Cardiothoracic Imaging for Outcome Prediction in Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Endarterectomy or Balloon Pulmonary Angioplasty: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Defining Outcomes and Analysis
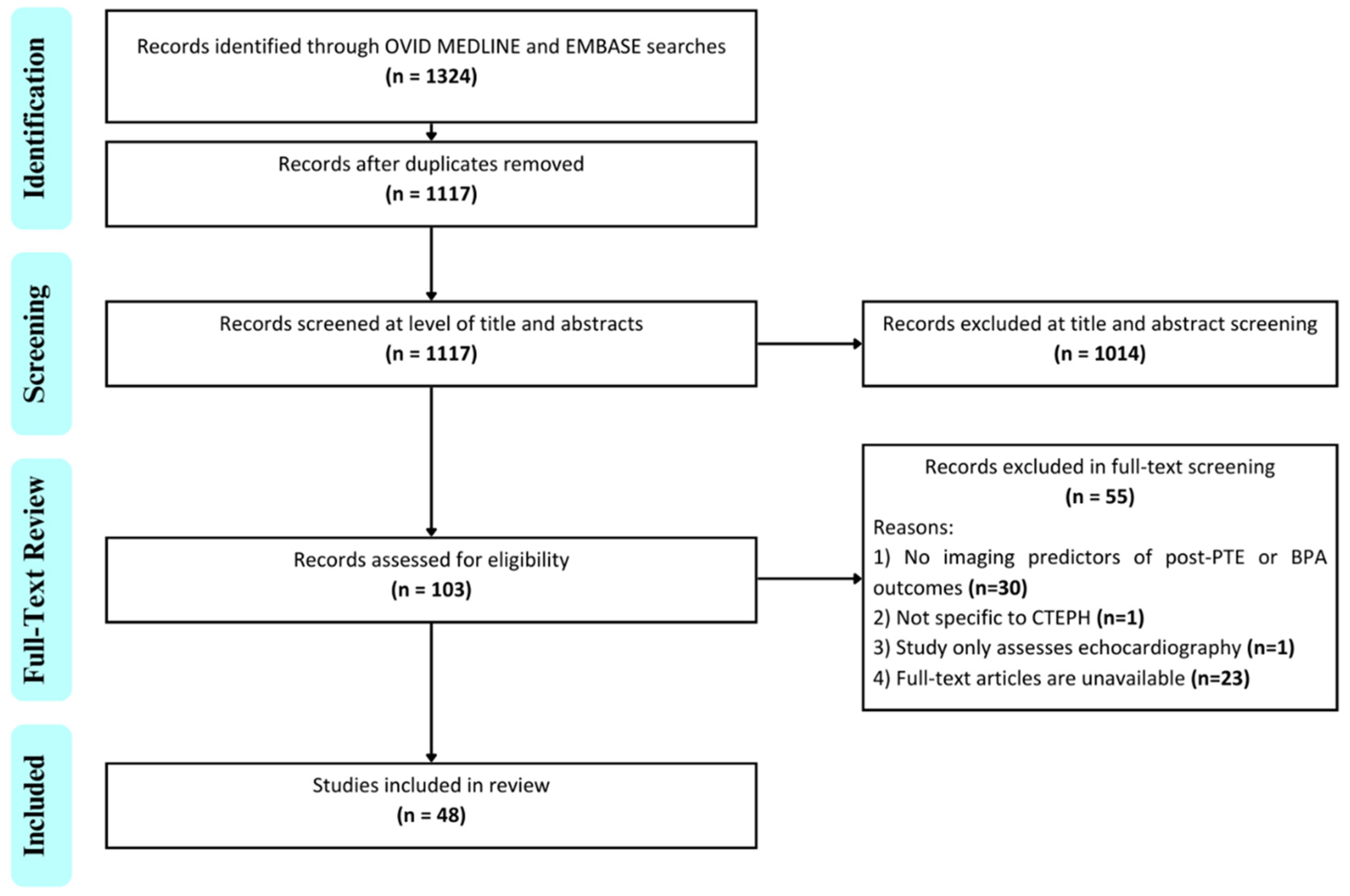
3. Results
3.1. Bibliometric Findings
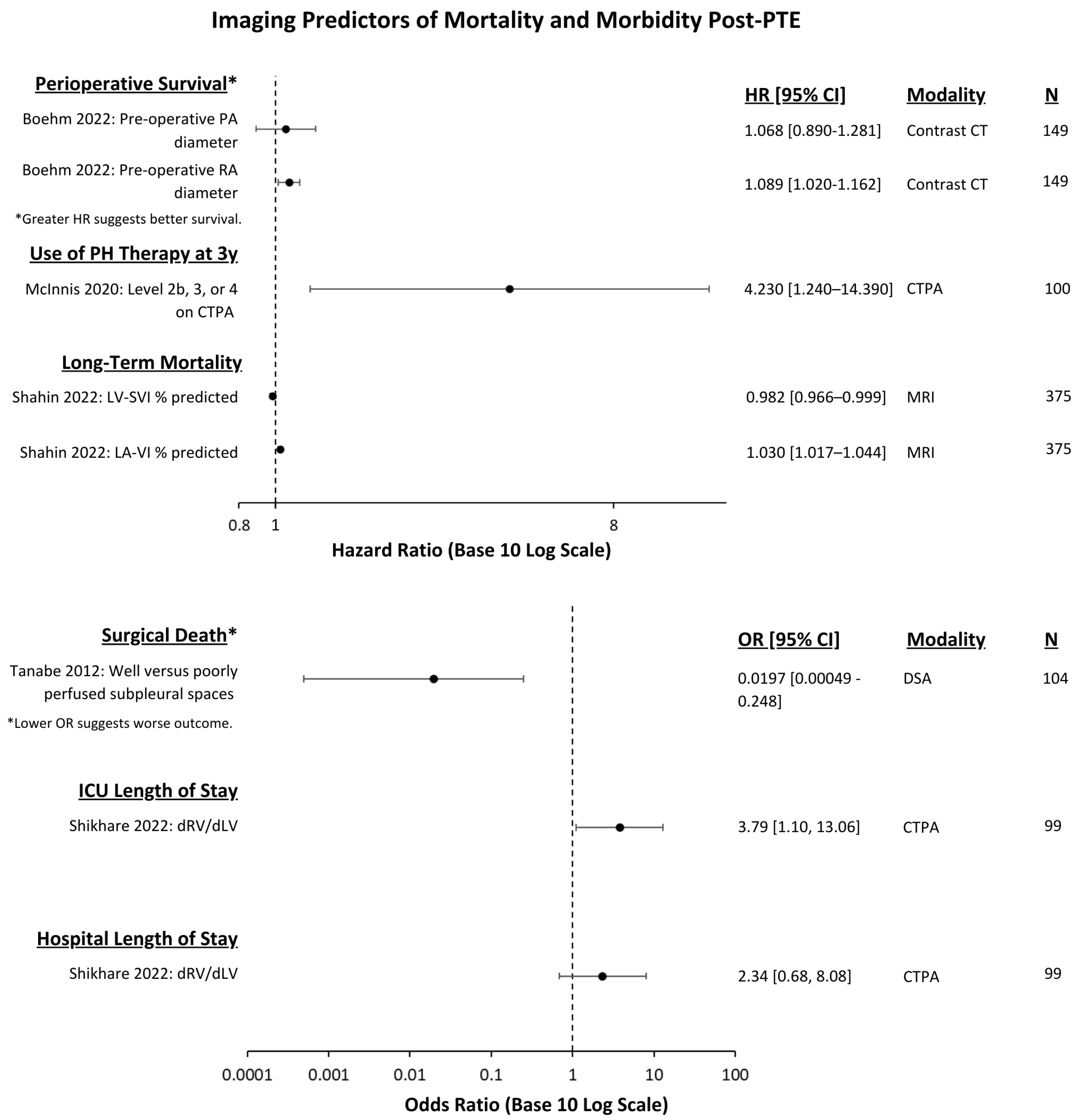
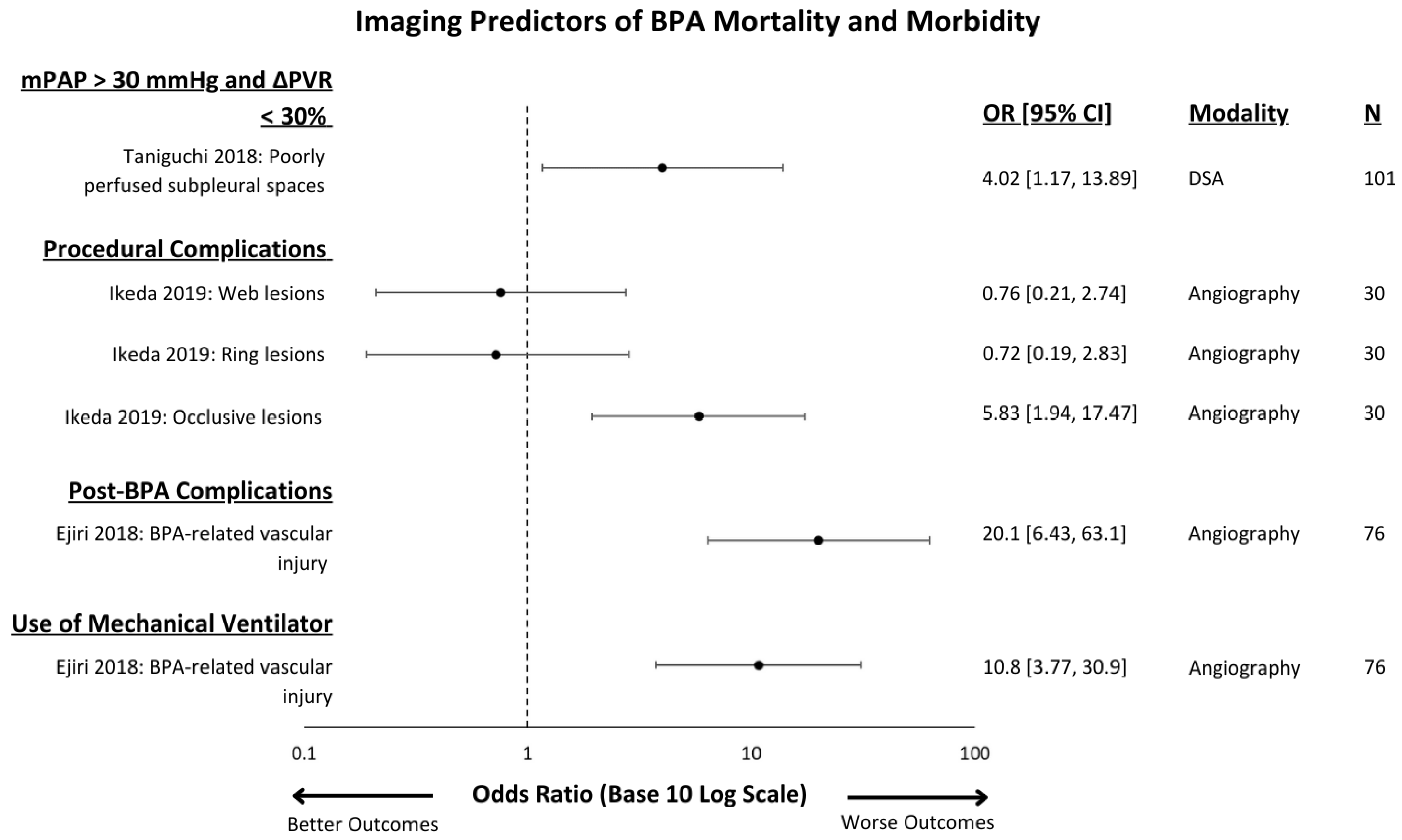
3.2. Outcomes Measured
3.3. Imaging Modality
4. Discussion
4.1. Future Directions
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ende-Verhaar, Y.M.; Cannegieter, S.C.; Noordegraaf, A.V.; Delcroix, M.; Pruszczyk, P.; Mairuhu, A.T.; Huisman, M.V.; Klok, F.A. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: A contemporary view of the published literature. Eur. Respir. J. 2017, 49, 1601792. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Remy-Jardin, M.; Ryerson, C.J.; Schiebler, M.L.; Leung, A.N.; Wild, J.M.; Hoeper, M.M.; Alderson, P.O.; Goodman, L.R.; Mayo, J.; Haramati, L.B.; et al. Imaging of pulmonary hypertension in adults: A position paper from the Fleischner Society. Eur. Respir. J. 2021, 57, 2004455. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhou, M.; Liu, D.; Long, X.; Guo, T.; Kong, X. Diagnostic accuracy of computed tomography for chronic thromboembolic pulmonary hypertension: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0126985. [Google Scholar] [CrossRef]
- Madani, M.; Mayer, E.; Fadel, E.; Jenkins, D.P. Pulmonary Endarterectomy. Patient Selection, Technical Challenges, and Outcomes. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S3), S240–S247. [Google Scholar] [CrossRef]
- Kennedy, M.K.; Kennedy, S.A.; Tan, K.T.; de Perrot, M.; Bassett, P.; McInnis, M.C.; Thenganatt, J.; Donahoe, L.; Granton, J.; Mafeld, S. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: A Systematic Review and Meta-analysis. Cardiovasc. Interv. Radiol. 2023, 46, 5–18. [Google Scholar] [CrossRef]
- Mayer, E.; Jenkins, D.; Lindner, J.; D’armini, A.; Kloek, J.; Meyns, B.; Ilkjaer, L.B.; Klepetko, W.; Delcroix, M.; Lang, I.; et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J. Thorac. Cardiovasc. Surg. 2011, 141, 702–710. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. IS 2010, 5, 69. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- OSF Registries|Applications of Thoracic Imaging in Outcome Prediction for Chronic Thromboembolic Pulmonary Hypertension after Surgery or Balloon Pulmonary Angioplasty: A Scoping Review Protocol. Available online: https://osf.io/g6vrn (accessed on 13 December 2023).
- Grob, D.; Smit, E.; Prince, J.; Kist, J.; Stöger, L.; Geurts, B.; Snoeren, M.M.; van Dijk, R.; Oostveen, L.J.; Prokop, M.; et al. Iodine Maps from Subtraction CT or Dual-Energy CT to Detect Pulmonary Emboli with CT Angiography: A Multiple-Observer Study. Radiology 2019, 292, 197–205. [Google Scholar] [CrossRef] [PubMed]
- McInnis, M.C.; Wang, D.; Donahoe, L.; Granton, J.; Thenganatt, J.; Tan, K.; Kavanagh, J.; de Perrot, M. Importance of computed tomography in defining segmental disease in chronic thromboembolic pulmonary hypertension. ERJ Open Res. 2020, 6, 00461–02020. [Google Scholar] [CrossRef]
- Eberhard, M.; McInnis, M.; de Perrot, M.; Lichtblau, M.; Ulrich, S.; Inci, I.; Opitz, I.; Frauenfelder, T. Dual-Energy CT Pulmonary Angiography for the Assessment of Surgical Accessibility in Patients with Chronic Thromboembolic Pulmonary Hypertension. Diagnostics 2022, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, A.; Dennie, C.J.; Muller, N.L.; Seely, J.M.; Matzinger, F.R.; Rubens, F.D. Chronic Thromboembolic Pulmonary Arterial Hypertension: Correlation of Postoperative Results of Thromboendarterectomy with Preoperative Helical Contrast-Enhanced Computed Tomography. J. Thorac. Imaging 2004, 19, 67–73. [Google Scholar] [CrossRef]
