Overview of the Use of Optical Coherence Tomography Angiography in Neovascular Age-Related Macular Degeneration
Abstract
:1. Introduction
- It is a non-invasive procedure with practically no local or systemic adverse effects; at worst, allergic shock;
- Less severe dazzling;
- Shorter examination time; and
- Reliable reproducibility.
2. Age-Related Macular Degeneration
3. Diagnosis of AMD
4. The Morphology of MNV in nAMD
5. Activity Parameters and Biomarkers
6. Non-Exudative MNV
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Chen, Z. Doppler variance imaging for three-dimensional retina and choroid angiography. J. Biomed. Opt. 2010, 15, 016029. [Google Scholar] [CrossRef] [PubMed]
- Waldstein, S.M.; Faatz, H.; Szimacsek, M.; Glodan, A.-M.; Podkowinski, D.; Montuoro, A.; Simader, C.; Gerendas, B.S.; Schmidt-Erfurth, U. Comparison of penetration depth in choroidal imaging using swept source vs spectral domain optical coherence tomography. Eye 2015, 29, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Giani, A.; Cigada, M.; Esmaili, D.D.; Salvetti, P.; Luccarelli, S.; Marziani, E.; Luiselli, C.; Sabella, P.; Cereda, M.; Eandi, C.; et al. Artifacts in automatic retinal segmentation using different optical coherence tomography instruments. Retina 2010, 30, 607–616. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Optical Coherence Tomography Angiography. Retina 2015, 35, 2161–2162. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.E.; Enders, C.; Loidl, M.; Lang, G.K.; Werner, J.U. Accurate OCT-angiography Interpretation—Detection and Exclusion of Artifacts. Klin. Monbl. Augenheilkd 2017, 234, 1109–1118. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Hyman, L.; Neborsky, R. Risk factors for age-related macular degeneration: An update. Curr. Opin. Ophthalmol. 2002, 13, 171–175. [Google Scholar] [CrossRef]
- Supanji, S.; Romdhoniyyah, D.F.; Sasongko, M.B.; Agni, A.N.; Wardhana, F.S.; Widayanti, T.W.; Prayogo, M.E.; Perdamaian, A.B.I.; Dianratri, A.; Kawaichi, M.; et al. Associations of ARMS2 and CFH Gene Polymorphisms with Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2021, 15, 1101–1108. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Wang, L.; Clark, M.E.; Crossman, D.K.; Kojima, K.; Messinger, J.D.; Mobley, J.A.; Curcio, C.A. Abundant lipid and protein components of drusen. PLoS ONE 2010, 5, e10329. [Google Scholar] [CrossRef]
