Atrial Functional Mitral Regurgitation: From Diagnosis to Current Interventional Therapies
Abstract
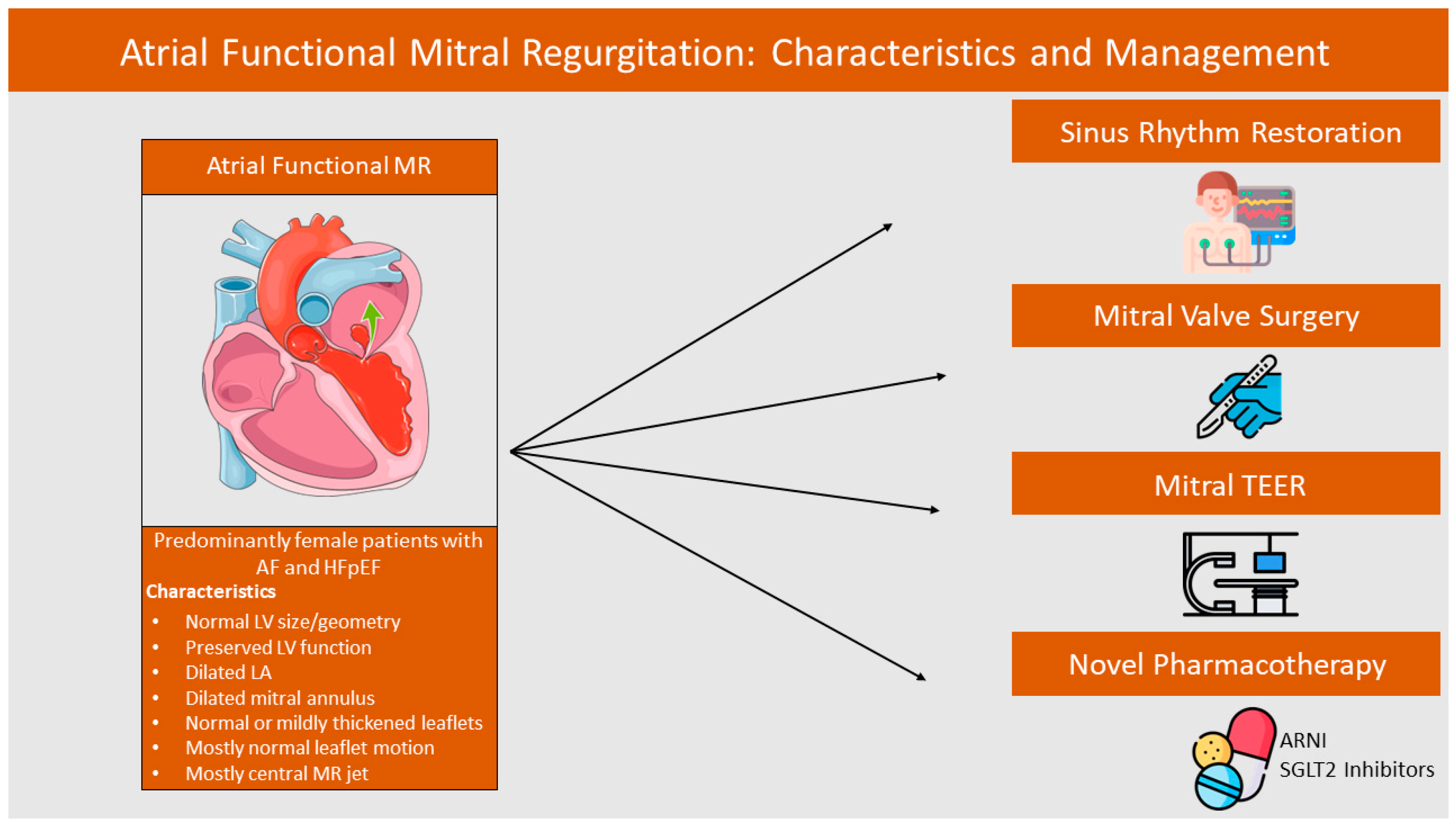
:1. Introduction
2. Pathophysiology and Epidemiology of AFMR
2.1. Epidemiology and Prognostic Evidence
2.2. Pathophysiology of AFMR
3. AFMR Assessment and Imaging Role
3.1. Echocardiographic Findings
3.2. Multimodality Imaging Assessment of AFMR
3.2.1. Cardiac Computed Tomography in AFMR Evaluation
3.2.2. Cardiac Magnetic Resonance in AFMR Evaluation
3.2.3. Global Longitudinal Strain in AFMR Assessment
4. Surgical and Pharmacotherapy Outcomes in AFMR
4.1. Current Guidelines Suggestions
4.2. Sinus Rhythm Restoration and AFMR
4.3. SGLT2 Inhibitors and Functional MR
4.4. Angiotensin Receptor-Neprilysin Inhibitors and FMR
4.5. Mitral Valve Surgery and AFMR
5. Transcatheter Edge-to-Edge Repair (TEER) Outcomes in AFMR
5.1. AFMR and VFMR Comparison
5.2. AFMR, VFMR, and DMR Comparison
5.3. Disproportionate AFMR
5.4. Data from Meta-Analyses
6. Clinical Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levine, R.A.; Schwammenthal, E. Ischemic Mitral Regurgitation on the Threshold of a Solution. Circulation 2005, 112, 745–758. [Google Scholar] [CrossRef]
- Otsuji, Y.; Handschumacher, M.D.; Schwammenthal, E.; Jiang, L.; Song, J.-K.; Guerrero, J.L.; Vlahakes, G.J.; Levine, R.A. Insights from Three-Dimensional Echocardiography into the Mechanism of Functional Mitral Regurgitation. Circulation 1997, 96, 1999–2008. [Google Scholar] [CrossRef]
- Akashi, J.; Otsuji, Y.; Nishimura, Y.; Levine, R.A.; Kataoka, M. Updated pathophysiological overview of functional MR (ventricular and atrial). Gen. Thorac. Cardiovasc. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Asgar, A.W.; Mack, M.J.; Stone, G.W. Secondary Mitral Regurgitation in Heart Failure. J. Am. Coll Cardiol. 2015, 65, 1231–1248. [Google Scholar] [CrossRef]
- Sannino, A.; Smith, R.L.; Schiattarella, G.G.; Trimarco, B.; Esposito, G.; Grayburn, P.A. Survival and Cardiovascular Outcomes of Patients with Secondary Mitral Regurgitation. JAMA Cardiol. 2017, 2, 1130. [Google Scholar] [CrossRef]
- Klimek, K.; Tworek, M.; Klocek, K.; Dołęga, J.; Majta, G.; Marcinkiewicz, K.; Wrona-Kolasa, K.; Cichoń, M.; Mizia-Stec, K. Functional tricuspid regurgitation and efficacy of electrical cardioversion in patients with atrial fibrillation and atrial functional mitral regurgitation. Cardiol. J. 2024. [Google Scholar] [CrossRef]
- Gertz, Z.M.; Raina, A.; Saghy, L.; Zado, E.S.; Callans, D.J.; Marchlinski, F.E.; Keane, M.G.; Silvestry, F.E. Evidence of Atrial Functional Mitral Regurgitation Due to Atrial Fibrillation. J. Am. Coll Cardiol. 2011, 58, 1474–1481. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Levine, R.A.; Flachskampf, F.; Grayburn, P.; Gillam, L.; Leipsic, J.; Thomas, J.D.; Kwong, R.Y.; Vandervoort, P.; Chandrashekhar, Y. Atrial Functional Mitral Regurgitation. JACC Cardiovasc. Imaging 2022, 15, 1870–1882. [Google Scholar] [CrossRef]
- Fan, Y.; Wan, S.; Wong, R.H.-L.; Lee, A.P.-W. Atrial functional mitral regurgitation: Mechanisms and surgical implications. Asian Cardiovasc. Thorac. Ann. 2020, 28, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Farhan, S.; Silbiger, J.J.; Halperin, J.L.; Zhang, L.; Dukkipati, S.R.; Vogel, B.; Kini, A.; Sharma, S.; Lerakis, S. Pathophysiology, Echocardiographic Diagnosis, and Treatment of Atrial Functional Mitral Regurgitation. J. Am. Coll Cardiol. 2022, 80, 2314–2330. [Google Scholar] [CrossRef] [PubMed]
- Kagiyama, N.; Hayashida, A.; Toki, M.; Fukuda, S.; Ohara, M.; Hirohata, A.; Yamamoto, K.; Isobe, M.; Yoshida, K. Insufficient Leaflet Remodeling in Patients with Atrial Fibrillation. Circ. Cardiovasc. Imaging 2017, 10, e005451. [Google Scholar] [CrossRef]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Bursi, F.; Faggiano, A.; Moscardelli, S.; Simeoli, P.S.; Guazzi, M.; Lorusso, R.; Carugo, S.; Faggiano, P. The Global Burden of Valvular Heart Disease: From Clinical Epidemiology to Management. J. Clin. Med. 2023, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Akamatsu, K.; Ito, K.; Matsumura, Y.; Shimeno, K.; Naruko, T.; Takahashi, Y.; Shibata, T.; Yoshiyama, M. Prevalence and Prognostic Significance of Functional Mitral and Tricuspid Regurgitation Despite Preserved Left Ventricular Ejection Fraction in Atrial Fibrillation Patients. Circ. J. 2018, 82, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Dziadzko, V.; Dziadzko, M.; Medina-Inojosa, J.R.; Benfari, G.; I Michelena, H.; A Crestanello, J.; Maalouf, J.; Thapa, P.; Enriquez-Sarano, M. Causes and mechanisms of isolated mitral regurgitation in the community: Clinical context and outcome. Eur. Heart J. 2019, 40, 2194–2202. [Google Scholar] [CrossRef]