- Heinrich, M.; Uder, M.; Tscholl, D.; Grgic, A.; Kramann, B.; Schafers, H.J. CT scan findings in chronic thromboembolic pulmonary hypertension: Predictors of hemodynamic improvement after pulmonary thromboendarterectomy. Chest 2005, 127, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Sueyoshi, E.; Sakamoto, I.; Uetani, M.; Nakata, T.; Maemura, K. Quantification of lung perfusion blood volume (lung PBV) by dual-energy CT in patients with chronic thromboembolic pulmonary hypertension (CTEPH) before and after balloon pulmonary angioplasty (BPA): Preliminary results. Eur. J. Radiol. 2016, 85, 1607–1612. [Google Scholar] [CrossRef]
- Koike, H.; Sueyoshi, E.; Nishimura, T.; Iwano, Y.; Oka, T.; Uetani, M.; Maemura, K. Effect of Balloon Pulmonary Angioplasty on Homogenization of Lung Perfusion Blood Volume by Dual-Energy Computed Tomography in Patients with Chronic Thromboembolic Pulmonary Hypertension. Lung 2021, 199, 475–483. [Google Scholar] [CrossRef]
- Ikeda, N.; Kubota, S.; Okazaki, T.; Iijima, R.; Hara, H.; Hiroi, Y.; Nakamura, M. The predictors of complications in balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Catheter. Cardiovasc. Interv. 2019, 93, E349–E356. [Google Scholar] [CrossRef]
- Boehm, P.M.; Schwarz, S.; Thanner, J.; Veraar, C.; Gerges, M.; Gerges, C.; Lang, I.; Apfaltrer, P.; Prosch, H.; Taghavi, S.; et al. Larger pulmonary artery to ascending aorta ratios are associated with decreased survival of patients undergoing pulmonary endarterectomy. JTCVS Open 2022, 10, 62–72. [Google Scholar] [CrossRef]
- Tsukada, J.; Yamada, Y.; Kawakami, T.; Matsumoto, S.; Inoue, M.; Nakatsuka, S.; Okada, M.; Fukuda, K.; Jinzaki, M. Treatment effect prediction using CT after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur. Radiol. 2021, 31, 5524–5532. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Nagao, M.; Abe, K.; Hosokawa, K.; Kawanami, S.; Kamitani, T.; Yamanouchi, T.; Horimoto, K.; Yabuuchi, H.; Honda, H. Balloon pulmonary angioplasty improves interventricular dyssynchrony in patients with inoperable chronic thromboembolic pulmonary hypertension: A cardiac MR imaging study. Int. J. Cardiovasc. Imaging 2017, 33, 229–239. [Google Scholar] [CrossRef]
- Nishina, Y.; Inami, T.; Kataoka, M.; Kariyasu, T.; Shimura, N.; Ishiguro, H.; Yokoyama, K.; Yoshino, H.; Satoh, T. Evaluation of Right Ventricular Function on Cardiac Magnetic Resonance Imaging and Correlation with Hemodynamics in Patients with Chronic Thromboembolic Pulmonary Hypertension. Circ. Rep. 2020, 2, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Kunihara, T.; Möller, M.; Langer, F.; Sata, F.; Tscholl, D.; Aicher, D.; Schäfers, H.-J. Angiographic Predictors of Hemodynamic Improvement After Pulmonary Endarterectomy. Ann. Thorac. Surg. 2010, 90, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, N.; Sugiura, T.; Jujo, T.; Sakao, S.; Kasahara, Y.; Kato, H.; Masuda, M.; Tatsumi, K. Subpleural Perfusion as a Predictor for a Poor Surgical Outcome in Chronic Thromboembolic Pulmonary Hypertension. Chest 2012, 141, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Ogawa, A.; Miyaji, K.; Mizoguchi, H.; Shimokawahara, H.; Naito, T.; Oka, T.; Yunoki, K.; Munemasa, M.; Matsubara, H. Novel Angiographic Classification of Each Vascular Lesion in Chronic Thromboembolic Pulmonary Hypertension Based on Selective Angiogram and Results of Balloon Pulmonary Angioplasty. Circ. Cardiovasc. Interv. 2016, 9, e003318. [Google Scholar] [CrossRef]
