Myocardial Fibrosis Quantification Methods by Cardiovascular Magnetic Resonance Imaging in Patients with Fabry Disease
Abstract
:1. Introduction
2. Materials and Methods
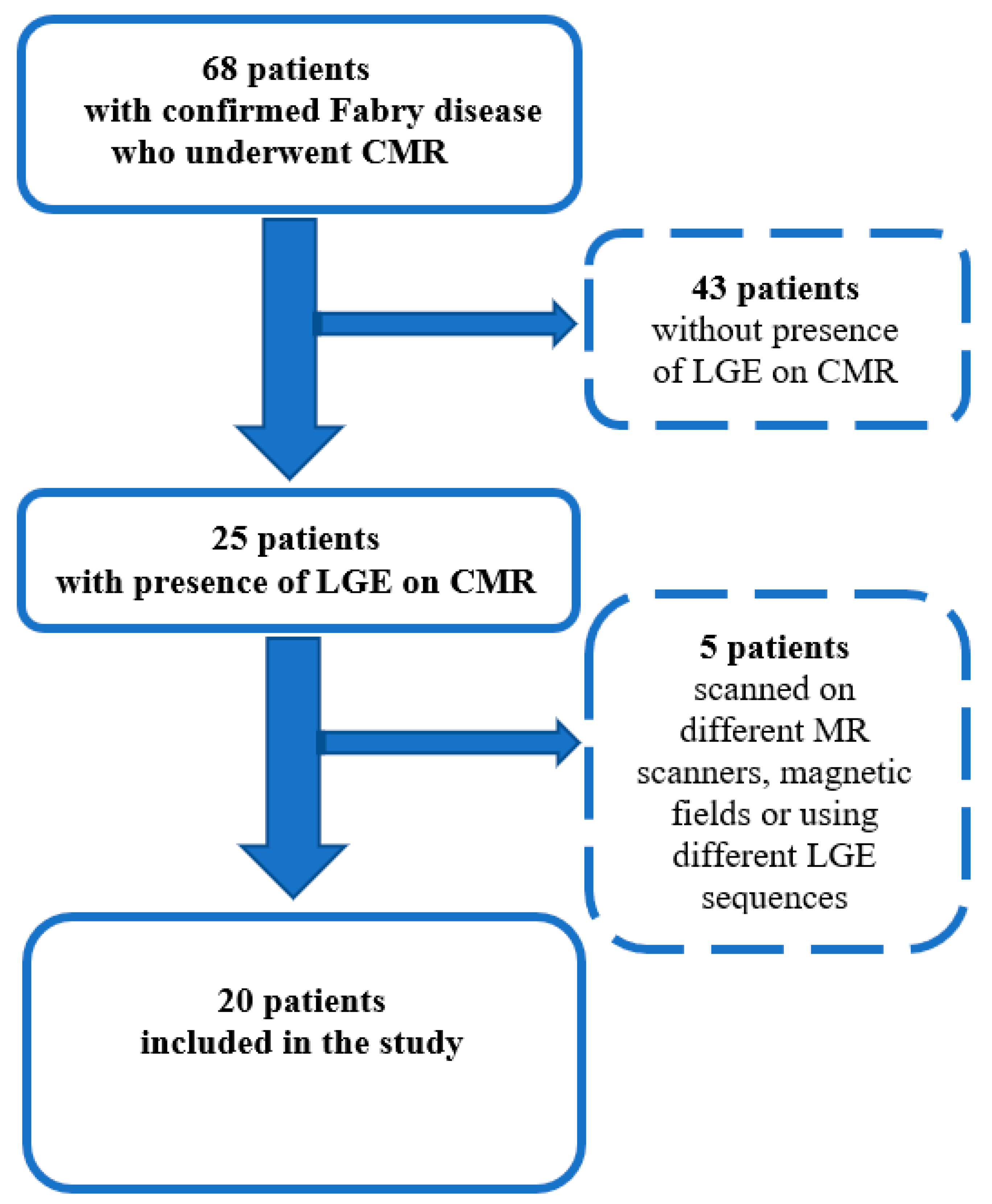
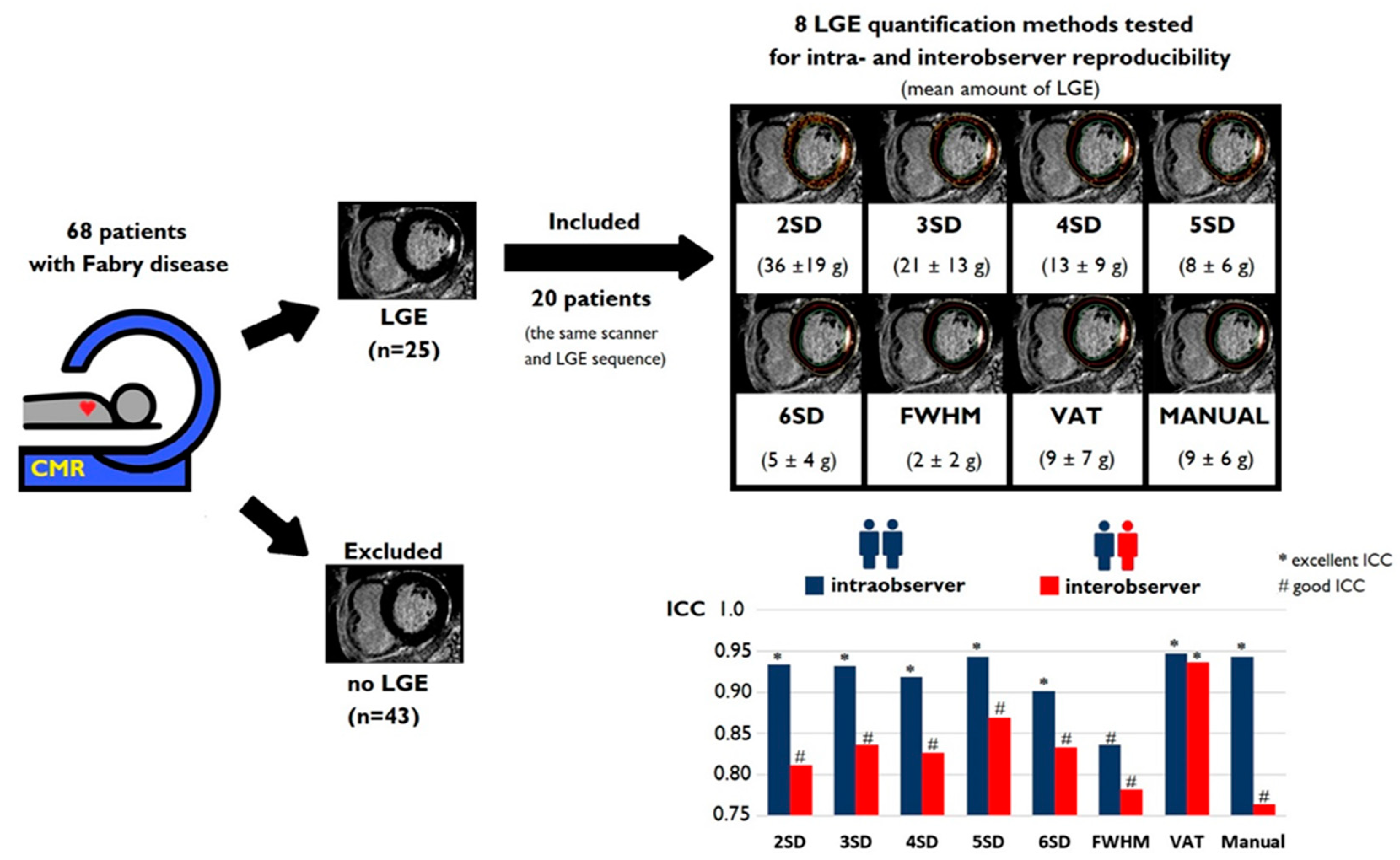
2.1. Study Population
2.2. CMR Imaging Data Acquisition
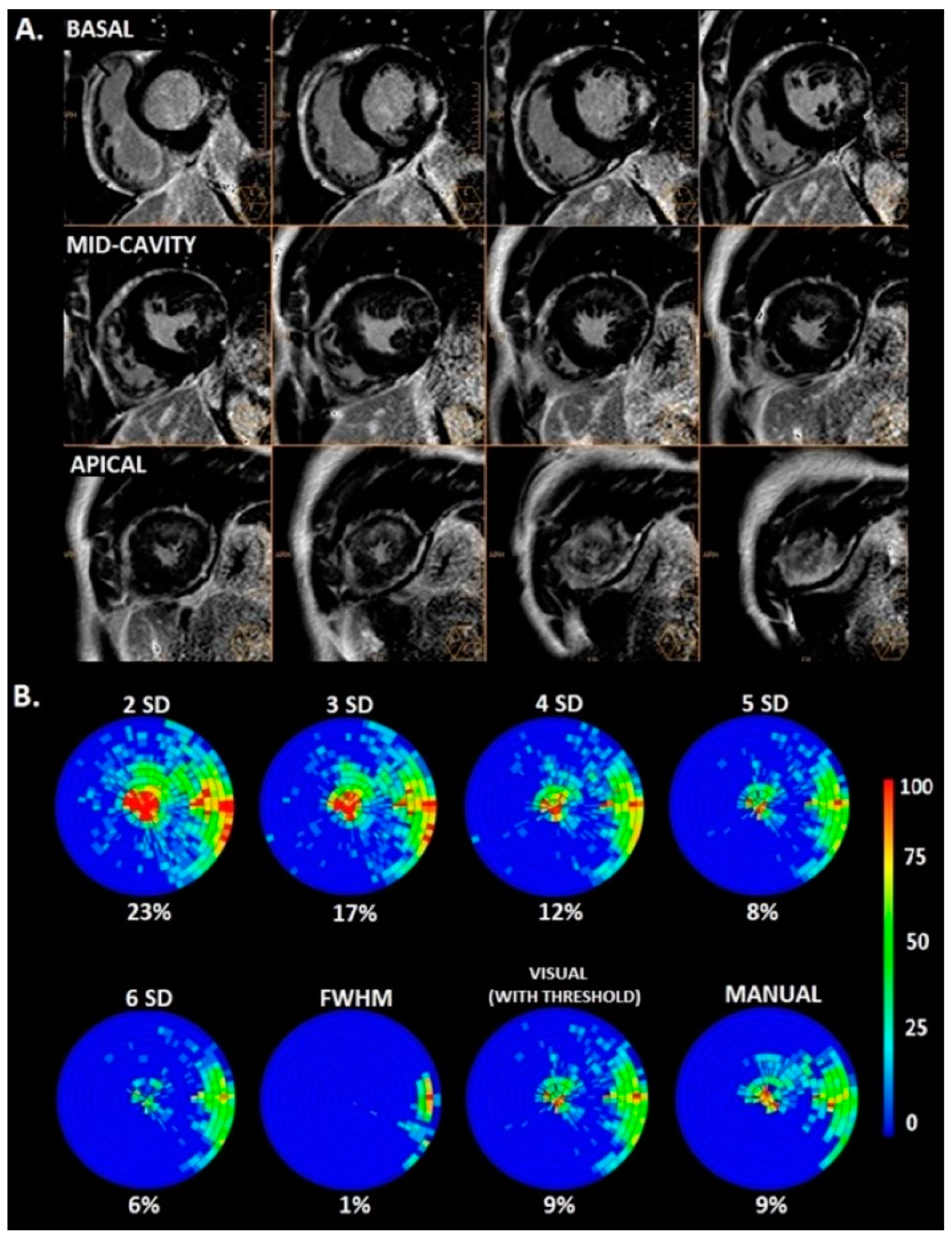
2.3. CMR Imaging Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Amount of LGE
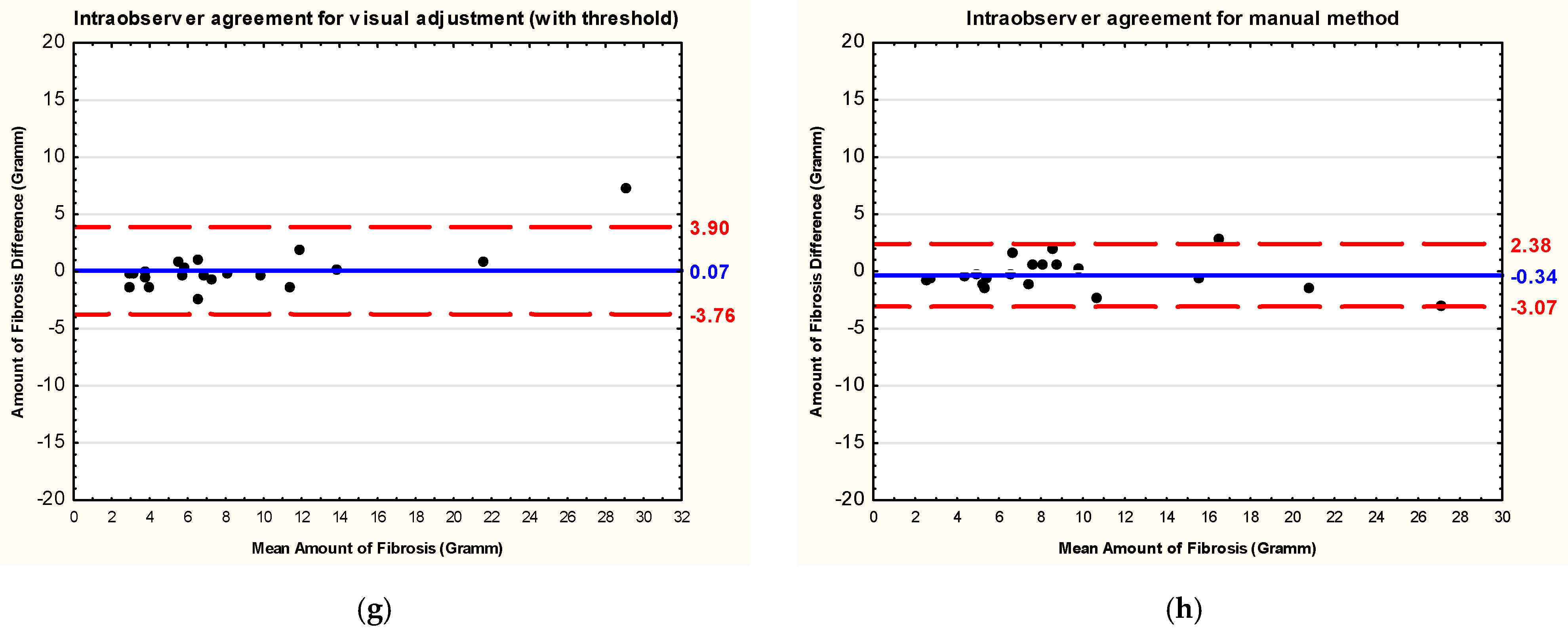
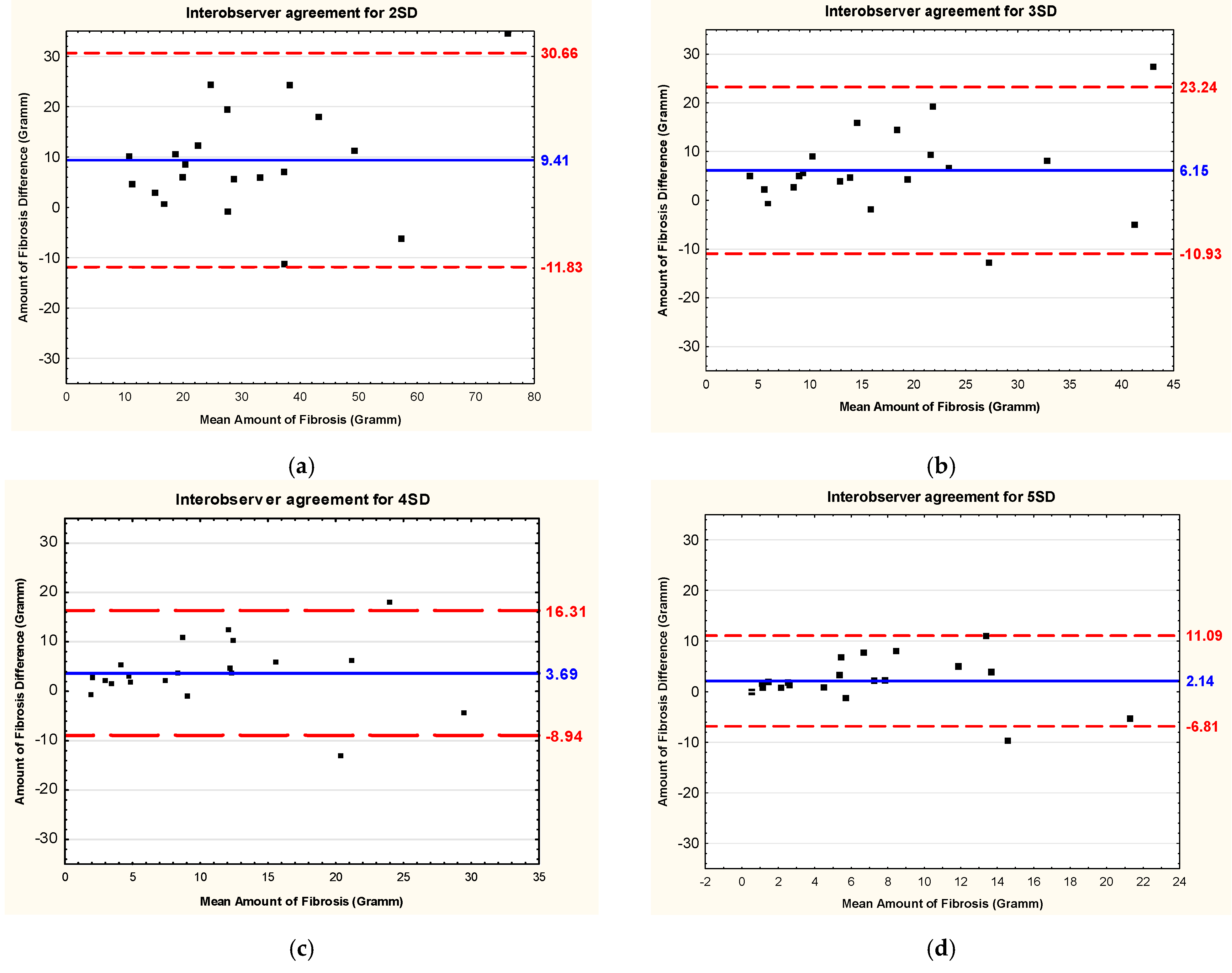
3.3. Intraobserver and Interobserver Reliability and Reproducibility
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy. Circulation 2020, 142, E558–E631. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.C.; McKenna, W.J.; McCrohon, J.A.; Elliott, P.M.; Smith, G.C.; Pennell, D.J. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2003, 41, 1561–1567. [Google Scholar] [CrossRef]
- Weng, Z.; Yao, J.; Chan, R.H.; He, J.; Yang, X.; Zhou, Y.; He, Y. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1392–1402. [Google Scholar] [CrossRef]
- Ismail, T.F.; Jabbour, A.; Gulati, A.; Mallorie, A.; Raza, S.; Cowling, T.E.; Das, B.; Khwaja, J.; Alpendurada, F.D.; Wage, R.; et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart 2014, 100, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Mentias, A.; Raeisi-Giglou, P.; Smedira, N.G.; Feng, K.; Sato, K.; Wazni, O.; Kanj, M.; Flamm, S.D.; Thamilarasan, M.; Popovic, Z.B.; et al. Late Gadolinium Enhancement in Patients with Hypertrophic Cardiomyopathy and Preserved Systolic Function. J. Am. Coll. Cardiol. 