- Moonen, A.; Ng, M.K.C.; Playford, D.; Strange, G.; Scalia, G.M.; Celermajer, D.S. Atrial functional mitral regurgitation: Prevalence, characteristics and outcomes from the National Echo Database of Australia. Open Heart 2023, 10, e002180. [Google Scholar] [CrossRef]
- Kim, K.; Kitai, T.; Kaji, S.; Pak, M.; Toyota, T.; Sasaki, Y.; Ehara, N.; Kobori, A.; Kinoshita, M.; Furukawa, Y. Outcomes and predictors of cardiac events in medically treated patients with atrial functional mitral regurgitation. Int. J. Cardiol. 2020, 316, 195–202. [Google Scholar] [CrossRef]
- Mesi, O.; Gad, M.M.; Crane, A.D.; Ramchand, J.; Puri, R.; Layoun, H.; Miyasaka, R.; Gillinov, M.A.; Wierup, P.; Griffin, B.P.; et al. Severe Atrial Functional Mitral Regurgitation: Clinical and Echocardiographic Characteristics, Management and Outcomes. JACC Cardiovasc. Imaging 2021, 14, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Naser, J.A.; Alexandrino, F.B.; Harada, T.; Michelena, H.I.; Borlaug, B.A.; Eleid, M.F.; Lin, G.; Scott, C.; Kennedy, A.M.; Pellikka, P.A.; et al. The Natural History of Atrial Functional Mitral Regurgitation. J. Am. Coll Cardiol. 2024, 83, 1495–1507. [Google Scholar] [CrossRef]
- Silbiger, J.J. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am. Heart J. 2012, 164, 163–176. [Google Scholar] [CrossRef]
- Salgo, I.S.; Gorman, J.H.; Gorman, R.C.; Jackson, B.M.; Bowen, F.W.; Plappert, T.; Sutton, M.G.S.J.; Edmunds, L.H. Effect of Annular Shape on Leaflet Curvature in Reducing Mitral Leaflet Stress. Circulation 2002, 106, 711–717. [Google Scholar] [CrossRef] [PubMed]
- A Timek, T.; Miller, D. Experimental and clinical assessment of mitral annular area and dynamics: What are we actually measuring? Ann. Thorac. Surg. 2001, 72, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, S.; Muraru, D.; Miglioranza, M.H.; Piasentini, E.; Peluso, D.; Cucchini, U.; Iliceto, S.; Vinereanu, D.; Badano, L.P. Normal mitral annulus dynamics and its relationships with left ventricular and left atrial function. Int. J. Cardiovasc. Imaging 2015, 31, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Silbiger, J.J.; Bazaz, R. The anatomic substrate of mitral annular contraction. Int. J. Cardiol. 2020, 306, 158–161. [Google Scholar] [CrossRef]
- Itabashi, Y.; Mihara, H.; Berdejo, J.; Utsunomiya, H.; Shiota, T. Distant Position of Chordae from Coaptation Causes Mitral Re-gurgitation in Patients with Atrial Fibrillation. J. Heart Valve Dis. 2016, 25, 323–331. [Google Scholar]
- Ring, L.; Dutka, D.P.; Wells, F.C.; Fynn, S.P.; Shapiro, L.M.; Rana, B.S. Mechanisms of atrial mitral regurgitation: Insights using 3D transoesophageal echo. Eur. Heart J. Cardiovasc. Imaging 2013, 15, 500–508. [Google Scholar] [CrossRef]
- Machino-Ohtsuka, T.; Seo, Y.; Ishizu, T.; Sato, K.; Sugano, A.; Yamamoto, M.; Hamada-Harimura, Y.; Aonuma, K. Novel Mechanistic Insights into Atrial Functional Mitral Regurgitation—3-Dimensional Echocardiographic Study. Circ. J. 2016, 80, 2240–2248. [Google Scholar] [CrossRef]
- Deferm, S.; Bertrand, P.B.; Verhaert, D.; Verbrugge, F.H.; Dauw, J.; Thoelen, K.; Giesen, A.; Bruckers, L.; Rega, F.; Thomas, J.D.; et al. Mitral Annular Dynamics in AF Versus Sinus Rhythm. JACC Cardiovasc. Imaging 2022, 15, 1–13. [Google Scholar] [CrossRef]
- Bai, W.; Chen, Y.; Zhong, Y.; Deng, L.; Li, D.; Zhu, W.; Rao, L. Assessment of mitral valve geometry in nonvalvular atrial fibrillation patients with or without ventricular dysfunction: Insights from high volume rate three-dimensional transesophageal echocardiography. Int. J. Cardiovasc. Imaging 2023, 39, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Cong, T.; Gu, J.; Lee, A.P.-W.; Shang, Z.; Sun, Y.; Sun, Q.; Wei, H.; Chen, N.; Sun, S.; Fu, T. Quantitative analysis of mitral valve morphology in atrial functional mitral regurgitation using real-time 3-dimensional echocardiography atrial functional mitral regurgitation. Cardiovasc. Ultrasound 2018, 16, 13. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Ausma, J.; Liu, G.S.; Allessie, M.A.; van Eys, G.J.; Borgers, M. Structural Changes of Atrial Myocardium During Chronic Atrial Fibrillation. Cardiovasc. Pathol. 2000, 9, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Dal-Bianco, J.P.; Aikawa, E.; Bischoff, J.; Guerrero, J.L.; Handschumacher, M.D.; Sullivan, S.; Johnson, B.; Titus, J.S.; Iwamoto, Y.; Wylie-Sears, J.; et al. Active Adaptation of the Tethered Mitral Valve. Circulation 2009, 120, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Heo, R.; Handschumacher, M.D.; Lee, S.; Choi, Y.-S.; Kim, K.-R.; Shin, Y.; Park, H.