Unlocking the Diagnostic Potential: A Systematic Review of Biomarkers in Spinal Tuberculosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Quality Assessment
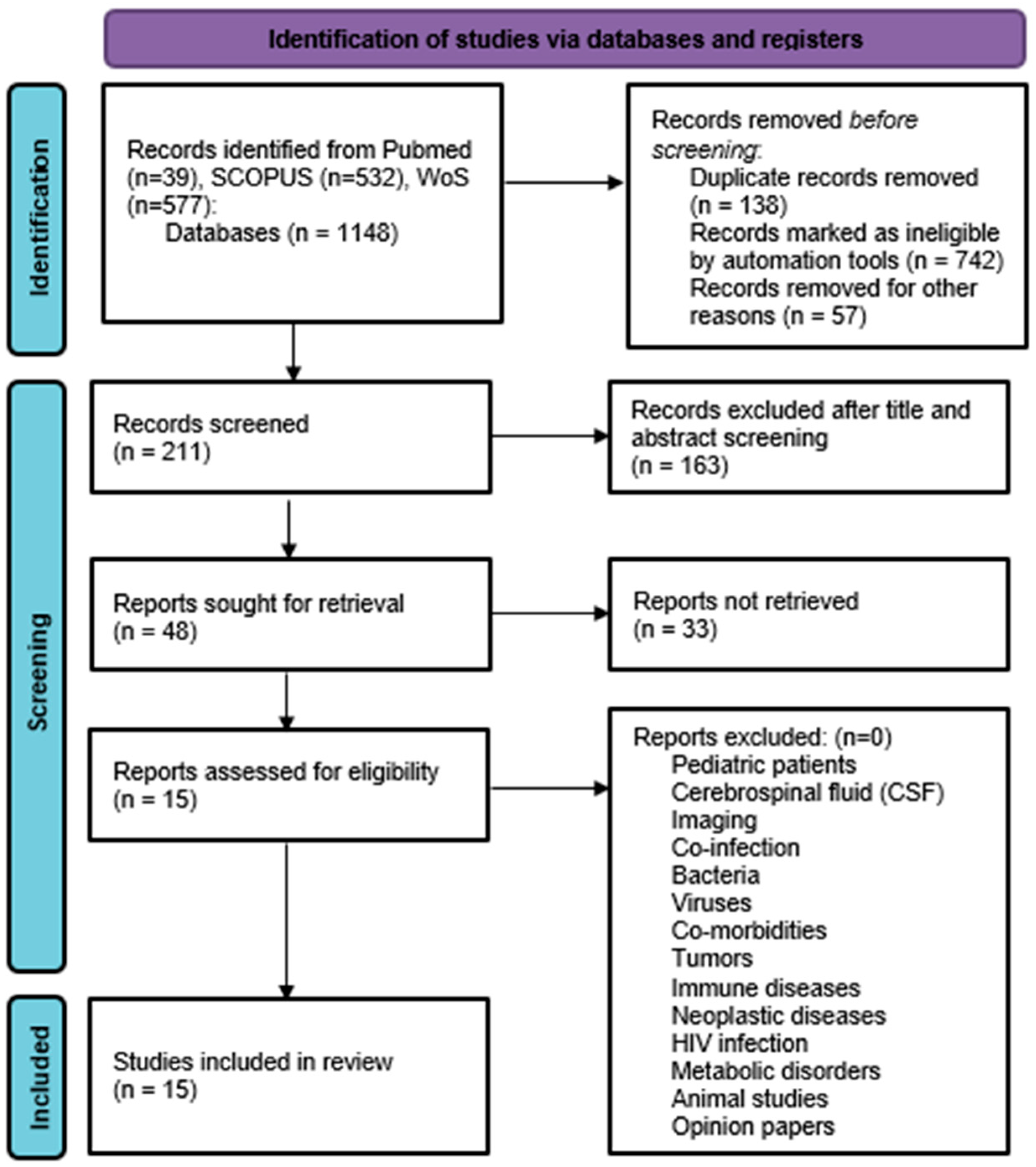
3. Results
3.1. Search Results
3.2. Study Characteristic and Demographics
3.3. Assessment of Risk of Bias and Applicability
3.4. Biomarker Categorization
3.4.1. Blood Cell Ratio and Complete Blood Count Parameters
3.4.2. Immunoproteasome
3.4.3. IFN-y, CXCR3, and Its Ligands (CXCL9 and CXCL10)
3.4.4. Fibrinogen, CRP, IFN-Gamma, NCAM, Ferritin, CXCL8, and GDF-15
3.4.5. ANGPTL-4 (Angiopoietin-like Protein 4)
3.4.6. Classically Activated Macrophages (M1) and Alternatively Activated (M2)
3.4.7. Lipopolysaccharide-Binding Protein (LBP)
3.4.8. Bacterial Antigen: Mycobacterium Tuberculosis-Specific Antigen/Phytohemagglutinin (TBAg/PHA) Ratio
3.4.9. RNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mann, T.N.; Davis, J.H.; Walzl, G.; du Toit, J.; Lamberts, R.P.; Chegou, N.N. Candidate Biomarkers to Distinguish Spinal Tuberculosis from Mechanical Back Pain in a Tuberculosis Endemic Setting. Front. Immunol. 2021, 12, 768040. [Google Scholar] [CrossRef] [PubMed]
- Hasan Khan, M.N.; Jamal, A.B.; Hafeez, A.; Sadiq, M.; Rasool, M.U. Is spinal tuberculosis changing with changing time? Ann. Med. Surg. 2021, 66, 102421. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Soundararajan, D.C.R.; Shetty, A.P.; Kanna, R.M. Spinal Tuberculosis: Current Concepts. Glob. Spine J. 2018, 8, 96S–108S. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, G.; Zhang, H.; Tang, M.; Liu, S.; Tang, B.; Xu, D.; Zhang, C.; Gao, Q. A predictive model for early clinical diagnosis of spinal tuberculosis based on conventional laboratory indices: A multicenter real-world study. Front. Cell. Infect. Microbiol. 2023, 13, 1150632. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Y.; Yi, J.; Zhou, W.; Zhao, S.; Yin, G. Neutrophil-lymphocyte ratio as a potential marker for differential diagnosis between spinal tuberculosis and pyogenic spinal infection. J. Orthop. Surg. Res. 2022, 17, 357. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, Z.; Liu, X.; Fang, Z.; Liu, Y.; Li, F. Tuberculosis-Specific Antigen/Phytohemagglutinin Ratio Combined with GeneXpert MTB/RIF for Early Diagnosis of Spinal Tuberculosis: A Prospective Cohort Study. Front. Cell. Infect. Microbiol. 2022, 12, 781315. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, X.; Shi, J.; Tian, J.; Chang, X.; Wang, X.; Ye, Q. Expression and Clinical Significance of lncRNA NEAT1 in Patients with Spinal Tuberculosis. Dis. Markers 2022, 2022, 5748756. [Google Scholar] [CrossRef]
- Liang, Q.; Jin, W.; Huang, Z.; Yin, H.; Liu, S.; Liu, L.; Song, X.; Wang, Z.; Fei, J. A plasma 3-marker microRNA biosignature distinguishes spinal tuberculosis from other spinal destructive diseases and pulmonary tuberculosis. Front. Cell. Infect. Microbiol. 2023, 13, 1125946. [Google Scholar] [CrossRef]
