; Ho, S.L.; Cheung, R.T.F.; Küker, W.; Mak, H.K.F.; Rothwell, P.M. Total Small Vessel Disease Score and Risk of Recurrent Stroke: Validation in 2 Large Cohorts. Neurology 2017, 88, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, G.; Qing, H.; Zhou, W.; Dobie, F.; Cai, F.; Staufenbiel, M.; Huang, L.E.; Song, W. Hypoxia Facilitates Alzheimer’s Disease Pathogenesis by up-Regulating BACE1 Gene Expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18727–18732. [Google Scholar] [CrossRef] [PubMed]
- Weingärtner, S. Magnetic Resonance Imaging-Based ID of the Vasculature across the Heart–Brain Axis. Eur. Heart J. 2023, 44, 2654–2656. [Google Scholar] [CrossRef]
- Wåhlin, A.; Ambarki, K.; Hauksson, J.; Birgander, R.; Malm, J.; Eklund, A. Phase Contrast MRI Quantification of Pulsatile Volumes of Brain Arteries, Veins, and Cerebrospinal Fluids Compartments: Repeatability and Physiological Interactions. J. Magn. Reson. Imaging 2012, 35, 1055–1062. [Google Scholar] [CrossRef]
- Gosselin, N.; Baril, A.-A.; Osorio, R.S.; Kaminska, M.; Carrier, J. Obstructive Sleep Apnea and the Risk of Cognitive Decline in Older Adults. Am. J. Respir. Crit. Care Med. 2019, 199, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Okamoto, S.; Lipton, S.A.; Xu, H. Oligomeric Aβ-Induced Synaptic Dysfunction in Alzheimer’s Disease. Mol. Neurodegener. 2014, 9, 48. [Google Scholar] [CrossRef]
- Costa, Y.S.; Lim, A.S.P.; Thorpe, K.E.; Colelli, D.R.; Mitchell, S.; Masellis, M.; Lam, B.; Black, S.E.; Boulos, M.I. Investigating Changes in Cognition Associated with the Use of CPAP in Cognitive Impairment and Dementia: A Retrospective Study. Sleep Med. 2023, 101, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Bratton, D.J.; Gaisl, T.; Wons, A.M.; Kohler, M. CPAP vs Mandibular Advancement Devices and Blood Pressure in Patients with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. JAMA 2015, 314, 2280–2293. [Google Scholar] [CrossRef]
- Martínez-García, M.-A.; Capote, F.; Campos-Rodríguez, F.; Lloberes, P.; Díaz de Atauri, M.J.; Somoza, M.; Masa, J.F.; González, M.; Sacristán, L.; Barbé, F.; et al. Effect of CPAP on Blood Pressure in Patients with Obstructive Sleep Apnea and Resistant Hypertension: The HIPARCO Randomized Clinical Trial. JAMA 2013, 310, 2407–2415. [Google Scholar] [CrossRef]
- Yetkin, O.; Kunter, E.; Gunen, H. CPAP Compliance in Patients with Obstructive Sleep Apnea Syndrome. Sleep Breath. 2008, 12, 365–367. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, C.; Moelter, S.; Fuentecilla, J.L.; Kincheloe, K.; Lozano, A.J.; Carter, P.; Gooneratne, N.; Richards, K.C. One Year of CPAP Adherence Improves Cognition in Older Adults with Mild Apnea and Mild Cognitive Impairment: A Secondary Analysis of Memories 1. Nurs. Res. 2020, 69, 157–164. [Google Scholar] [CrossRef]