2018, 72, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Papanastasiou, C.A.; Kokkinidis, D.G.; Kampaktsis, P.N.; Bikakis, I.; Cunha, D.K.; Oikonomou, E.K.; Greenwood, J.P.; Garcia, M.J.; Karamitsos, T.D. The Prognostic Role of Late Gadolinium Enhancement in Aortic Stenosis: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2020, 13, 385–392. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Qin, S. Prognostic value of cardiac magnetic resonance in patients with aortic stenosis: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0263378. [Google Scholar] [CrossRef] [PubMed]
- Barone-Rochette, G.; Piérard, S.; De Meester de Ravenstein, C.; Seldrum, S.; Melchior, J.; Maes, F.; Pouleur, A.C.; Vancraeynest, D.; Pasquet, A.; Vanoverschelde, J.L.; et al. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J. Am. Coll. Cardiol. 2014, 64, 144–154. [Google Scholar] [CrossRef]
- Alba, A.C.; Gaztañaga, J.; Foroutan, F.; Thavendiranathan, P.; Merlo, M.; Alonso-Rodriguez, D.; Vallejo-García, V.; Vidal-Perez, R.; Corros-Vicente, C.; Barreiro-Pérez, M.; et al. Prognostic Value of Late Gadolinium Enhancement for the Prediction of Cardiovascular Outcomes in Dilated Cardiomyopathy: An International, Multi-Institutional Study of the MINICOR Group. Circ. Cardiovasc. Imaging 2020, 13, 10105. [Google Scholar] [CrossRef]