- Ou, F.S.; Michiels, S.; Shyr, Y.; Adjei, A.A.; Oberg, A.L. Biomarker Discovery and Validation: Statistical Considerations. J. Thorac. Oncol. 2021, 16, 537–545. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. Available online: https://www.acpjournals.org/doi/abs/10.7326/0003-4819-155-8-201110180-00009 (accessed on 15 April 2024). [CrossRef]
- Wu, S.; Liang, T.; Jiang, J.; Zhu, J.; Chen, T.; Zhou, C.; Huang, S.; Yao, Y.; Guo, H.; Ye, Z.; et al. Proteomic analysis to identification of hypoxia related markers in spinal tuberculosis: A study based on weighted gene co-expression network analysis and machine learning. BMC Med. Genom. 2023, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Liu, J.; Ren, Z.; Ji, J.; Ma, H.; Dong, H.; Wang, L.; Zhang, X.; Niu, N. Analysis of the Value of Serum Biomarker LBP in the Diagnosis of Spinal Tuberculosis. Infect. Drug Resist. 2022, 15, 4915–4926. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Pang, X.; Wang, X.; Zeng, H. Differential expression analysis of miRNAs in macrophage-derived exosomes in the tuberculosis-infected bone microenvironment. Front. Microbiol. 2023, 14, 1236012. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Wang, L.; Liu, Y.; Liu, X.; Lv, J.; Zhou, X.; Wang, H.; Nazierhan, S.; Wang, J.; Ma, X. Diagnostic value of CXCR3 and its ligands in spinal tuberculosis. Exp. Ther. Med. 2020, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liang, T.; Jiang, J.; Chen, J.; Chen, T.; Huang, S.; Chen, L.; Sun, X.; Chen, W.; Zhu, J.; et al. MMP9 and STAT1 are biomarkers of the change in immune infiltration after anti-tuberculosis therapy, and the immune status can identify patients with spinal tuberculosis. Int. Immunopharmacol. 2023, 116, 109588. [Google Scholar] [CrossRef]
- Siregar, O.; Augustinus, Y.; Benny, B.; Rahyussalim, A.J. Comparison of Serum Matrix Metalloproteinase-9 Value in Spondylitis Tuberculous with Degenerative Spine Disease. Open Access Maced. J. Med. Sci. 2020, 8, 646–648. [Google Scholar] [CrossRef]
- Lan, S.; He, Y.; Tiheiran, M.; Liu, W.; Guo, H. The Angiopoietin-like protein 4: A promising biomarker to distinguish brucella spondylitis from tuberculous spondylitis. Clin. Rheumatol. 2021, 40, 4289–4294. [Google Scholar] [CrossRef]
- Daniel, K.; Dunn, R. Comparison of platelet count in tuberculosis spine to other spine pathology. Eur. Spine J. 2013, 22, 2810–2814. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Liang, T.; Ye, Z.; Huang, S.; Chen, J.; Sun, X.; Yi, M.; Jiang, J.; Chen, T.; et al. Monocyte-to-Lymphocyte Ratio Was an Independent Factor of the Severity of Spinal Tuberculosis. Oxidative Med. Cell. Longev. 2022, 2022, 7340330. [Google Scholar] [CrossRef]