- Thurman, J.M.; Renner, B.; Kunchithapautham, K.; Ferreira, V.P.; Pangburn, M.K.; Ablonczy, Z.; Tomlinson, S.; Holers, V.M.; Rohrer, B. Oxidative Stress Renders Retinal Pigment Epithelial Cells Susceptible to Complement-mediated Injury. J. Biol. Chem. 2009, 284, 16939–16947. [Google Scholar] [CrossRef]
- Pauleikhoff, D.; Harper, C.A.; Marshall, J.; Bird, A.C. Aging Changes in Bruch’s Membrane: A Histochemical and Morphologic Study. Ophthalmology 1990, 97, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Matthé, E.; Sandner, D. Early treatment of exudative age-related macular degeneration with ranibizumab (Lucentis®): The key to success. Ophthalmologe 2011, 108, 237–243. [Google Scholar] [CrossRef]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S.; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Jung, J.J.; Balaratnasingam, C.; Dansingani, K.K.; Dhrami-Gavazi, E.; Suzuki, M.; de Carlo, T.E.; Shahlaee, A.; Klufas, M.A.; El Maftouhi, A.; et al. A Comparison between Optical Coherence Tomography Angiography and Fluorescein Angiography for the Imaging of Type 1 Neovascularization. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT314–OCT323. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Yu, S.; Gong, Y.; Wang, F.; Sun, X. The Diagnostic Accuracy of Optical Coherence Tomography Angiography for Neovascular Age-Related Macular Degeneration: A Comparison with Fundus Fluorescein Angiography. J. Ophthalmol. 2016, 2016, 7521478. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.C.; de Carlo, T.E.; Baumal, C.R.; Reichel, E.; Waheed, N.K.; Duker, J.S.; Witkin, A.J. Correlation of Spectral Domain Optical Coherence Tomography Angiography and Clinical Activity in Neovascular Age-Related Macular Degeneration. Retina 2016, 36, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, A.; Cicinelli, M.V.; Capuano, V.; Corvi, F.; Mazzaferro, A.; Querques, L.; Scorcia, V.; Souied, E.H.; Bandello, F.; Querques, G. Optical Coherence Tomography Angiography: A Useful Tool for Diagnosis of Treatment-Naïve Quiescent Choroidal Neovascularization. Am. J. Ophthalmol. 2016, 169, 189–198. [Google Scholar] [CrossRef]
- Ploner, S.B.; Moult, E.M.; Choi, W.; Waheed, N.K.; Lee, B.; Novais, E.A.; Cole, E.D.; Potsaid, B.; Husvogt, L.; Schottenhamml, J.; et al. Toward Quantitative Optical Coherence Tomography Angiography: Visualizing Blood Flow Speeds in Ocular Pathology Using Variable Interscan Time Analysis. Retina 2016, 36 (Suppl. 1), S118–S126. [Google Scholar] [CrossRef]
- Costanzo, E.; Miere, A.; Querques, G.; Capuano, V.; Jung, C.; Souied, E.H. Type 1 Choroidal Neovascularization Lesion Size: Indocyanine Green Angiography Versus Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT307–OCT313. [Google Scholar] [CrossRef]