-K.; Bischoff, J.; Aikawa, E.; et al. Mitral Valve Adaptation to Isolated Annular Dilation. JACC Cardiovasc. Imaging 2019, 12, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Silbiger, J.J. Does Left Atrial Enlargement Contribute to Mitral Leaflet Tethering in Patients with Functional Mitral Regurgitation? Proposed Role of Atriogenic Leaflet Tethering. Echocardiography 2014, 31, 1310–1311. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Abe, Y.; Takahashi, Y.; Shimada, Y.; Fukumoto, H.; Matsumura, Y.; Naruko, T.; Shibata, T.; Yoshiyama, M.; Yoshikawa, J. Mechanism of atrial functional mitral regurgitation in patients with atrial fibrillation: A study using three-dimensional transesophageal echocardiography. J. Cardiol. 2017, 70, 584–590. [Google Scholar] [CrossRef]
- Kagiyama, N.; Mondillo, S.; Yoshida, K.; Mandoli, G.E.; Cameli, M. Subtypes of Atrial Functional Mitral Regurgitation: Imaging Insights into Their Mechanisms and Therapeutic Implications. JACC Cardiovasc. Imaging 2020, 13, 820–835. [Google Scholar] [CrossRef]
- Tang, Z.; Fan, Y.-T.; Wang, Y.; Jin, C.-N.; Kwok, K.-W.; Lee, A.P.-W. Mitral Annular and Left Ventricular Dynamics in Atrial Functional Mitral Regurgitation: A Three-Dimensional and Speckle-Tracking Echocardiographic Study. J. Am. Soc. Echocardiogr. 2019, 32, 503–513. [Google Scholar] [CrossRef]
- Silbiger, J.J. Mechanistic insights into atrial functional mitral regurgitation: Far more complicated than just left atrial remodeling. Echocardiography 2019, 36, 164–169. [Google Scholar] [CrossRef]
- Doherty, J.U.; Kort, S.; Mehran, R.; Schoenhagen, P.; Soman, P.; Dehmer, G.J.; Bashore, T.M.; Bhave, N.M.; Calnon, D.A.; Carabello, B.; et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 Appropriate Use Criteria for Multimodality Imaging in the Assessment of Cardiac Structure and Function in Nonvalvular Heart Disease. J. Am. Coll Cardiol. 2019, 73, 488–516. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Izumi, C.; Eishi, K.; Ashihara, K.; Arita, T.; Otsuji, Y.; Kunihara, T.; Komiya, T.; Shibata, T.; Seo, Y.; Daimon, M.; et al. JCS/JSCS/JATS/JSVS 2020 Guidelines on the Management of Valvular Heart Disease. Circ. J. 2020, 84, 2037–2119. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Levine, R.A.; Hua, L.; Morris, E.L.; Kang, Y.; Flaherty, M.; Morgan, N.V.; Hung, J. Diagnostic value of vena contracta area in the quantification of mitral regurgitation severity by color doppler 3D echocardiography. Circ. Cardiovasc. Imaging 2011, 4, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.M.; Soliman, O.I.I.; Cate, F.J.T.; Nemes, A.; McGhie, J.S.; Krenning, B.J.; van Geuns, R.-J.; Galema, T.W.; Geleijnse, M.L. True mitral annulus diameter is underestimated by two-dimensional echocardiography as evidenced by real-time three-dimensional echocardiography and magnetic resonance imaging. Int. J. Cardiovasc. Imaging 2007, 23, 541–547. [Google Scholar] [CrossRef]
- Naoum, C.; Blanke, P.; Cavalcante, J.L.; Leipsic, J. Cardiac Computed Tomography and Magnetic Resonance Imaging in the Evaluation of Mitral and Tricuspid Valve Disease. Circ. Cardiovasc. Imaging 2017, 10, e005331. [Google Scholar] [CrossRef]
- Mak, G.J.; Blanke, P.; Ong, K.; Naoum, C.; Thompson, C.R.; Webb, J.G.; Moss, R.; Boone, R.; Ye, J.; Cheung, A.; et al. Three-Dimensional Echocardiography Compared with Computed Tomography to Determine Mitral Annulus Size Before Transcatheter Mitral Valve Implantation. Circ. Cardiovasc. Imaging 2016, 9, e004176. [Google Scholar] [CrossRef] [PubMed]
- Coisne, A.; Pontana, F.; Aghezzaf, S.; Mouton, S.; Ridon, H.; Richardson, M.; Polge, A.-S.; Longère, B.; Silvestri, V.; Pagniez, J.; et al. Utility of Three-Dimensional Transesophageal Echocardiography for Mitral Annular Sizing in Transcatheter Mitral Valve Replacement Procedures: A Cardiac Computed Tomographic Comparative Study. J. Am. Soc. Echocardiogr. 2020, 33, 1245–1252. [Google Scholar] [CrossRef]
- Salemi, V.M.; Rochitte, C.E.; Shiozaki, A.A.; Andrade, J.M.; Parga, J.R.; de Ávila, L.F.; Benvenuti, L.A.; Cestari, I.N.; Picard, M.H.; Kim, R.J.; et al. Late Gadolinium Enhancement Magnetic Resonance Imaging in the Diagnosis and Prognosis of Endomyocardial Fibrosis Patients. Circ. Cardiovasc. Imaging 2011, 4, 304–311. [Google Scholar] [CrossRef]
- Cavalcante, J.L.; Kusunose, K.; Obuchowski, N.A.; Jellis, C.; Griffin, B.P.; Flamm, S.D.; Kwon, D.H. Prognostic Impact of Ischemic Mitral Regurgitation Severity and Myocardial Infarct Quantification by Cardiovascular Magnetic Resonance. JACC Cardiovasc. Imaging 2020, 13, 1489–1501. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef]