- Hanneman, K.; Karur, G.R.; Wasim, S.; Morel, C.F.; Iwanochko, R.M. Prognostic Significance of Cardiac Magnetic Resonance Imaging Late Gadolinium Enhancement in Fabry Disease. Circulation 2018, 138, 2579–2581. [Google Scholar] [CrossRef]
- Krämer, J.; Niemann, M.; Störk, S.; Frantz, S.; Beer, M.; Ertl, G.; Wanner, C.; Weidemann, F. Relation of burden of myocardial fibrosis to malignant ventricular arrhythmias and outcomes in Fabry disease. Am. J. Cardiol. 2014, 114, 895–900. [Google Scholar] [CrossRef]
- Hiestand, R.; Nowak, A.; Sokolska, J.M.; Chan, R.; Ruschitzka, F.; Manka, R.; Gruner, C. Clinical and CMR characteristics associated with cardiac events in patients with Fabry disease. Int. J. Cardiol. 2023, 382, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Flett, A.S.; Hasleton, J.; Cook, C.; Hausenloy, D.; Quarta, G.; Ariti, C.; Muthurangu, V.; Moon, J.C. Evaluation of Techniques for the Quantification of Myocardial Scar of Differing Etiology Using Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2011, 4, 150–156. [Google Scholar] [CrossRef]
- Mikami, Y.; Kolman, L.; Joncas, S.X.; Stirrat, J.; Scholl, D.; Rajchl, M.; Lydell, C.P.; Weeks, S.G.; Howarth, A.G.; White, J.A. Accuracy and reproducibility of semi-automated late gadolinium enhancement quantification techniques in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2014, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A.C. Assessment of Myocardial Fibrosis with Cardiac Magnetic Resonance. J. Am. Coll. Cardiol. 2011, 57, 891. [Google Scholar] [CrossRef]