- Lindner, M.; Fang, P.P.; Steinberg, J.S.; Domdei, N.; Pfau, M.; Krohne, T.U.; Schmitz-Valckenberg, S.; Holz, F.G.; Fleckenstein, M. OCT Angiography-Based Detection and Quantification of the Neovascular Network in Exudative AMD. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6342–6348. [Google Scholar] [CrossRef]
- Kuehlewein, L.; Bansal, M.; Lenis, T.L.; Iafe, N.A.; Sadda, S.R.; Bonini Filho, M.A.; De Carlo, T.E.; Waheed, N.K.; Duker, J.S.; Sarraf, D. Optical Coherence Tomography Angiography of Type 1 Neovascularization in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2015, 160, 739–748.e2. [Google Scholar] [CrossRef]
- El Ameen, A.; Cohen, S.Y.; Semoun, O.; Miere, A.; Srour, M.; Quaranta-El Maftouhi, M.; Oubraham, H.; Blanco-Garavito, R.; Querques, G.; Souied, E.H. Type 2 Neovascularization Secondary to Age-Related Macular Degeneration Imaged by Optical Coherence Tomography Angiography. Retina 2015, 35, 2212–2218. [Google Scholar] [CrossRef]
- Sulzbacher, F.; Pollreisz, A.; Kaider, A.; Kickinger, S.; Sacu, S.; Schmidt-Erfurth, U.; Vienna Eye Study Center. Identification and clinical role of choroidal neovascularization characteristics based on optical coherence tomography angiography. Acta Ophthalmol. 2017, 95, 414–420. [Google Scholar] [CrossRef]
- Pilotto, E.; Frizziero, L.; Daniele, A.R.; Convento, E.; Longhin, E.; Guidolin, F.; Parrozzani, R.; Cavarzeran, F.; Midena, E. Early OCT angiography changes of type 1 CNV in exudative AMD treated with anti-VEGF. Br. J. Ophthalmol. 2019, 103, 67–71. [Google Scholar] [CrossRef]
- Nakano, Y.; Kataoka, K.; Takeuchi, J.; Fujita, A.; Kaneko, H.; Shimizu, H.; Ito, Y.; Terasaki, H. Vascular maturity of type 1 and type 2 choroidal neovascularization evaluated by optical coherence tomography angiography. PLoS ONE 2019, 14, e0216304. [Google Scholar] [CrossRef]
- Faatz, H.; Rothaus, K.; Ziegler, M.; Book, M.; Heimes-Bussmann, B.; Pauleikhoff, D.; Lommatzsch, A. Vascular Analysis of Type 1, 2, and 3 Macular Neovascularization in Age-Related Macular Degeneration Using Swept-Source Optical Coherence Tomography Angiography Shows New Insights into Differences of Pathologic Vasculature and May Lead to a More Personalized Understanding. Biomedicines 2022, 10, 694. [Google Scholar] [CrossRef]
- Zarbin, M.A. Age-related macular degeneration: Review of pathogenesis. Eur. J. Ophthalmol. 1998, 8, 199–206. [Google Scholar] [CrossRef]