- Chelu, M.G.; King, J.B.; Kholmovski, E.G.; Ma, J.; Gal, P.; Marashly, Q.; AlJuaid, M.A.; Kaur, G.; Silver, M.A.; Johnson, K.A.; et al. Atrial Fibrosis by Late Gadolinium Enhancement Magnetic Resonance Imaging and Catheter Ablation of Atrial Fibrillation: 5-Year Follow-Up Data. J. Am. Heart Assoc. 2018, 7, e006313. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Sekiya, K.; Asai, M.; Iida, O.; Okamoto, S.; Ishihara, T.; Nanto, K.; Kanda, T.; Tsujimura, T.; Matsuda, Y.; et al. Influence of catheter ablation for atrial fibrillation on atrial and ventricular functional mitral regurgitation. ESC Heart Fail. 2022, 9, 1901–1913. [Google Scholar] [CrossRef]
- Cawley, P.J.; Hamilton-Craig, C.; Owens, D.S.; Krieger, E.V.; Strugnell, W.E.; Mitsumori, L.; D’jang, C.L.; Schwaegler, R.G.; Nguyen, K.Q.; Nguyen, B.; et al. Prospective Comparison of Valve Regurgitation Quantitation by Cardiac Magnetic Resonance Imaging and Transthoracic Echocardiography. Circ. Cardiovasc. Imaging 2013, 6, 48–57. [Google Scholar] [CrossRef]
- Delgado, V.; Hundley, W.G. Added Value of Cardiovascular Magnetic Resonance in Primary Mitral Regurgitation. Circulation 2018, 137, 1361–1363. [Google Scholar] [CrossRef]
- Kim, H.M.; Cho, G.-Y.; Hwang, I.-C.; Choi, H.-M.; Park, J.-B.; Yoon, Y.E.; Kim, H.-K. Myocardial Strain in Prediction of Outcomes After Surgery for Severe Mitral Regurgitation. JACC Cardiovasc. Imaging 2018, 11, 1235–1244. [Google Scholar] [CrossRef]
- Kamperidis, V.; Marsan, N.A.; Delgado, V.; Bax, J.J. Left ventricular systolic function assessment in secondary mitral regurgitation: Left ventricular ejection fraction vs. speckle tracking global longitudinal strain. Eur. Heart J. 2016, 37, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Koukos, M.; Dimitroglou, Y.; Tsartsalis, D.; Beneki, E.; Tolis, E.; Patsourakos, D.; Kalompatsou, A.; Aggeli, C.; Tsioufis, K. Left Atrium: A New Prognostic Marker and Therapeutic Target in Secondary Mitral Regurgitation? Eur. Cardiol. 2024, 19, e04. [Google Scholar] [CrossRef]
- Cramariuc, D.; Alfraidi, H.; Nagata, Y.; Levine, R.A.; van Kampen, A.; Andrews, C.; Hung, J. Atrial Dysfunction in Significant Atrial Functional Mitral Regurgitation: Phenotypes and Prognostic Implications. Circ. Cardiovasc. Imaging 2023, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, A.; Rossi, L.; Zanni, A.; Sticozzi, C.; Piepoli, M.F.; Benfari, G. Quantified mitral regurgitation and left atrial function in heart failure with reduced ejection fraction: Interplay and outcome implications. Eur. J. Heart Fail. 2022, 24, 694–702. [Google Scholar] [CrossRef]
- Matta, M.; Ayoub, C.; Hassan, O.K.A.; Layoun, H.; Cremer, P.C.; Hussein, A.; Schoenhagen, P.; Saliba, W.I.; Rodriguez, L.L.; Griffin, B.P.; et al. Anatomic and Functional Determinants of Atrial Functional Mitral Regurgitation. Struct. Heart 2021, 5, 498–507. [Google Scholar] [CrossRef]
- Deferm, S.; Bertrand, P.B.; Verbrugge, F.H.; Verhaert, D.; Rega, F.; Thomas, J.D.; Vandervoort, P.M. Atrial Functional Mitral Regurgitation. J. Am. Coll Cardiol. 2019, 73, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, V.; Raafs, A.; Manca, P.; Henkens, M.T.; Gregorio, C.; Boscutti, A.; Verdonschot, J.; Hazebroek, M.; Knackstedt, C.; Merlo, M.; et al. Left Atrial Reverse Remodeling in Dilated Cardiomyopathy. J. Am. Soc. Echocardiogr. 2023, 36, 154–162. [Google Scholar] [CrossRef]
- Deferm, S.; Bertrand, P.B.; Verhaert, D.; Dauw, J.; Van Keer, J.M.; Van De Bruaene, A.; Herregods, M.-C.; Meuris, B.; Verbrugghe, P.; Rex, S.; et al. Outcome and durability of mitral valve annuloplasty in atrial secondary mitral regurgitation. Heart 2021, 107, 1503–1509. [Google Scholar] [CrossRef]
- Soulat-Dufour, L.; Lang, S.; Addetia, K.; Ederhy, S.; Adavane-Scheuble, S.; Chauvet-Droit, M.; Jean, M.-L.; Nhan, P.; Ben Said, R.; Kamami, I.; et al. Restoring Sinus Rhythm Reverses Cardiac Remodeling and Reduces Valvular Regurgitation in Patients with Atrial Fibrillation. J. Am. Coll Cardiol. 2022, 79, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Baalman, S.W.E.; Berg, N.W.E.v.D.; Neefs, J.; Berger, W.R.; Meulendijks, E.R.; de Bruin-Bon, R.H.A.C.M.; Bouma, B.J.; van Boven, W.J.P.; Driessen, A.H.G.; de Groot, J.R. Left atrial strain and recurrence of atrial fibrillation after thoracoscopic surgical ablation: A subanalysis of the AFACT study. Int. J. Cardiovasc. Imaging 2022, 38, 2615–2624. [Google Scholar] [CrossRef]
- Klocek, K.; Klimek, K.; Tworek, M.; Wrona-Kolasa, K.; Cichoń, M.; Wybraniec, M.; Mizia-Stec, K. Efficacy of Electrical Cardioversion in Relation to Occurrence and Type of Functional Mitral Regurgitation in Patients with Atrial Fibrillation. J. Clin. Med. 2022, 11, 2069. [Google Scholar] [CrossRef]