- Hashimoto, H.; Oka, T.; Nakanishi, R.; Mizumura, S.; Dobashi, S.; Hashimoto, Y.; Okamura, Y.; Ota, K.; Ikeda, T. Evaluation of balloon pulmonary angioplasty using lung perfusion SPECT in patients with chronic thromboembolic pulmonary hypertension. J. Nucl. Cardiol. 2022, 29, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, Y.; Nagao, M.; Baba, S.; Isoda, T.; Kitamura, Y.; Yamazaki, Y.; Abe, K.; Sasaki, M.; Abe, K.; Honda, H. Three-dimensional fractal analysis of 99mTc-MAA SPECT images in chronic thromboembolic pulmonary hypertension for evaluation of response to balloon pulmonary angioplasty: Association with pulmonary arterial pressure. Nucl. Med. Commun. 2017, 38, 480–486. [Google Scholar] [CrossRef]
- Schölzel, B.E.; Post, M.C.; van de Bruaene, A.; Dymarkowski, S.; Wuyts, W.; Meyns, B.; Budts, W.; Delcroix, M. Prediction of hemodynamic improvement after pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension using non-invasive imaging. Int. J. Cardiovasc. Imaging. 2015, 31, 143–150. [Google Scholar] [CrossRef]
- Shahin, Y.; Alabed, S.; Quadery, S.R.; Lewis, R.A.; Johns, C.; Alkhanfar, D.; Sukhanenko, M.; Alandejani, F.; Garg, P.; Elliot, C.A.; et al. CMR Measures of Left Atrial Volume Index and Right Ventricular Function Have Prognostic Value in Chronic Thromboembolic Pulmonary Hypertension. Front. Med. 2022, 9, 840196. [Google Scholar] [CrossRef]
- Shikhare, S.; Balki, I.; Shi, Y.; Kavanagh, J.; Donahoe, L.; Xu, W.; Rozenberg, D.; de Perrot, M.; McInnis, M. Right-to-left ventricle ratio determined by machine learning algorithms on CT pulmonary angiography images predicts prolonged ICU length of stay in operated chronic thromboembolic pulmonary hypertension. Br. J. Radiol. 2022, 95, 20210722. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Brenot, P.; Jais, X.; Garcia, C.; Weatherald, J.; Planche, O.; Fadel, E.; Humbert, M.; Simonneau, G. Poor Subpleural Perfusion Predicts Failure After Balloon Pulmonary Angioplasty for Nonoperable Chronic Thromboembolic Pulmonary Hypertension. Chest 2018, 154, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Ejiri, K.; Ogawa, A.; Fujii, S.; Ito, H.; Matsubara, H. Vascular Injury Is a Major Cause of Lung Injury After Balloon Pulmonary Angioplasty in Patients with Chronic Thromboembolic Pulmonary Hypertension. Circ. Cardiovasc. Interv. 2018, 11, e005884. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Swift, A.J.; Capener, D.; Telfer, A.; Davies, C.; Hill, C.; Condliffe, R.; Elliot, C.; Hurdman, J.; Kiely, D.G.; et al. Diagnostic accuracy of contrast-enhanced MR angiography and unenhanced proton MR imaging compared with CT pulmonary angiography in chronic thromboembolic pulmonary hypertension. Eur. Radiol. 2012, 22, 310–317. [Google Scholar] [CrossRef]
- Rajaram, S.; Swift, A.J.; Telfer, A.; Hurdman, J.; Marshall, H.; Lorenz, E.; Capener, D.; Davies, C.; Hill, C.; Elliot, C.; et al. 3D contrast-enhanced lung perfusion MRI is an effective screening tool for chronic thromboembolic pulmonary hypertension: Results from the ASPIRE Registry. Thorax 2013, 68, 677–678. [Google Scholar] [CrossRef]
- Grothues, F.; Moon, J.C.; Bellenger, N.G.; Smith, G.S.; Klein, H.U.; Pennell, D.J. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am. Heart J. 2004, 147, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, K.R.; Ansari-Gilani, K. Quantification in Cardiovascular MRI: A Primer for Radiology Residents. RadioGraphics 2020, 40, 1832–1833. [Google Scholar] [CrossRef]