- Spiewak, M.; Malek, L.A.; Misko, J.; Chojnowska, L.; Milosz, B.; Klopotowski, M.; Petryka, J.; Dabrowski, M.; Kepka, C.; Ruzyllo, W. Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur. J. Radiol. 2010, 74, e149–e153. [Google Scholar] [CrossRef]
- Gräni, C.; Eichhorn, C.; Bière, L.; Kaneko, K.; Murthy, V.L.; Agarwal, V.; Aghayev, A.; Steigner, M.; Blankstein, R.; Jerosch-Herold, M.; et al. Comparison of myocardial fibrosis quantification methods by cardiovascular magnetic resonance imaging for risk stratification of patients with suspected myocarditis. J. Cardiovasc. Magn. Reson. 2019, 21, 14. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Perry, R.; Shah, R.; Saiedi, M.; Patil, S.; Ganesan, A.; Linhart, A.; Selvanayagam, J.B. The Role of Cardiac Imaging in the Diagnosis and Management of Anderson-Fabry Disease. JACC Cardiovasc. Imaging 2019, 12, 1230–1242. [Google Scholar] [CrossRef]
- Pieroni, M.; Moon, J.C.; Arbustini, E.; Barriales-Villa, R.; Camporeale, A.; Vujkovac, A.C.; Elliott, P.M.; Hagege, A.; Kuusisto, J.; Linhart, A.; et al. Cardiac Involvement in Fabry Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 922–936. [Google Scholar] [CrossRef] [PubMed]
- Linhart, A.; Germain, D.P.; Olivotto, I.; Akhtar, M.M.; Anastasakis, A.; Hughes, D.; Namdar, M.; Pieroni, M.; Hagège, A.; Cecchi, F.; et al. An expert consensus document on the management of cardiovascular manifestations of Fabry disease. Eur. J. Heart Fail. 2020, 22, 1076–1096. [Google Scholar] [CrossRef]