- Huang, Z.S.; Fan, R.; Xie, P.H.; Zhong, J.L.; Zhang, S.Z.; Zhuang, X.D.; Liao, X.X. Dapagliflozin effect on functional mitral regurgitation and myocardial remodeling (DEFORM trial). Eur. Heart J. 2023, 44, ehad655.1751. [Google Scholar] [CrossRef]
- Kang, D.-H.; Park, S.-J.; Shin, S.-H.; Hong, G.-R.; Lee, S.; Kim, M.-S.; Yun, S.-C.; Song, J.-M.; Park, S.-W.; Kim, J.-J. Angiotensin Receptor Neprilysin Inhibitor for Functional Mitral Regurgitation. Circulation 2019, 139, 1354–1365. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Omar, A.M.S.; Liu, Y.; Murphy, S.; Butler, J.; Felker, G.M.; Piña, I.L.; Ward, J.; Solomon, S.; Contreras, J. Association Between Sacubitril/Valsartan Initiation and Mitral Regurgitation Severity in Heart Failure with Reduced Ejection Fraction: The PROVE-HF Study. Circulation 2022, 146, 1638–1640. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Z.; Liu, L.; Kong, H.; Wang, H.; Dong, W.; Bai, L.; Wang, J.; Sun, Z.; Zhang, J.; et al. ARNI or ARB Treats Residual Left Ventricular Remodelling after Surgery for Valvular Regurgitation: ReReRe study protocol. ESC Heart Fail. 2022, 9, 3585–3592. [Google Scholar] [CrossRef]
- Amabile, A.; Fereydooni, S.; Mori, M.; Hameed, I.; Jung, J.; Krane, M.; Geirsson, A. Variable definitions and treatment approaches for atrial functional mitral regurgitation: A scoping review of the literature. J. Card. Surg. 2022, 37, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Domene, R.; Canovas, S.J. Going further of mitral ring annuloplasty: The role of surgery in atrial functional mitral regurgitation. J. Thorac. Dis. 2023, 15, 2381–2384. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Shibata, T.; Hattori, K.; Kato, Y.; Motoki, M.; Morisaki, A.; Nishimura, S. Extended posterior leaflet extension for mitral regurgitation in giant left atrium. J. Heart Valve Dis. 2014, 23, 88–90. [Google Scholar] [PubMed]
- Shibata, T.; Takahashi, Y.; Fujii, H.; Morisaki, A.; Abe, Y. Surgical considerations for atrial functional regurgitation of the mitral and tricuspid valves based on the etiological mechanism. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Vohra, H.A.; Whistance, R.N.; Magan, A.; Sadeque, S.A.; Livesey, S.A. Mitral valve repair for severe mitral regurgitation secondary to lone atrial fibrillation. Eur. J. Cardio-Thoracic Surg. 2012, 42, 634–637. [Google Scholar] [CrossRef]
- Takahashi, Y.; Abe, Y.; Sasaki, Y.; Bito, Y.; Morisaki, A.; Nishimura, S.; Shibata, T. Mitral valve repair for atrial functional mitral regurgitation in patients with chronic atrial fibrillation. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 163–168. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Totsugawa, T.; Orihashi, K.; Kihara, K.; Tamura, K.; Hiraoka, A.; Chikazawa, G.; Yoshitaka, H. Mitral annuloplasty for atrial functional mitral regurgitation in patients with chronic atrial fibrillation. J. Card. Surg. 2019, 34, 767–773. [Google Scholar] [CrossRef]
- Takahashi, Y.; Abe, Y.; Takashi, M.; Fujii, H.; Morisaki, A.; Nishimura, S.; Sakon, Y.; Ito, K.; Shintani, A.; Shibata, T. Mid-term results of valve repairs for atrial functional mitral and tricuspid regurgitations. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 467–476. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Lv, M.; Yang, Z.; Zhu, S.; Wei, L.; Hong, T.; Ding, W.; Lin, Y.; Wang, C. Mitral valve repair and surgical ablation for atrial functional mitral regurgitation. Ann. Transl. Med. 2020, 8, 1420. [Google Scholar] [CrossRef]
- Matsumori, M.; Kawashima, M.; Aihara, T.; Fujisue, J.; Fujimoto, M.; Fukase, K.; Nomura, Y.; Tanaka, H.; Murakami, H.; Mukohara, N. Efficacy of left atrial plication for atrial functional mitral regurgitation. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 458–465. [Google Scholar] [CrossRef] [PubMed]
- El-Andari, R.; Watkins, A.R.; Fialka, N.M.; Kang, J.J.; Bozso, S.J.; Hassanabad, A.F.; Vasanthan, V.; Adams, C.; Cook, R.; Moon, M.C.; et al. Minimally Invasive Approaches to Mitral Valve Surgery: Where Are We Now? A Narrative Review. Can. J. Cardiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Balogh, Z.; Mizukami, T.; Bartunek, J.; Collet, C.; Beles, M.; Albano, M.; Katbeh, A.; Casselman, F.; Vanderheyden, M.; Van Camp, G.; et al. Mitral Valve Repair of Atrial Functional Mitral Regurgitation in Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2020, 9, 3432. [Google Scholar] [CrossRef] [PubMed]
- Hirji, S.A.; Cote, C.L.; Javadikasgari, H.; Malarczyk, A.; McGurk, S.; Kaneko, T. Atrial functional versus ventricular functional mitral regurgitation: Prognostic implications. J. Thorac. Cardiovasc. Surg. 2022, 164, 1808–1815.e4. [Google Scholar] [CrossRef]