- Alabed, S.; Garg, P.; Johns, C.S.; Alandejani, F.; Shahin, Y.; Dwivedi, K.; Zafar, H.; Wild, J.M.; Kiely, D.G.; Swift, A.J.; et al. Cardiac Magnetic Resonance in Pulmonary Hypertension—An Update. Curr. Cardiovasc. Imaging Rep. 2020, 13, 30. [Google Scholar] [CrossRef]
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.H.; Ismail, N.A.; Dweck, M.R.; et al. Association of Fibrosis with Mortality and Sudden Cardiac Death in Patients with Nonischemic Dilated Cardiomyopathy. JAMA 2013, 309, 896–908. [Google Scholar] [CrossRef]
- Roller, F.C.; Wiedenroth, C.; Breithecker, A.; Liebetrau, C.; Mayer, E.; Schneider, C.; Rolf, A.; Hamm, C.; Krombach, G.A. Native T1 mapping and extracellular volume fraction measurement for assessment of right ventricular insertion point and septal fibrosis in chronic thromboembolic pulmonary hypertension. Eur. Radiol. 2017, 27, 1980–1991. [Google Scholar] [CrossRef]
- Leong, K.; Howard, L.; Giudice, F.L.; Davies, R.; Haji, G.; Gibbs, S.; Gopalan, D. Utility of cardiac magnetic resonance feature tracking strain assessment in chronic thromboembolic pulmonary hypertension for prediction of REVEAL 2.0 high risk status. Pulm. Circ. 2023, 13, e12116. [Google Scholar] [CrossRef]
- Kamada, H.; Ota, H.; Nakamura, M.; Sun, W.; Aoki, T.; Sato, H.; Sugimura, K.; Takase, K. Quantification of vortex flow in pulmonary arteries of patients with chronic thromboembolic pulmonary hypertension. Eur. J. Radiol. 2022, 148, 110142. [Google Scholar] [CrossRef]
- de Perrot, M.; Gopalan, D.; Jenkins, D.; Lang, I.M.; Fadel, E.; Delcroix, M.; Benza, R.; Heresi, G.A.; Kanwar, M.; Granton, J.T.; et al. Evaluation and management of patients with chronic thromboembolic pulmonary hypertension—Consensus statement from the ISHLT. J. Heart Lung Transplant. 2021, 40, 1301–1326. [Google Scholar] [CrossRef] [PubMed]
- Remy-Jardin, M.; Guiffault, L.; Oufriche, I.; Duhamel, A.; Flohr, T.; Schmidt, B.; Remy, J. Image quality of lung perfusion with photon-counting-detector CT: Comparison with dual-source, dual-energy CT. Eur. Radiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, J.B.; von Falck, C.; Hoeper, M.M.; Olsson, K.M.; Wacker, F.K.; Meyer, B.C.; Renne, J. Pulmonary Artery Imaging in Patients with Chronic Thromboembolic Pulmonary Hypertension: Comparison of Cone-Beam CT and 64-Row Multidetector CT. J. Vasc. Interv. Radiol. 2016, 27, 361–368.e2. [Google Scholar] [CrossRef] [PubMed]
- Grafham, G.K.; Bambrick, M.; Houbois, C.; Mafeld, S.; Donahoe, L.; de Perrot, M.; McInnis, M.C. Enhancing preoperative assessment in chronic thromboembolic pulmonary hypertension: A comprehensive analysis of interobserver agreement and proximity-based CT pulmonary angiography scoring. Heliyon 2023, 9, e20899. [Google Scholar] [CrossRef]
- Lambert, L.; Michalek, P.; Burgetova, A. The diagnostic performance of CT pulmonary angiography in the detection of chronic thromboembolic pulmonary hypertension-systematic review and meta-analysis. Eur. Radiol. 2022, 32, 7927–7935. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Luo, J.; Chen, J.; Luo, P.; Li, J. Comparative Efficacy and Safety of Targeted Therapies for Chronic Thromboembolic Pulmonary Hypertension: A Systematic Review and Network Meta-Analysis. Can. Respir. J. 2021, 2021, 1626971. [Google Scholar] [CrossRef]