- Wang, L.; Shang, X.; Qi, X.; Ba, D.; Lv, J.; Zhou, X.; Wang, H.; Shaxika, N.; Wang, J.; Ma, X. Clinical Significance of M1/M2 Macrophages and Related Cytokines in Patients with Spinal Tuberculosis. Dis. Markers 2020, 2020, 2509454. [Google Scholar] [CrossRef] [PubMed]
- Ethier, J.L.; Desautels, D.; Templeton, A.; Shah, P.S.; Amir, E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Adane, T.; Melku, M.; Ayalew, G.; Bewket, G.; Aynalem, M.; Getawa, S. Accuracy of monocyte to lymphocyte ratio for tuberculosis diagnosis and its role in monitoring anti-tuberculosis treatment Systematic review and meta-analysis. Medicine 2022, 101, E31539. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix Metalloproteinase-9: Many Shades of Function in Cardiovascular Disease. Physiology 2013, 28, 391. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, C.; Li, M.; Gao, S.; Li, S.; Sun, H. Expression of TNF-α, IFN-γ, TGF-β, and IL-4 in the spinal tuberculous focus and its impact on the disease. Cell. Biochem. Biophys. 2014, 70, 1759–1764. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, L.; Lei, N.; Chen, P.; Zhang, Y. Crohn’s Disease Patients with Depression Exhibit Alterations in Monocyte/Macrophage Phenotype and Increased Proinflammatory Cytokine Production. Dig. Dis. 2020, 38, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Oishi, E.; Sakata, S. Serum Lipopolysaccharide-Binding Protein Levels and the Incidence of Cardiovascular Disease in a General Japanese Population: The Hisayama Study. J. Am. Heart Assoc. 2019, 8, e013628. [Google Scholar] [CrossRef]
- Luo, Y.; Xue, Y.; Lin, Q.; Tang, G.; Yuan, X.; Mao, L.; Song, H.; Wang, F.; Sun, Z. A combination of iron metabolism indexes and tuberculosis-specific antigen/phytohemagglutinin ratio for distinguishing active tuberculosis from latent tuberculosis infection. Int. J. Infect. Dis. 2020, 97, 190–196. [Google Scholar] [CrossRef]
- Kumar, R.; Das, R.K.; Mahapatra, A.K. Role of interferon gamma release assay in the diagnosis of Pott disease. J. Neurosurg. Spine 2010, 12, 462–466. [Google Scholar] [CrossRef]
- Wang, F.; Yu, J.; Zhou, Y.; Luo, Y.; Wu, S.; Huang, M.; Yin, B.; Huang, J.; Mao, L.; Sun, Z. The use of TB-specific antigen/phytohemagglutinin ratio for diagnosis and treatment monitoring of extrapulmonary tuberculosis. Front. Immunol. 2018, 9, 1047. [Google Scholar] [CrossRef]
- Rus, V.; Via, C.S. Cytokines in Systemic Lupus Erythematosus. In Systemic Lupus Erythematosus E-Book: A Companion Rheumatology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 109–120. [Google Scholar]
- Howe, C.L.; Rodriguez, M. Remyelination as Neuroprotection. In Multiple Sclerosis as a Neuronal Disease; Elsevier: Amsterdam, The Netherlands, 2005; pp. 89–419. [Google Scholar]
- Ryu, S.Y.; Patel, R. Microbiology of Bone and Joint Infections. In Bone and Joint Infections: From Microbiology to Diagnostics and Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 5–20. [Google Scholar]