- Carino, D.; Lapenna, E.; Ascione, G.; Ruggeri, S.; Del Forno, B.; Castiglioni, A.; Alfieri, O.; De Bonis, M. Is mitral annuloplasty an effective treatment for severe atrial functional mitral regurgitation? J. Card. Surg. 2021, 36, 596–602. [Google Scholar] [CrossRef]
- Kawamoto, N.; Fukushima, S.; Kainuma, S.; Ikuta, A.; Tadokoro, N.; Kakuta, T.; Fujita, T. Mitral valve surgery for atrial functional mitral regurgitation: Predicting recurrent mitral regurgitation and mid-term outcome. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 761–769. [Google Scholar] [CrossRef]
- Kwon, M.H.; Lee, L.S.; Cevasco, M.; Couper, G.S.; Shekar, P.S.; Cohn, L.H.; Chen, F.Y. Recurrence of mitral regurgitation after partial versus complete mitral valve ring annuloplasty for functional mitral regurgitation. J. Thorac. Cardiovasc. Surg. 2013, 146, 616–622. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, Y.; Bai, C.; Liu, K.; Zhao, C.; Liu, Y.; Li, Y.; Wang, J. Outcome of mitral repair combined with Cox-maze procedure for atrial functional mitral regurgitation with heart failure with recovered ejection fraction. Eur. J. Cardio-Thoracic Surg. 2023, 64, ezad273. [Google Scholar] [CrossRef]
- Song, K.; Lee, J.H.; Woo, H.T.; Kim, Y.S.; Jang, W.S.; Chung, S.; Cho, Y.H.; Kim, W.S.; Sung, K. Surgical Outcome of Mitral Valve Surgery in Atrial Functional Mitral Regurgitation Compared with Degenerative Etiology. J. Thorac. Cardiovasc. Surg. 2024. [Google Scholar] [CrossRef]
- Stone, G.W.; Abraham, W.T.; Lindenfeld, J.; Kar, S.; Grayburn, P.A.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Rinaldi, M.; Kapadia, S.R.; et al. Five-Year Follow-up after Transcatheter Repair of Secondary Mitral Regurgitation. New Engl. J. Med. 2023, 388, 2037–2048. [Google Scholar] [CrossRef]
- Ailawadi, G.; Lim, D.S.; Mack, M.J.; Trento, A.; Kar, S.; Grayburn, P.A.; Glower, D.D.; Wang, A.; Foster, E.; Qasim, A.; et al. One-Year Outcomes After MitraClip for Functional Mitral Regurgitation. Circulation 2019, 139, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Alqeeq, B.F.; Al-Tawil, M.; Hamam, M.; Aboabdo, M.; Elrayes, M.I.; Leick, J.; Zeinah, M.; Haneya, A.; Harky, A. Transcatheter edge-to-edge repair in mitral regurgitation: A comparison of device systems and recommendations for tailored device selection. A systematic review and meta-analysis. Prog. Cardiovasc. Dis. 2023, 81, 98–104. [Google Scholar] [CrossRef]
- Makkar, R.R.; Chikwe, J.; Chakravarty, T.; Chen, Q.; O’gara, P.T.; Gillinov, M.; Mack, M.J.; Vekstein, A.; Patel, D.; Stebbins, A.L.; et al. Transcatheter Mitral Valve Repair for Degenerative Mitral Regurgitation. JAMA 2023, 329, 1778. [Google Scholar] [CrossRef]
- Lim, D.S.; Smith, R.L.; Gillam, L.D.; Zahr, F.; Chadderdon, S.; Makkar, R.; von Bardeleben, R.S.; Kipperman, R.M.; Rassi, A.N.; Szerlip, M.; et al. Randomized Comparison of Transcatheter Edge-to-Edge Repair for Degenerative Mitral Regurgitation in Prohibitive Surgical Risk Patients. JACC Cardiovasc. Interv. 2022, 15, 2523–2536. [Google Scholar] [CrossRef]
- Tanaka, T.; Sugiura, A.; Öztürk, C.; Vogelhuber, J.; Tabata, N.; Wilde, N.; Zimmer, S.; Nickenig, G.; Weber, M. Transcatheter Edge-to-Edge Repair for Atrial Secondary Mitral Regurgitation. JACC Cardiovasc. Interv. 2022, 15, 1731–1740. [Google Scholar] [CrossRef]
- Rubbio, A.P.; Testa, L.; Grasso, C.; Sisinni, A.; Tusa, M.; Agricola, E.; De Marco, F.; Petronio, A.S.; Montorfano, M.; Citro, R.; et al. Transcatheter edge-to-edge mitral valve repair in atrial functional mitral regurgitation: Insights from the multi-center MITRA-TUNE registry. Int. J. Cardiol. 2022, 349, 39–45. [Google Scholar] [CrossRef]
- Claeys, M.J.; Debonnaire, P.; Bracke, V.; Bilotta, G.; Shkarpa, N.; Vanderheyden, M.; Coussement, P.; Vanderheyden, J.; Van de Heyning, C.M.; Cosyns, B.; et al. Clinical and Hemodynamic Effects of Percutaneous Edge-to-Edge Mitral Valve Repair in Atrial Versus Ventricular Functional Mitral Regurgitation. Am. J. Cardiol. 2021, 161, 70–75. [Google Scholar] [CrossRef]
- Benito-González, T.; Carrasco-Chinchilla, F.; Estévez-Loureiro, R.; Pascual, I.; Arzamendi, D.; Garrote-Coloma, C.; Nombela-Franco, L.; Pan, M.; Serrador, A.; Freixa, X.; et al. Clinical and echocardiographic outcomes of transcatheter mitral valve repair in atrial functional mitral regurgitation. Int. J. Cardiol. 2021, 345, 29–35. [Google Scholar] [CrossRef]