- Li, W.; Yang, T.; Quan, R.L.; Chen, X.X.; An, J.; Zhao, Z.H.; Liu, Z.H.; Xiong, C.M.; He, J.G.; Gu, Q. Balloon pulmonary angioplasty reverse right ventricular remodelling and dysfunction in patients with inoperable chronic thromboembolic pulmonary hypertension: A systematic review and meta-analysis. Eur. Radiol. 2021, 31, 3898–3908. [Google Scholar] [CrossRef]
- Jorge, E.; Baptista, R.; Calisto, J.; Faria, H.; Monteiro, P.; Pan, M.; Pêgo, M. Optical coherence tomography of the pulmonary arteries: A systematic review. J. Cardiol. 2016, 67, 6–14. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Y.; Wang, X.; Liu, X.; Zheng, X.; Sun, G.; Zhen, Y.; Liu, M.; Ye, Z.; Wen, J.; et al. Radiomics signature of epicardial adipose tissue for predicting postoperative atrial fibrillation after pulmonary endarterectomy. Front. Cardiovasc. Med. 2023, 9, 1046931. [Google Scholar] [CrossRef]
- Niznansky, M.; Kavan, J.; Zemankova, P.; Prskavec, T.; Ambroz, D.; Jansa, P.; Lindner, J. Computed tomography angiographic parameters of pulmonary artery as prognostic factors of residual pulmonary hypertension after pulmonary endarterectomy. J. Int. Med. Res. 2021, 49, 3000605211002024. [Google Scholar] [CrossRef]
- Ruigrok, D.; Braams, N.J.; Nossent, E.J.; Bonta, P.I.; Boonstra, A.; Lely, R.J.; Klok, F.A.; Noordegraaf, A.V.; Symersky, P.; Bogaard, H.; et al. Dynamic vascular changes in chronic thromboembolic pulmonary hypertension after pulmonary endarterectomy. Pulm. Circ. 2020, 10, 2045894020907883. [Google Scholar] [CrossRef]
- Saito, T.; Kasai, H.; Sugiura, T.; Takahashi, Y.; Tajima, H.; Shigeta, A.; Sakao, S.; Tanabe, N.; Tatsumi, K. Effects of pulmonary endarterectomy on pulmonary hemodynamics in chronic thromboembolic pulmonary hypertension, evaluated by interventricular septum curvature. Pulm. Circ. 2020, 10, 2045894019897502. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.B.; Giannotta, M.; Palazzini, M.; Cefarelli, M.; Suàrez, S.M.; Gotti, E.; Reggiani, M.L.B.; Zompatori, M.; Galiè, N. A new CT-score as index of hemodynamic changes in patients with chronic thromboembolic pulmonary hypertension. Radiol Med (Torino) 2017, 122, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Tanabe, N.; Terada, J.; Masuda, M.; Sakao, S.; Kasahara, Y.; Takiguchi, Y.; Tatsumi, K.; Kuriyama, T. Dilatation of Bronchial Arteries Correlates with Extent of Central Disease in Patients with Chronic Thromboembolic Pulmonary Hypertension. Circ. J. 2008, 72, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Bergin, C.J.; Sirlin, C.; Deutsch, R.; Fedullo, P.; Hauschildt, J.; Huynh, T.; Auger, W.; Brown, M. Predictors of patient response to pulmonary thromboendarterectomy. AJR Am. J. Roentgenol. 2000, 174, 509–515. [Google Scholar] [CrossRef]
- Dong, M.L.; Azarine, A.; Haddad, F.; Amsallem, M.; Kim, Y.-W.; Yang, W.; Fadel, E.; Aubrege, L.; Loecher, M.; Ennis, D.; et al. 4D flow cardiovascular magnetic resonance recovery profiles following pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. J. Cardiovasc. Magn. Reson. 2022, 24, 59. [Google Scholar] [CrossRef]
- Frederiksen, C.A.; Waziri, F.; Ringgaard, S.; Mellemkjær, S.; Clemmensen, T.S.; Hjortdal, V.E.; Nielsen, S.L.; Poulsen, S.H. Reverse remodeling of tricuspid valve morphology and function in chronic thromboembolic pulmonary hypertension patients following pulmonary thromboendarterectomy: A cardiac magnetic resonance imaging and invasive hemodynamic study. BMC Cardiovasc. Disord. 2021, 21, 450. [Google Scholar] [CrossRef]
- Czerner, C.P.; Schoenfeld, C.; Cebotari, S.; Renne, J.; Kaireit, T.F.; Winther, H.B.; Pöhler, G.H.; Olsson, K.M.; Hoeper, M.M.; Wacker, F.; et al. Perioperative CTEPH patient monitoring with 2D phase-contrast MRI reflects clinical, cardiac and pulmonary perfusion changes after pulmonary endarterectomy. PLoS ONE. 2020, 15, e0238171. [Google Scholar] [CrossRef]