- Caredda, G.; Bassareo, P.P.; Cherchi, M.V.; Pontone, G.; Suri, J.S.; Saba, L. Anderson-fabry disease: Role of traditional and new cardiac MRI techniques. Br. J. Radiol. 2021, 94, 20210020. [Google Scholar] [CrossRef]
- Moon, J.C.; Sheppard, M.; Reed, E.; Lee, P.; Elliott, P.M.; Pennell, D.J. The histological basis of late gadolinium enhancement cardiovascular magnetic resonance in a patient with Anderson-Fabry disease. J. Cardiovasc. Magn. Reson. 2006, 8, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Deva, D.P.; Hanneman, K.; Li, Q.; Ng, M.Y.; Wasim, S.; Morel, C.; Iwanochko, R.M.; Thavendiranathan, P.; Crean, A.M. Cardiovascular magnetic resonance demonstration of the spectrum of morphological phenotypes and patterns of myocardial scarring in Anderson-Fabry disease. J. Cardiovasc. Magn. Reson. 2016, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Positano, V.; Pingitore, A.; Strata, E.; Bella GDi Formisano, F.; Spirito, P.; Lombardi, M. Quantitative analysis of late gadolinium enhancement in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2010, 12, 21. [Google Scholar] [CrossRef]
- Liu, D.; Ma, X.; Liu, J.; Zhao, L.; Chen, H.; Xu, L.; Sun, Z.; Fan, Z. Quantitative analysis of late gadolinium enhancement in hypertrophic cardiomyopathy: Comparison of diagnostic performance in myocardial fibrosis between gadobutrol and gadopentetate dimeglumine. Int. J. Cardiovasc. Imaging 2017, 33, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Seydelmann, N.; Wanner, C.; Störk, S.; Ertl, G.; Weidemann, F. Fabry disease and the heart. Best. Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 195–204. [Google Scholar] [CrossRef]
- Gilchrist, M.; Casanova, F.; Tyrrell, J.S.; Cannon, S.; Wood, A.R.; Fife, N.; Young, K.; Oram, R.A.; Weedon, M.N. Prevalence of Fabry disease-causing variants in the UK Biobank. J. Med. Genet. 2023, 60, 391–396. [Google Scholar] [CrossRef]
- Goldman, M.R.; Brady, T.J.; Pykett, I.L.; Burt, C.T.; Buonanno, F.S.; Kistler, J.P.; Newhouse, J.H.; Hinshaw, W.S.; Pohost, G.M. Quantification of experimental myocardial infarction using nuclear magnetic resonance imaging and paramagnetic ion contrast enhancement in excised canine hearts. Circulation 1982, 66, 1012–1016. [Google Scholar] [CrossRef]
- Moravsky, G.; Ofek, E.; Rakowski, H.; Butany, J.; Williams, L.; Ralph-Edwards, A.; Wintersperger, B.J.; Crean, A. Myocardial fibrosis in hypertrophic cardiomyopathy: Accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging JACC Cardiovasc Imaging 2013, 6, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Treibel, T.A.; White, S.K.; Moon, J.C. Myocardial Tissue Characterization: Histological and Pathophysiological Correlation. Curr. Cardiovasc. Imaging Rep. 2014, 7, 9254. [Google Scholar] [CrossRef] [PubMed]



| Patients with Fabry Disease and Presence of LGE Included in the Study n = 20 | |