- Doldi, P.; Stolz, L.; Orban, M.; Karam, N.; Praz, F.; Kalbacher, D.; Lubos, E.; Braun, D.; Adamo, M.; Giannini, C.; et al. Transcatheter Mitral Valve Repair in Patients with Atrial Functional Mitral Regurgitation. JACC Cardiovasc. Imaging 2022, 15, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Sugiura, A.; Vogelhuber, J.; Öztürk, C.; Böhm, L.; Wilde, N.; Zimmer, S.; Nickenig, G.; Weber, M. Outcomes of transcatheter edge-to-edge repair for atrial functional mitral regurgitation. EuroIntervention 2024, 20, e250–e260. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Makar, M.; Kar, S.; Chakravarty, T.; Oakley, L.; Sekhon, N.; Koseki, K.; Nakamura, M.; Hamilton, M.; Patel, J.K.; et al. Outcomes After Transcatheter Edge-to-Edge Mitral Valve Repair According to Mitral Regurgitation Etiology and Cardiac Remodeling. JACC Cardiovasc. Interv. 2022, 15, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Simard, T.; Reddy, Y.N.V.; Thaden, J.J.; Padang, R.; Michelena, H.I.; Nkomo, V.T.; Lloyd, J.W.; El Sabbagh, A.; Nishimura, R.A.; Reeder, G.S.; et al. Atrial mitral regurgitation: Characteristics and outcomes of transcatheter mitral valve edge-to-edge repair. Catheter. Cardiovasc. Interv. 2022, 100, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Masiero, G.; Montonati, C.; Rubbio, A.P.; Adamo, M.; Grasso, C.; Denti, P.; Giordano, A.; Godino, C.; Bartorelli, A.L.; De Felice, F.; et al. Impact of Transcatheter Edge-to-Edge Mitral Valve Repair on Atrial Functional Mitral Regurgitation from the GIOTTO Registry. Am. J. Cardiol. 2024, 211, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Doldi, P.M.; Stolz, L.; Kassar, M.; Kalbacher, D.; Petronio, A.S.; Butter, C.; von Bardeleben, R.S.; Iliadis, C.; Grayburn, P.; Hausleiter, J. Paradox of disproportionate atrial functional mitral regurgitation and survival after transcatheter edge-to-edge repair. ESC Heart Fail. 2024, 11, 2447–2450. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, N.; Asch, F.M.; Ruf, T.; Petrescu, A.; von Bardeleben, S.; Lim, D.S.; Maisano, F.; Kar, S.; Price, M.J. Clinical Outcomes with Transcatheter Edge-to-Edge Repair in Atrial Functional MR From the EXPAND Study. JACC Cardiovasc. Interv. 2022, 15, 1723–1730. [Google Scholar] [CrossRef]
- Hamada, S.; Ueyama, H.; Aikawa, T.; Kampaktsis, P.N.; Misumida, N.; Takagi, H.; Kuno, T.; Latib, A. Outcomes of transcatheter edge-to-edge repair for atrial functional mitral regurgitation: A meta-analysis of observational studies. Catheter. Cardiovasc. Interv. 2023, 102, 751–760. [Google Scholar] [CrossRef]
- Moras, E.; Gandhi, K.; Koshy, A.N.; Bhatia, K.; Krittanawong, C.; Dominguez, A.C.; Argulian, E.; Stone, G.W. Mitral Transcatheter Edge-to-Edge Repair in Patients with Atrial Functional Mitral Regurgitation. J. Am. Coll Cardiol. 2024, 83, 1253–1255. [Google Scholar] [CrossRef]
- Pyrpyris, N.; Dimitriadis, K.; Theofilis, P.; Iliakis, P.; Beneki, E.; Pitsiori, D.; Tsioufis, P.; Shuvy, M.; Aznaouridis, K.; Tsioufis, K. Transcatheter Structural Heart Interventions in the Acute Setting: An Emerging Indication. J. Clin. Med. 2024, 13, 3528. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Lim, D.S.; Wang, J. Transcatheter mitral valve repair in patient with atrial functional mitral regurgitation using novel DragonFly™ device. Catheter. Cardiovasc. Interv. 2022, 99, 1691–1695. [Google Scholar] [CrossRef]
- Rottländer, D.; Gödde, M.; Degen, H.; Ögütcü, A.; Haude, M. Percutaneous Coronary Sinus-Based Mitral Valve Annuloplasty in Atrial Functional Mitral Regurgitation. JACC Cardiovasc. Interv. 2020, 13, 2947–2949. [Google Scholar] [CrossRef]
- Rottländer, D.; Golabkesh, M.; Degen, H.; Ögütcü, A.; Saal, M.; Haude, M. Mitral valve edge-to-edge repair versus indirect mitral valve annuloplasty in atrial functional mitral regurgitation. Catheter. Cardiovasc. Interv. 2022, 99, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Badano, L.P.; Hahn, R.T.; Lang, R.M.; Delgado, V.; Wunderlich, N.C.; Donal, E.; Taramasso, M.; Duncan, A.; Lurz, P.; et al. Atrial secondary tricuspid regurgitation: Pathophysiology, definition, diagnosis, and treatment. Eur. Heart J. 2024, 45, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Galloo, X.; Dietz, M.F.; Fortuni, F.; A Prihadi, E.; Cosyns, B.; Delgado, V.; Bax, J.J.; Marsan, N.A. Prognostic implications of atrial vs. ventricular functional tricuspid regurgitation. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 733–741. [Google Scholar] [CrossRef]
- Schlotter, F.; Dietz, M.F.; Stolz, L.; Kresoja, K.-P.; Besler, C.; Sannino, A.; Rommel, K.-P.; Unterhuber, M.; von Roeder, M.; Delgado, V.; et al. Atrial Functional Tricuspid Regurgitation: Novel Definition and Impact on Prognosis. Circ. Cardiovasc. Interv. 2022, 15, e011958. [Google Scholar] [CrossRef]
- Scotti, A.; Curio, J.; Leone, P.P.; Ludwig, S.; Coisne, A.; Sturla, M.; Murthy, S.; Chau, M.; Granada, J.F.; Jorde, U.P.; et al. Management of Volume Overload in Severe Atrial-Functional Tricuspid Regurgitation. JACC Case Rep. 2023, 12, 101776. [Google Scholar] [CrossRef]