- Xiang, Y.; Huang, C.; He, Y.; Zhang, Q. Cancer or Tuberculosis: A Comprehensive Review of the Clinical and Imaging Features in Diagnosis of the Confusing Mass. Front. Oncol. 2021, 11, 644150. [Google Scholar] [CrossRef] [PubMed]


| Authors | Country | Sample | Reference Standard | Index Test | Population Size | Biomarker Performance |
|---|---|---|---|---|---|---|
| Liu et al., 2022 [5] | China | Blood | Clinical, laboratory, and radiologic evaluations and clinical response to anti-TB drugs or antimicrobial therapy | NLR (neutrophil to lymphocyte ratio) | STB (n = 146; male n = 78; female n = 68; mean age = 55.70 ± 17.16). Control group (PSI) (n = 60; male n = 34; female n = 26; mean age = 63.68 ± 11.52) |
|
| Qi et al., 2022 [6] | China | Tissue | Culture and histopathology | Mycobacterium tuberculosis-specific antigen/phytohemagglutinin (TBAg/PHA) ratio with AFBS and GeneXpert MTB/RIF | A total of 519 STB (319 subjects at Tongji Hospital (male n = 200; female n = 119) and 200 subjects at Sino-French New City Hospital (male n = 125; female n = 75) |
|
| Liang et al., 2023 [8] | China | Blood | Clinical manifestations, imaging, laboratory examinations, histopathology | Three-plasma miRNA combination (hsa-miR-506-3p, hsamiR-543, hsa-miR-195-5p) | A total of 423 subjects were recruited with 157 cases of STB, 30 cases of active PTB, 83 cases of SDD, and 153 cases of healthy controls |
|
| Zheng et al., 2022 [7] | China | Blood | Imaging, laboratory, histopathology | NEAT1 lncRNA in granulomatous tissue vs peripheral blood | STB (n = 120; male n = 63; female n = 57; age range = 14–91 y; (<60 y n = 78; ≥60 y n = 42)) Healthy control group (n = 60; male n = 37; female n = 28; age range = 20–80 y) |
|
| Wu et al., 2023 [12] | China | Tissue | Not specified | Proteasome 20 S subunit beta 9 (PSMB9), signal transducer and activator of transcription 1 (STAT1), and transporter 1 (TAP1) | STB (n = 5) Thoracolumbar disk herniation as control group (n = 5) |
|
| Lou et al., 2022 [13] | China | Blood | Culture and histopathology | LBP | STB (n = 100; male n = 50; female n = 50; mean age = 49.47 ± 16.32 y; age range = 18–77 y). Healthy control group (n = 30; male n = 13; female n = 17, mean age = 53.39 ± 9.67 y; age range = 40–72 y) |
|
| Sun et al., 2023 [14] | China | Blood | Not specified | miRNA | STB (n = 10; male n = 3; female n = 7) Control group with disc generation (n = 10; male n = 3; female n = 7) |
|
| Shang et al., 2021 [15] | China | Tissue | Laboratory, imaging, and histopathology | IFN-gamma, CXCR3, and its ligands (CXCL9 and CXCL10) | STB (n = 36; male n = 18; female n = 18, mean age 43.14 ± 15.36 y; age range 18–77 y). Healthy control group (n = 20; male n = 10; female n = 10; mean age 46.65 ± 11.82 y) |
|