- Richards, K.C.; Gooneratne, N.; Dicicco, B.; Hanlon, A.; Moelter, S.; Onen, F.; Wang, Y.; Sawyer, A.; Weaver, T.; Lozano, A.; et al. CPAP Adherence May Slow 1-Year Cognitive Decline in Older Adults with Mild Cognitive Impairment and Apnea. J. Am. Geriatr. Soc. 2019, 67, 558–564. [Google Scholar] [CrossRef] [PubMed]
| Diabetes | Inflammation | Obesity | Genetics | Age | Gender | Smoking & Alcohol | |
|---|---|---|---|---|---|---|---|
| A | T2D in OSA 1.5 (1.2–2.0) [57] | CRP in OSA 0.58 (0.42–0.73) [61] | 10% Weight gain 6.0 (2.2–17) [63] | TNF-α in OSA 1.82 (1.26–2.61) [66] | Age < 60: 35.0 (33.3–36.8) | NDD in women with AHI ≥ 15 3.4 (1.4–8.1) [71] | Alcohol in OSA 1.33 (1.10–1.62) [78] |
| B | OSA in T2D 1.48 (1.42–1.55) [58] | CRP in OSA 1.77 (1.28–2.26) [55] | BMI > 30 in OSA Male: 18 (14–23) | APOE variant in OSA 1.41 (1.06–1.87) [67] | Age > 60: 67.6 (65.2–70.0) | NDD in Women with APOE: 4.6 (1.0–20.7) [71] | Alcohol in OSA 2.03 (1.30–3.17) [73] |
| C | T2D + APOE 1.48 (1.36–1.60) [59] | TNF-α in NDD 2.4(0.3–4.5) [62] | BMI > 30 in OSA Female: 5.6(3.4–9.2) [64] | APOE in NDD [68] EA: 4.54 (3.99–5.17) | 10-yr age increase, AHI ≥ 15 [69] Male: 1.94 (1.66–2.27) | Early menopause: 1.22 (1.11–1.34) | Smoking in OSA 2.5 (1.3–4.7) [74] |
| D | T2D onset in NDD 2.12 (1.50–3.0) [60] | IM + MetS in NDD 1.66 (1.19–2.32) [50] | PAF in NDD Obesity: 20.9 (13–28.8) [65] | White: 3.46 (3.27–3.65) | 10-yr age increase, AHI ≥ 15 [69] Female: 3.20 (2.42–4.25) | Suppressed Androgen: 1.18 (1.08–1.29) | Alcohol in NDD, >14 drinks D.17.0 (4.0–32.0) [75] |
| E | NDD in Obesity w/o MetS before age 60 1.69 (1.16–2.45) [53] | Black: 2.18 (1.90–2.49) | NDD in Age + OSA [70] Age < 60: 3.30 (1.68–6.48) | Gender in NDD incidence [72] Female: 16.4 (15.2–17.6) | Alcohol in NDD, >14 drinks 1.72 (0.87–3.40) [76] | ||
| F | Hispanic: 1.90 (1.65–2.18) | Age > 60: 2.40 (1.65–3.48) | Gender in NDD incidence [72] Male: 12.3 (11.1–13.5) | Smoking in NDD 2.14 (1.65–2.78) [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogbu, I.; Menon, T.; Chahil, V.; Kahlon, A.; Devanand, D.; Kalra, D.K. Sleep Disordered Breathing and Neurocognitive Disorders. J. Clin. Med. 2024, 13, 5001. https://doi.org/10.3390/jcm13175001
Ogbu I, Menon T, Chahil V, Kahlon A, Devanand D, Kalra DK. Sleep Disordered Breathing and Neurocognitive Disorders. Journal of Clinical Medicine. 2024; 13(17):5001. https://doi.org/10.3390/jcm13175001
Chicago/Turabian StyleOgbu, Ikechukwu, Tushar Menon, Vipanpreet Chahil, Amrit Kahlon, Dakshinkumaar Devanand, and Dinesh K. Kalra. 2024. "Sleep Disordered Breathing and Neurocognitive Disorders" Journal of Clinical Medicine 13, no. 17: 5001. https://doi.org/10.3390/jcm13175001
APA StyleOgbu, I., Menon, T., Chahil, V., Kahlon, A., Devanand, D., & Kalra, D. K. (2024). Sleep Disordered Breathing and Neurocognitive Disorders. Journal of Clinical Medicine, 13(17), 5001. https://doi.org/10.3390/jcm13175001