|---|---|
| Demographics and clinical variables | |
| Age, years | 54 ± 11 |
| Male, n (%) | 9 (45) |
| Angina pectoris symptoms, n (%) | 5 (25) |
| NYHA class I/II, n (%) | 14 (70)/6 (30) |
| ECG-RBBB, n (%) | 2 (10) |
| ECG-LBBB, n (%) | 3 (15) |
| ECG-short PQ, n (%) | 2 (10) |
| Renal involvement, n (%) | 15 (75) |
| Dialysis, n (%) | 2 (10) |
| Kidney transplantation, n (%) | 1 (5) |
| Diabetes, n (%) | 0 |
| Coronary heart diseases, n (%) | 1 (5) |
| Arterial hypertension, n (%) | 3 (15) |
| Smoking, n (%) | 6 (30) |
| Laboratory findings | |
| Troponin level above the normal range, n (%) | 16 (80) |
| NT-proBNP level above the normal range, n (%) | 13 (65) |
| NT-proBNP level, ng/L | 563 ± 730 [342, 244–640] |
| eGFR, mL/min/1.73 m2 | 77 ± 25 [87, 61–92] |
| Albuminuria, n (%) | 15 (75) |
| Proteinuria, n (%) | 10 (50) |
| Treatment | |
| Beta-blocker, n (%) | 4 (20) |
| ACEI/ARB, n (%) | 10 (50) |
| Calcium-channel blocker, n (%) | 3 (15) |
| Amiodarone, n (%) | 1 (5) |
| Antiplatelet drug, n (%) | 6 (30) |
| Oral anticoagulation, n (%) | 2 (10) |
| Diuretics, n (%) | 0 |
| Aldosterone antagonist, n (%) | 0 |
| Statin, n (%) | 3 (15) |
| Antidepressants, n (%) | 1 (5) |
| ERT, n (%) | 18 (90) |
| Duration of ERT, years | 5 ± 4 |
| CMR findings | |
| LVEF, % | 69 ± 9 |
| LV maximal wall thickness, mm | 13.2 ± 4.7 [12.5, 10.5–14.0] |
| LV-EDV, mL | 137 ± 37 |
| LV-ESV, mL | 44 ± 21 |
| LGE Quantification Method | Mean Difference | SD | ICC | Lower CI | Upper CI | CV (%) |