- Coscas, G.J.; Lupidi, M.; Coscas, F.; Cagini, C.; Souied, E.H. Optical Coherence Tomography Angiography versus Traditional Multimodal Imaging in Assessing the Activity of Exudative Age-Related Macular Degeneration: A New Diagnostic Challenge. Retina 2015, 35, 2219–2228. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, J.; Nah, S.K.; Lee, J.; Kim, C.G.; Kim, J.W. Nonexudative morphologic changes of neovascularization on optical coherence tomography angiography as predictive factors for exudative recurrence in age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 839–848. [Google Scholar] [CrossRef]
- Hikichi, T.; Agarie, M.; Kubo, N.; Yamauchi, M. Predictors of Recurrent Exudation in Choroidal Neovascularization in Age-Related Macular Degeneration during a Treatment-Free Period. Retina 2020, 40, 2158–2165. [Google Scholar] [CrossRef]
- Faatz, H.; Farecki, M.-L.; Rothaus, K.; Gutfleisch, M.; Pauleikhoff, D.; Lommatzsch, A. Changes in the OCT angiographic appearance of type 1 and type 2 CNV in exudative AMD during anti-VEGF treatment. BMJ Open Ophthalmol. 2019, 4, e000369. [Google Scholar] [CrossRef]
- Kuehlewein, L.; Sadda, S.R.; Sarraf, D. OCT angiography and sequential quantitative analysis of type 2 neovascularization after ranibizumab therapy. Eye 2015, 29, 932–935. [Google Scholar] [CrossRef]
- Tan, A.C.S.; Dansingani, K.K.; Yannuzzi, L.A.; Sarraf, D.; Freund, K.B. Type 3 Neovascularization Imaged with Cross-Sectional and En Face Optical Coherence Tomography Angiography. Retina 2017, 37, 234–246. [Google Scholar] [CrossRef]
- Xu, D.; Dávila, J.P.; Rahimi, M.; Rebhun, C.B.; Alibhai, A.Y.; Waheed, N.K.; Sarraf, D. Long-term Progression of Type 1 Neovascularization in Age-related Macular Degeneration Using Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2018, 187, 10–20. [Google Scholar] [CrossRef]
- Faatz, H.; Gunnemann, M.-L.; Rothaus, K.; Book, M.; Gutfleisch, M.; Lommatzsch, A.; Pauleikhoff, D. Influence of CNV vascular morphology in exudative age-related macular degeneration on development of visual acuity and need for anti-VEGF therapy after 1 year. Ophthalmologe 2021, 118, 154–161. [Google Scholar] [CrossRef]
- Faatz, H.; Rothaus, K.; Ziegler, M.; Book, M.; Spital, G.; Heimes-Bussmann, B.; Pauleikhoff, D.; Lommatzsch, A. Correlation of retinal alterations with vascular structure of macular neovascularisation in swept-source optical coherence tomography angiography in age-related macular degeneration. Int. Ophthalmol. 2022, 42, 1553–1562. [Google Scholar] [CrossRef]