| Study, Year | Participants (n) | Mean Age (years) | Gender (Female) | AF | Device Type | Post-Procedure Echocardiographic Assessment | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Technical Success | MR < 2+ | MR < 1+ | NYHA Class I/II | CV Mortality | All-Cause Mortality | HF | Adverse Events | ||||||
| Tanaka et al. [95], 2022 | 118 AFMR patients | 80 ± 8 | 60.2% | 90.7% | MitraClip (all generations) | 94.1% | 90.7% | 79.7% | NR | 0% | 2.5% | NR | None |
| Rubbio et al. [96], 2022 | 87 AFMR patients | 81 (78–83) | 61% | 100% | MitraClip (all generations) | 97% | 89% | NR | 79% | 4% | 5% | 4% | AKI:8% Major Bleeding: 4.5% |
| Claeys et al. [97], 2021 | 52 AFMR and 307 V-FMR patients | 79 ± 8 vs. 72 ±10 | 54% vs. 28% | 63% vs. 49% | MitraClip | 92% vs. 92% | 94% vs. 82% (pnon-inferiority < 0.001) | NR | 90% vs. 80% (p = NS) | NR | 0% vs. 3.3% (p = NS) | 95% vs. 87% | No significant difference |
| Benito-Gonzalez et al. [98], 2021 | 48 AFMR patients vs. 624 VFMR patients | 78.0 ± 7.5 vs. 70.7 ± 10.4 | 52.1% vs. 24% | 100% vs. 56.1% | MitraClip | 97.9% vs. 96% (p = NS) | 94.6% vs. 95.3% | 74.5% vs. 61.7% | 78.2% vs. 74.7% | AFMR: 74.9% free of events | No difference | ||
| Doldi et al. [99], 2022 | 126 AFMR patients vs. 1349 VFMR patients vs. 133 non-AFMR | 80.3 (76.3–83.4) vs. 74.0 (68.0–79.0) vs. 78.5 (74.6–82.0) | 61.1% vs. 30.2% vs. 50.4% | 78.6% vs. 58.2% vs. 64.7% | MitraClip | 87.2% vs. 94% vs. 93.3% | 87.2% vs. 94% vs. 93.3% | NR | AFMR: 73.4% | No difference between groups | |||
| Tanaka et al. [100], 2023 | 125 AFMR patients vs. 316 VFMR patients | 80 ± 6 vs. 76 ± 8 | 66.8% vs. 38.0% | 92.8% vs. 73.1% | Mitraclip (92%), PASCAL: 8% | NR | 92.0% vs. 93.9% | 77.6% vs. 71.2% | NR | AFMR: Residual MR with an MPG ≥ 5 mmHg was associated with a higher risk of the composite outcome (HR 2.31, 95% CI: 1.11–4.83; p = 0.025) (VFMR: Residual MR ≤ 1+ was also associated with a lower risk of the composite outcome (HR 0.56, 95% CI: 0.35–0.88; p = 0.012); | |||
| Yoon et al. [101], 2022 | 116 AFMR patients vs. 505 VFMR patients vs. 423 DMR patients | 78.8 ± 9.7 vs. 72.1 ± 12.8 vs. 80.6 ± 10.5 | 56.0% vs. 38.0% vs. 40.4% | 72.4% vs. 52.5% vs. 52.2% | MitraClip | NR | 94.8% vs. 96.8% vs. 97.4% | 86.2% vs. 84.7% vs. 86.0% | NR | VFMR vs. AFMR vs. DMR at 2 years: 31.1% vs. 26.8% vs. 17.7%, respectively | NR | NR | |
| Simard et al. [102], 2022 | 21 AFMR patients vs. 37 VFMR patients vs. 191 DMR patients | 82.4 ± 5.9 vs. 74 ± 9.4 vs. 79.8 ± 12.7 | 23.8% vs. 21.6% vs. 39.3% | 76.2% vs. 67.6% vs. 63.9% | MitraClip | NR | 90.4% vs. 89.1% vs. 90.6% | 52.3% vs. 64.8% vs. 67% | 63.6% vs. 51.9% vs. 67.5% | NR | 14% vs. 27% vs. 18% | NR | MACE at 1 year: 48% vs. 46% vs. 32% |
| Masiero et al. [103], 2024 | 71 AFMR patients vs. 541 iFMR patients vs. 541 niFMR patients | 79 [75–84] vs. 73 [69–79] vs. 72 [67–79] | 58% vs. 21% vs. 35% | 76% vs. 43% vs. 54% | MitraClip (all generations) | 100% vs. 98% vs. 99% | 97.2% vs. 91.1% vs. 90.4% | 67.6% vs. 57.5% vs. 54% | 80% vs. 79% vs. 90% | 3.1% vs. 2.4% vs. 2.7% | 4.8% vs. 3.2% vs. 4.4% | 1.7% vs. 3.1% vs. 2.8% | No difference in periprocedural adverse events |
| Doldi et al. [104], 2024 | 98 patients with AFMR (77 included, 52 proportionate, 25 disproportionate) | 80.0 [76.1, 84.0] vs. 81.0 [77.5, 83.5] | 65.4% vs. 76% | 76.9% vs. 84% | MitraClip | NR | Similar between groups | 84.3% vs. 80% | NR | NR | Disproportionate MR was associated with increased 2-year mortality (56% vs. 68%; p < 0.001) | NR | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliakis, P.; Dimitriadis, K.; Pyrpyris, N.; Beneki, E.; Theofilis, P.; Tsioufis, P.; Kamperidis, V.; Aznaouridis, K.; Aggeli, K.; Tsioufis, K. Atrial Functional Mitral Regurgitation: From Diagnosis to Current Interventional Therapies. J. Clin. Med. 2024, 13, 5035. https://doi.org/10.3390/jcm13175035
Iliakis P, Dimitriadis K, Pyrpyris N, Beneki E, Theofilis P, Tsioufis P, Kamperidis V, Aznaouridis K, Aggeli K, Tsioufis K. Atrial Functional Mitral Regurgitation: From Diagnosis to Current Interventional Therapies. Journal of Clinical Medicine. 2024; 13(17):5035. https://doi.org/10.3390/jcm13175035
Chicago/Turabian StyleIliakis, Panagiotis, Kyriakos Dimitriadis, Nikolaos Pyrpyris, Eirini Beneki, Panagiotis Theofilis, Panagiotis Tsioufis, Vasileios Kamperidis, Konstantinos Aznaouridis, Konstantina Aggeli, and Konstantinos Tsioufis. 2024. "Atrial Functional Mitral Regurgitation: From Diagnosis to Current Interventional Therapies" Journal of Clinical Medicine 13, no. 17: 5035. https://doi.org/10.3390/jcm13175035