| C. Zhou et al., 2023 [16] | China | Tissue | Laboratory, imaging, and histopathology | MMP9 (matrix metallopeptidase 9) and STAT1 (signal transducer and activator of transcription 1) | STB (n = 164; male n = 95; female n = 69, mean age = 45.16 ± 17.04 y) Control group with lumbar spinal stenosis or disc herniation (n = 162; male n = 93; female n = 69; mean age 47.99 ± 17.21 y) |
|
| Siregar et al., 2020 [17] | Indonesia | Blood | Not specified | Matrix metalloproteinase-9 (MMP-9) | STB (n = 5; male n = 1; female n = 4; mean age 41.6 ± 18.8 y). Control group with degenerative spinal disease (n = 5; male n = 3; female n = 2; mean age = 44.79 ± 16.98 years) |
|
| T.N. Mann et al., 2021 [1] | South Africa | Blood | Culture and histopathology | Fibrinogen, CRP, IFN-gamma, NCAM, CRP, ferritin, and CXCL8m GDF-15 | STB (n = 26; male n = 12; female n = 14) Control group with mechanical back pain (n = 17; male n = 7; female n = 10) |
|
| Lan et al., 2020 [18] | China | Blood | Culture, imaging, and histopathology | ANGPTL-4 (angiopoietin-like protein 4) | STB (n = 27; male n = 10; female n = 17; mean age = 47.33 ± 15.43 y; age range = 18–69 y) Brucellosis spinal (n = 17; male n = 15; female n = 11; mean age was 50.95 ± 13.41 y; age range = 31–72 y) |
|
| Daniel, K. and Dunn, R., 2013 [19] | South Africa | Blood | Histopathology, TB culture, and TB PCR | Platelet count | STB (n = 160; male n = 69; female n = 91; mean age = 40.5 y; age range = 13–79 y) Non-STB as control group (n = 210; male n = 85; female n = 125; mean age = 54.5 y; age range = 13–86) |
|
| Chen et al., 2022 [20] | China | Blood | Histopathology | Monocyte-to-lymphocyte ratio (MLR) | STB (n = 247; male n = 202; female n = 145; mean age = 49.4 ± 17.3 y) Non-STB as a control group (n = 353; male n = 307; female n = 46; mean age = 35.4 ± 10.4 y) |
|
| Wang et al., 2020 [21] | China | Blood | Histopathology | M1 and M2 | Patients with STB (n = 36; male n = 17; female n = 19; mean age = 56.20 ± 5.80 y; age range = 4–77 y) Healthy control group (n = 25; male n = 12; female n = 13; mean age = 44.20 ± 11.50 y) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siahaan, A.M.P.; Ivander, A.; Tandean, S.; Indharty, R.S.; Fernando, E.T.; Nugroho, S.A.; Milenia, V.; Az Zahra, D.O. Unlocking the Diagnostic Potential: A Systematic Review of Biomarkers in Spinal Tuberculosis. J. Clin. Med. 2024, 13, 5028. https://doi.org/10.3390/jcm13175028
Siahaan AMP, Ivander A, Tandean S, Indharty RS, Fernando ET, Nugroho SA, Milenia V, Az Zahra DO. Unlocking the Diagnostic Potential: A Systematic Review of Biomarkers in Spinal Tuberculosis. Journal of Clinical Medicine. 2024; 13(17):5028. https://doi.org/10.3390/jcm13175028
Chicago/Turabian StyleSiahaan, Andre Marolop Pangihutan, Alvin Ivander, Steven Tandean, Rr. Suzy Indharty, Eric Teo Fernando, Stefanus Adi Nugroho, Viria Milenia, and Dhea Olivia Az Zahra. 2024. "Unlocking the Diagnostic Potential: A Systematic Review of Biomarkers in Spinal Tuberculosis" Journal of Clinical Medicine 13, no. 17: 5028. https://doi.org/10.3390/jcm13175028