- Boyer, D.S.; Antoszyk, A.N.; Awh, C.C.; Bhisitkul, R.B.; Shapiro, H.; Acharya, N.R.; MARINA Study Group. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology 2007, 114, 246–252. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Shapiro, H.; Tuomi, L.; Webster, M.; Elledge, J.; Blodi, B.; MARINA and ANCHOR Study Groups. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology 2011, 118, 523–530. [Google Scholar] [CrossRef]
- Ying, G.; Huang, J.; Maguire, M.G.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Daniel, E.; Klein, M.; Pieramici, D.; Wells, J.; et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology 2013, 120, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Steinle, N.C.; Du, W.; Gibson, A.; Saroj, N. Outcomes by Baseline Choroidal Neovascularization Features in Age-Related Macular Degeneration: A Post Hoc Analysis of the VIEW Studies. Ophthalmol. Retina 2021, 5, 141–150. [Google Scholar] [CrossRef]
- Arrigo, A.; Romano, F.; Aragona, E.; Di Nunzio, C.; Battista, M.; Bandello, F.; Battaglia Parodi, M. Optical Coherence Tomography Angiography Can Categorize Different Subgroups of Choroidal Neovascularization Secondary to Age-Related Macular Degeneration. Retina 2020, 40, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Aragona, E.; Bordato, A.; Amato, A.; Borghesan, F.; Bandello, F.; Battaglia Parodi, M. Morphological and Functional Relationship between OCTA and FA/ICGA Quantitative Features in AMD-Related Macular Neovascularization. Front. Med. (Lausanne) 2021, 8, 758668. [Google Scholar] [CrossRef] [PubMed]
- Faatz, H.; Rothaus, K.; Gunnemann, M.-L.; Book, M.; Wilming, P.; Gutfleisch, M.; Spital, G.; Lommatzsch, A.; Pauleikhoff, D. Morphologic analysis of macular neovascularizations by OCT angiography-Technical limitations in the comparison of 3×3mm and 6×6mm images. PLoS ONE 2020, 15, e0237785. [Google Scholar] [CrossRef] [PubMed]
- Faatz, H.; Rothaus, K.; Ziegler, M.; Book, M.; Lommatzsch, C.; Spital, G.; Gutfleisch, M.; Pauleikhoff, D.; Lommatzsch, A. Quantitative Comparison of the Vascular Structure of Macular Neovascularizations between Swept-Source and Spectral-Domain Optical Coherence Tomography Angiography. Clin. Ophthalmol. 2020, 14, 3179–3186. [Google Scholar] [CrossRef]
- Inoue, M.; Balaratnasingam, C.; Freund, K.B. Optical Coherence Tomography Angiography of Polypoidal Choroidal Vasculopathy and Polypoidal Choroidal Neovascularization. Retina 2015, 35, 2265–2274. [Google Scholar] [CrossRef]