- Pöhler, G.H.; Klimes, F.; Voskrebenzev, A.; Behrendt, L.; Czerner, C.; Gutberlet, M.; Cebotari, S.; Ius, F.; Fegbeutel, C.; Schoenfeld, C.; et al. Chronic Thromboembolic Pulmonary Hypertension Perioperative Monitoring Using Phase-Resolved Functional Lung (PREFUL)-MRI. J. Magn. Reson. Imaging. 2020, 52, 610–619. [Google Scholar] [CrossRef]
- Waziri, F.; Ringgaard, S.; Mellemkjær, S.; Bøgh, N.; Kim, W.Y.; Clemmensen, T.S.; Hjortdal, V.E.; Nielsen, S.L.; Poulsen, S.H. Long-term changes of right ventricular myocardial deformation and remodeling studied by cardiac magnetic resonance imaging in patients with chronic thromboembolic pulmonary hypertension following pulmonary thromboendarterectomy. Int. J. Cardiol. 2020, 300, 282–288. [Google Scholar] [CrossRef]
- Berman, M.; Gopalan, D.; Sharples, L.; Screaton, N.; Maccan, C.; Sheares, K.; Pepke-Zaba, J.; Dunning, J.; Tsui, S.; Jenkins, D.P. Right Ventricular Reverse Remodeling after Pulmonary Endarterectomy: Magnetic Resonance Imaging and Clinical and Right Heart Catheterization Assessment. Pulm. Circ. 2014, 4, 36–44. [Google Scholar] [CrossRef]
- Reesink, H.J.; Marcus, J.T.; Tulevski, I.I.; Jamieson, S.; Kloek, J.J.; Noordegraaf, A.V.; Bresser, P. Reverse right ventricular remodeling after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: Utility of magnetic resonance imaging to demonstrate restoration of the right ventricle. J. Thorac. Cardiovasc. Surg. 2007, 133, 58–64. [Google Scholar] [CrossRef]
- Zhai, Z.; Ota, H.; Staring, M.; Stolk, J.; Sugimura, K.; Takase, K.; Stoel, B.C. Treatment Effect of Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension Quantified by Automatic Comparative Imaging in Computed Tomography Pulmonary Angiography. Investig. Radiol. 2018, 53, 286. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Kubota, S.; Okazaki, T.; Iijima, R.; Hara, H.; Hiroi, Y.; Nakamura, M. The impact of computerised tomography for prediction of clinical adverse events after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J. Am. Coll. Cardiol. 2018, 71 (Suppl. 11), A1936. [Google Scholar] [CrossRef]
- Sugimoto, K.; Nakazato, K.; Sakamoto, N.; Yamaki, T.; Kunii, H.; Yoshihisa, A.; Suzuki, H.; Saitoh, S.-I.; Takeishi, Y. Pulmonary Artery Diameter Predicts Lung Injury After Balloon Pulmonary Angioplasty in Patients with Chronic Thromboembolic Pulmonary Hypertension. Int. Heart J. 2017, 58, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Abe, K.; Kamitani, T.; Hosokawa, K.; Kawakubo, M.; Sagiyama, K.; Hida, T.; Matsuura, Y.; Murayama, Y.; Funatsu, R.; et al. Balloon pulmonary angioplasty improves right atrial reservoir and conduit functions in chronic thromboembolic pulmonary hypertension. Eur. Heart J.-Cardiovasc. Imaging. 2020, 21, 855–862. [Google Scholar] [CrossRef]
- Roller, F.C.; Schüssler, A.; Hasse, A.; Kriechbaum, S.; Richter, M.; Guth, S.; Tello, K.; Breithecker, A.; Liebetrau, C.; Hamm, C.W.; et al. Effects of BPA on right ventricular mechanical dysfunction in patients with inoperable CTEPH—A cardiac magnetic resonance study. Eur. J. Radiol. 2022, 147, 110111. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, M.; Yamasaki, Y.; Kamitani, T.; Sagiyama, K.; Matsuura, Y.; Hino, T.; Abe, K.; Hosokawa, K.; Yabuuchi, H.; Honda, H. Clinical usefulness of right ventricular 3D area strain in the assessment of treatment effects of balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension: Comparison with 2D feature-tracking MRI. Eur. Radiol. 2019, 29, 4583–4592. [Google Scholar] [CrossRef]
- Schoenfeld, C.; Hinrichs, J.B.; Olsson, K.M.; Kuettner, M.-A.; Renne, J.; Kaireit, T.; Czerner, C.; Wacker, F.; Hoeper, M.M.; Meyer, B.C.; et al. Cardio-pulmonary MRI for detection of treatment response after a single BPA treatment session in CTEPH patients. Eur. Radiol. 2019, 29, 1693–1702. [Google Scholar] [CrossRef]
- Nagao, M.; Yamasaki, Y.; Abe, K.; Hosokawa, K.; Kawanami, S.; Kamitani, T.; Yamanouchi, T.; Yabuuchi, H.; Fukushima, K.; Honda, H. Energy efficiency and pulmonary artery flow after balloon pulmonary angioplasty for inoperable, chronic thromboembolic pulmonary hypertension: Analysis by phase-contrast MRI. Eur. J. Radiol. 2017, 87, 99–104. [Google Scholar] [CrossRef]
- Kriechbaum, S.D.; Vietheer, J.M.; Wiedenroth, C.B.; Rudolph, F.; Barde, M.A.; Wolter, J.; Haas, M.; Fischer-Rasokat, U.; Weferling, M.M.; Rolf, A.; et al. Cardiac biomarkers as indicators of right ventricular dysfunction and recovery in chronic thromboembolic pulmonary hypertension patients after balloon pulmonary angioplasty therapy—A cardiac magnetic resonance imaging cohort study. Pulm. Circ. 2021, 11, 20458940211056500. [Google Scholar] [CrossRef] [PubMed]
- Maschke, S.K.; Renne, J.; Werncke, T.; Olsson, K.M.; Hoeper, M.M.; Wacker, F.K.; Meyer, B.C.; Hinrichs, J.B. Chronic thromboembolic pulmonary hypertension: Evaluation of 2D-perfusion angiography in patients who undergo balloon pulmonary angioplasty. Eur. Radiol. 2017, 27, 4264–4270. [Google Scholar] [CrossRef] [PubMed]
- Kinutani, H.; Shinke, T.; Nakayama, K.; Taniguchi, Y.; Otake, H.; Takaya, T.; Osue, T.; Konishi, A.; Emoto, N.; Hirata, K.-I. High perfusion pressure as a predictor of reperfusion pulmonary injury after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. IJC Heart Vasc. 2016, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]

| Imaging Modality | Hemodynamics | Mortality and Complications | Performance Capacity | |
|---|---|---|---|---|
| Computed Tomography | CTPA | (n = 7/48) | (n = 3/48) | (n = 1/48) |
| Other contrast-enhanced CT | (n = 4/48) | (n = 2/48) | (n = 1/48) | |
| Non-contrast | (n = 1/48) | (n = 2/48) | - | |
| Dual energy | (n = 2/48) | - | (n = 2/48) | |
| DSA | (n = 4/48) | (n = 5/48) | - | |
| Magnetic resonance imaging | (n = 17/48) | (n = 3/48) | (n = 6/48) | |
| SPECT | (n = 2/48) | - | - | |
| V/Q scintigraphy | - | - | - | |
| Hemodynamics | Complications | PC | |||||
|---|---|---|---|---|---|---|---|
| Significant Imaging Predictors | mPAP | PVR | CO | Mortality | Procedural Complication | Length of Stay | 6MWT |
| PULMONARY THROMBOENDARTERECTOMY | |||||||
| CT level of disease (n = 4) | (n = 2) | (n = 3) | (n = 1) | ||||
| Interventricular septal curvature (n = 1) | (n = 1) | (n = 1) | (n = 1) | ||||
| Composite CT score (n = 1) | (n = 1) | (n = 1) | |||||
| Right to left ventricle ratio (n = 1) | (n = 1) | ||||||
| Small vessel disease (n = 1) | (n = 1) | ||||||
| PA to aortic diameter ratio (n = 1) | (n = 1) | ||||||
| PA diameter (n = 1) | (n = 1) | ||||||
| BALLOON PULMONARY ANGIOPLASTY | |||||||
| Whole-lung PBV (n = 2) | (n = 1) | (n = 2) | (n = 2) | ||||
| Long-axis pulmonary bleeding (n = 1) | (n = 1) | ||||||
| Short-axis pulmonary bleeding (n = 1) | (n = 1) | ||||||
| Pulmonary bleeding volume (n = 1) | (n = 1) | ||||||
| PA diameter (n = 1) | (n = 1) | ||||||
| RA diameter (n = 1) | (n = 1) | ||||||
| Density changes in vascular centerlines (n = 1) | (n = 1) | (n = 1) | (n = 1) | ||||