- Rispoli, M.; Savastano, M.C.; Lumbroso, B. Quantitative Vascular Density Changes in Choriocapillaris around CNV after Anti-VEGF Treatment: Dark Halo. Ophthalmic. Surg. Lasers Imaging Retina 2018, 49, 918–924. [Google Scholar] [CrossRef]
- Viggiano, P.; Grassi, M.O.; Pignataro, M.; Boscia, G.; Borrelli, E.; Molfetta, T.; Evangelista, F.; Alessio, G.; Boscia, F. Topographical Analysis of the Choriocapillaris Reperfusion After Loading Anti-VEGF Therapy in Neovascular AMD. Transl. Vis. Sci. Technol. 2022, 11, 18. [Google Scholar] [CrossRef]
- Fossataro, F.; Cennamo, G.; Montorio, D.; Clemente, L.; Costagliola, C. Dark halo, a new biomarker in macular neovascularization: Comparison between OCT angiography and ICGA—A pilot prospective study. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 3205–3211. [Google Scholar] [CrossRef]
- Wang, J.; Hormel, T.T.; Gao, L.; Zang, P.; Guo, Y.; Wang, X.; Bailey, S.T.; Jia, Y. Automated diagnosis and segmentation of choroidal neovascularization in OCT angiography using deep learning. Biomed. Opt. Express 2020, 11, 927–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hormel, T.T.; Tsuboi, K.; Wang, X.; Ding, X.; Peng, X.; Huang, D.; Bailey, S.T.; Jia, Y. Deep Learning for Diagnosing and Segmenting Choroidal Neovascularization in OCT Angiography in a Large Real-World Data Set. Transl. Vis. Sci. Technol. 2023, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Yan, Y.; Chen, M.; Wang, J.; Pan, X.; Liu, X.; Liu, M.; Lou, L.; Wang, Y.; Ye, J. Multimodal deep learning with feature level fusion for identification of choroidal neovascularization activity in age-related macular degeneration. Acta Ophthalmol. 2021, 100, e512–e520. [Google Scholar] [CrossRef]
- Silva, R.; Cachulo, M.L.; Fonseca, P.; Bernardes, R.; Nunes, S.; Vilhena, N.; Faria de Abreu, J.R. Age-related macular degeneration and risk factors for the development of choroidal neovascularisation in the fellow eye: A 3-year follow-up study. Ophthalmologica 2011, 226, 110–118. [Google Scholar] [CrossRef]
- Macular Photocoagulation Study Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch. Ophthalmol. 1997, 115, 741–747. [Google Scholar] [CrossRef]
- Ferris, F.L.; Davis, M.D.; Clemons, T.E.; Lee, L.-Y.; Chew, E.Y.; Lindblad, A.S.; Milton, R.C.; Bressler, S.B.; Klein, R.; Age-Related Eye Disease Study (AREDS) Research Group. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch. Ophthalmol. 2005, 123, 1570–1574. [Google Scholar] [CrossRef]
- Roisman, L.; Zhang, Q.; Wang, R.K.; Gregori, G.; Zhang, A.; Chen, C.-L.; Durbin, M.K.; An, L.; Stetson, P.F.; Robbins, G.; et al. Optical Coherence Tomography Angiography of Asymptomatic Neovascularization in Intermediate Age-Related Macular Degeneration. Ophthalmology 2016, 123, 1309–1319. [Google Scholar] [CrossRef]
- de Oliveira Dias, J.R.; Zhang, Q.; Garcia, J.M.B.; Zheng, F.; Motulsky, E.H.; Roisman, L.; Miller, A.; Chen, C.-L.; Kubach, S.; de Sisternes, L.; et al. Natural History of Subclinical Neovascularization in Nonexudative Age-Related Macular Degeneration Using Swept-Source OCT Angiography. Ophthalmology 2018, 125, 255–266. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Motulsky, E.H.; Thulliez, M.; Shi, Y.; Lyu, C.; de Sisternes, L.; Durbin, M.K.; Feuer, W.; Wang, R.K.; et al. Two-Year Risk of Exudation in Eyes with Nonexudative Age-Related Macular Degeneration and Subclinical Neovascularization Detected with Swept Source Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2019, 208, 1–11. [Google Scholar] [CrossRef]
- Bailey, S.T.; Thaware, O.; Wang, J.; Hagag, A.M.; Zhang, X.; Flaxel, C.J.; Lauer, A.K.; Hwang, T.S.; Lin, P.; Huang, D.; et al. Detection of Nonexudative Choroidal Neovascularization and Progression to Exudative Choroidal Neovascularization Using OCT Angiography. Ophthalmol. Retina 2019, 3, 629–636. [Google Scholar] [CrossRef]
- Corvi, F.; Cozzi, M.; Invernizzi, A.; Pace, L.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography for detection of macular neovascularization associated with atrophy in age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef] [PubMed]
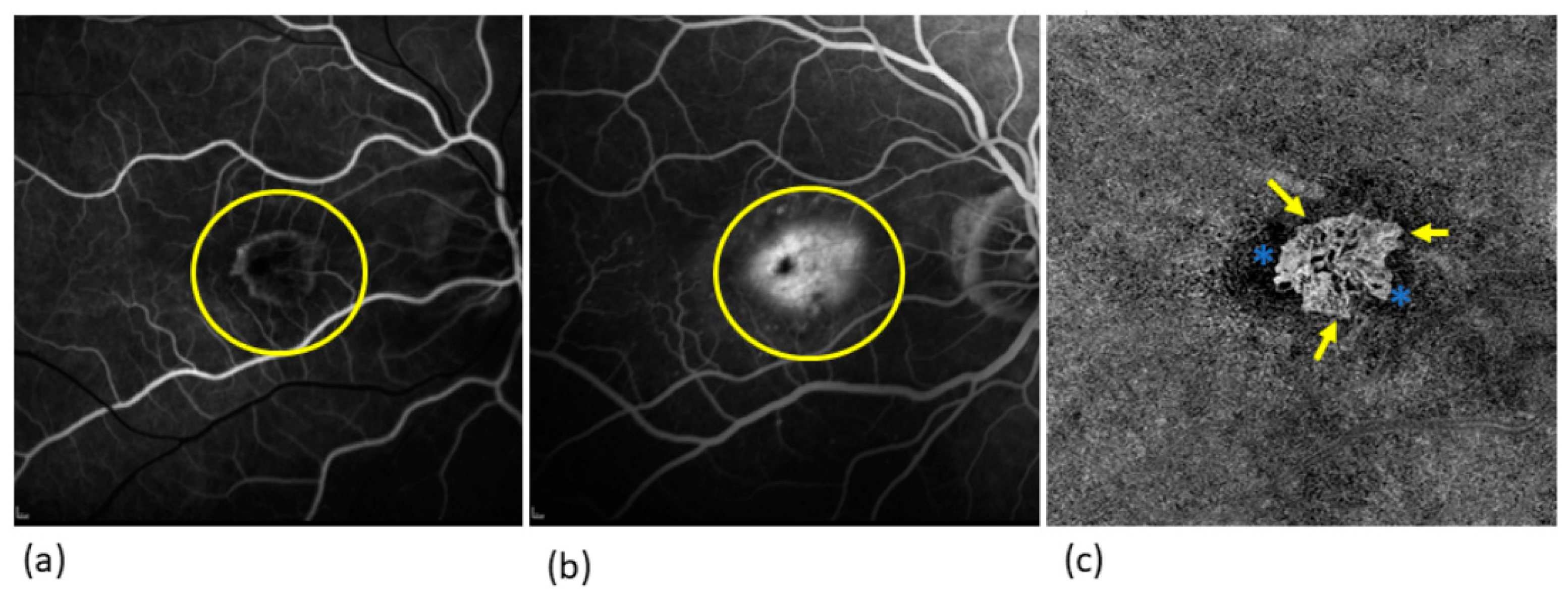

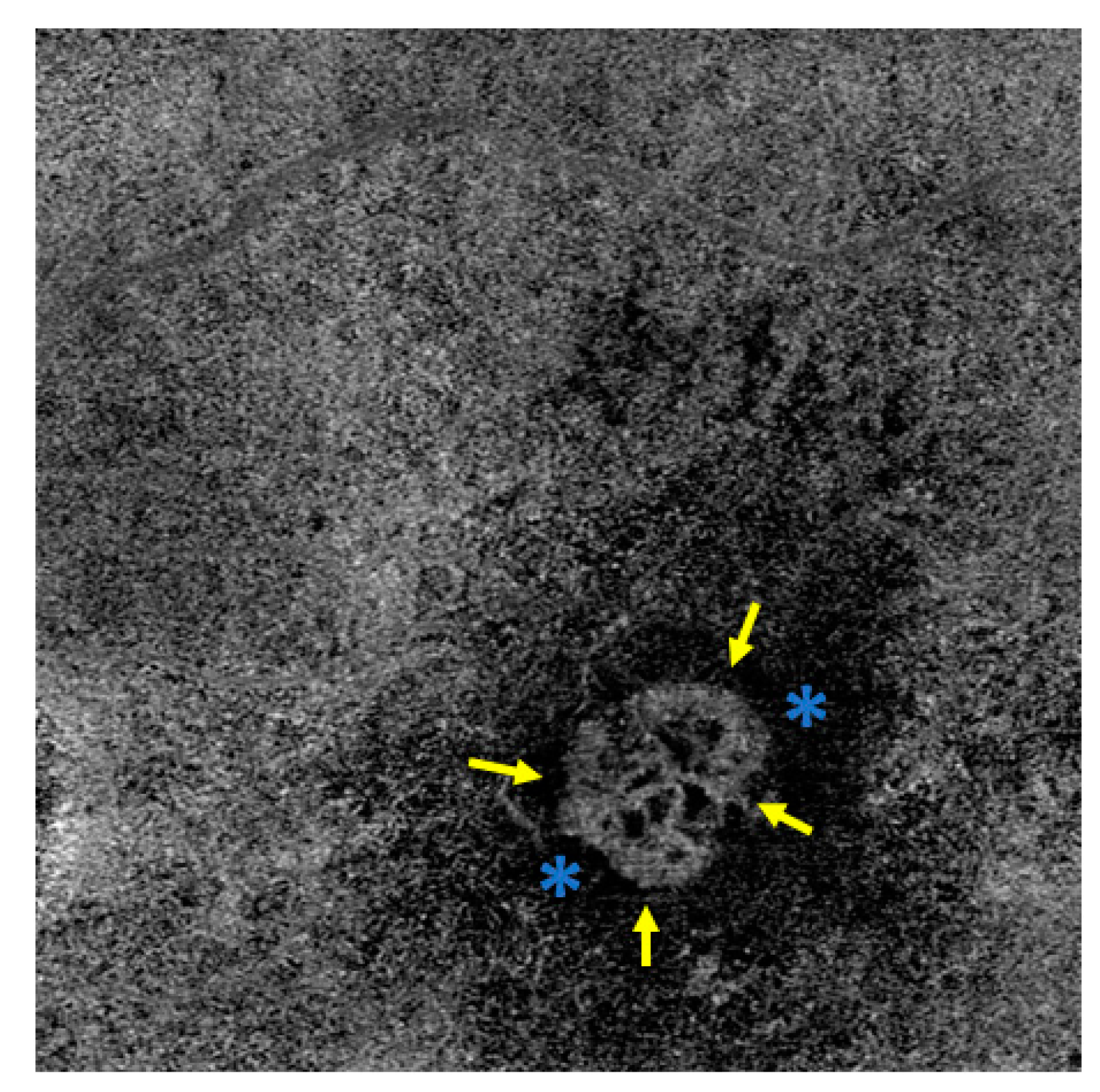

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faatz, H.; Lommatzsch, A. Overview of the Use of Optical Coherence Tomography Angiography in Neovascular Age-Related Macular Degeneration. J. Clin. Med. 2024, 13, 5042. https://doi.org/10.3390/jcm13175042
Faatz H, Lommatzsch A. Overview of the Use of Optical Coherence Tomography Angiography in Neovascular Age-Related Macular Degeneration. Journal of Clinical Medicine. 2024; 13(17):5042. https://doi.org/10.3390/jcm13175042
Chicago/Turabian StyleFaatz, Henrik, and Albrecht Lommatzsch. 2024. "Overview of the Use of Optical Coherence Tomography Angiography in Neovascular Age-Related Macular Degeneration" Journal of Clinical Medicine 13, no. 17: 5042. https://doi.org/10.3390/jcm13175042
APA StyleFaatz, H., & Lommatzsch, A. (2024). Overview of the Use of Optical Coherence Tomography Angiography in Neovascular Age-Related Macular Degeneration. Journal of Clinical Medicine, 13(17), 5042. https://doi.org/10.3390/jcm13175042