| Density changes in parenchymal areas (n = 1) | (n = 1) | ||||||
| Hemodynamics | Complications | PC | |||||
|---|---|---|---|---|---|---|---|
| Significant Imaging Predictors | mPAP | PVR | CO | Mortality | Procedural Complication | Length of Stay | 6MWT |
| PULMONARY THROMBOENDARTERECTOMY | |||||||
| Minimum main PA area; minimum main PA volume; mean right PA centerline velocity; and maximum right PA spatial average helical flow index (each n = 1) | (n = 1) | ||||||
| Right heart strain (n = 1) | (n = 1) | (n = 1) | |||||
| LV-SVI; LAVI (n = 1) | (n = 1) | ||||||
| Δ Coaptation height (n = 1) | (n = 1) | ||||||
| Max mean velocity through main PA (n = 1) | (n = 1) | (n = 1) | |||||
| Average mean velocity in main PA (n = 1) | (n = 1) | ||||||
| Deceleration volume in main PA (n = 1) | (n = 1) | ||||||
| Median pPTT of whole lung; change in QDPpreful (each n = 1) | (n = 1) | ||||||
| Change in PREFULq (n = 1) | (n = 1) | ||||||
| RV-EDV; RV-ESV; diastolic RV mass; systolic RV mass (each n = 1) | (n = 1) | (n = 1) | |||||
| Flow per beat; flow per minute (each n = 1) | (n = 1) | ||||||
| BALLOON PULMONARY ANGIOPLASTY | |||||||
| ΔRA max volume; ΔRA min volume; ΔRA ejection fraction; ΔRA peak longitudinal strain; Δ peak LSR; ΔRA early LSR (each n = 1) | (n = 1) | ||||||
| Global longitudinal strain (n = 1) | (n = 1) | (n = 1) | |||||
| Apical area strain at RV apex (n = 1) | (n = 1) | ||||||
| Pulmonary blood flow ratio (n = 1) | (n = 1) | ||||||
| RV-EDVI (n = 2) | (n = 2) | ||||||
| RV-ESVI (n = 2) | (n = 2) | ||||||
| RV-EF (n = 1) | (n = 1) | ||||||
| Septal inversion ratio (n = 1) | (n = 1) | ||||||
| Hemodynamics | Complications | PC | |||||
|---|---|---|---|---|---|---|---|
| Significant Imaging Predictors | mPAP | PVR | CO | Mortality | Procedural Complication | Length of Stay | 6MWT |
| PULMONARY THROMBOENDARTERECTOMY | |||||||
| Pouch or membrane segments (n = 1) | (n = 1) | ||||||
| Subpleural perfusion (n = 1) | (n = 1) | (n = 1) | |||||
| BALLOON PULMONARY ANGIOPLASTY | |||||||
| Occlusive lesions (n = 1) | (n = 1) | ||||||
| Subtotal lesions (n = 1) | (n = 1) | ||||||
| Hemodynamics | Complications | PC | |||||
|---|---|---|---|---|---|---|---|
| Significant Imaging Predictors | mPAP | PVR | CO | Mortality | Procedural Complication | Length of Stay | 6MWT |
| BALLOON PULMONARY ANGIOPLASTY | |||||||
| Functional volume of lung (n = 1) | (n = 1) | ||||||
| Fractal dimension (n = 1) | (n = 1) | ||||||
| Total uptake volume (n = 1) | (n = 1) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, M.; Malik, S.; Karur, G.R.; Mafeld, S.; de Perrot, M.; McInnis, M.C. Cardiothoracic Imaging for Outcome Prediction in Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Endarterectomy or Balloon Pulmonary Angioplasty: A Scoping Review. J. Clin. Med. 2024, 13, 5045. https://doi.org/10.3390/jcm13175045
Malik M, Malik S, Karur GR, Mafeld S, de Perrot M, McInnis MC. Cardiothoracic Imaging for Outcome Prediction in Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Endarterectomy or Balloon Pulmonary Angioplasty: A Scoping Review. Journal of Clinical Medicine. 2024; 13(17):5045. https://doi.org/10.3390/jcm13175045
Chicago/Turabian StyleMalik, Mikail, Shamir Malik, Gauri R. Karur, Sebastian Mafeld, Marc de Perrot, and Micheal C. McInnis. 2024. "Cardiothoracic Imaging for Outcome Prediction in Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Endarterectomy or Balloon Pulmonary Angioplasty: A Scoping Review" Journal of Clinical Medicine 13, no. 17: 5045. https://doi.org/10.3390/jcm13175045