|---|---|---|---|---|---|---|
| 2 SD | 3.56 | 6.09 | 0.934 * | 0.838 | 0.974 | 18.1 |
| 3 SD | 2.02 | 4.18 | 0.932 * | 0.833 | 0.973 | 20.9 |
| 4 SD | 1.53 | 3.12 | 0.919 * | 0.803 | 0.968 | 26.0 |
| 5 SD | 1.04 | 0.43 | 0.943 * | 0.859 | 0.978 | 5.8 |
| 6 SD | 0.88 | 0.39 | 0.901 * | 0.763 | 0.961 | 8.0 |
| FWHM | 0.06 | 1.17 | 0.836 * | 0.625 | 0.933 | 63.0 |
| Visual adjustment (with threshold) | 0.07 | 1.95 | 0.947 * | 0.868 | 0.979 | 22.8 |
| Manual | −0.34 | 1.39 | 0.943 * | 0.859 | 0.978 | 15.0 |
| LGE Quantification Method | Mean Difference | SD | ICC | Lower CI | Upper CI | CV (%) |
|---|---|---|---|---|---|---|
| 2 SD | 9.41 | 10.84 | 0.811 * | 0.575 | 0.923 | 35.3 |
| 3 SD | 6.15 | 8.72 | 0.836 * | 0.625 | 0.933 | 48.5 |
| 4 SD | 3.69 | 6.44 | 0.826 * | 0.604 | 0.929 | 59.1 |
| 5 SD | 2.14 | 4.57 | 0.869 * | 0.693 | 0.947 | 66.3 |
| 6 SD | 1.27 | 3.50 | 0.833 * | 0.619 | 0.932 | 74.8 |
| FWHM | 0.59 | 1.50 | 0.782 * | 0.519 | 0.910 | 94.2 |
| Visual adjustment (with threshold) | 2.34 | 2.79 | 0.937 * | 0.845 | 0.975 | 37.5 |
| Manual | 3.28 | 3.00 | 0.764 * | 0.486 | 0.902 | 40.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolska, J.M.; Károlyi, M.; Hiestand, D.R.; Gastl, M.; Weber, L.; Sokolski, M.; Kosmala, W.; Alkadhi, H.; Gruner, C.; Manka, R. Myocardial Fibrosis Quantification Methods by Cardiovascular Magnetic Resonance Imaging in Patients with Fabry Disease. J. Clin. Med. 2024, 13, 5047. https://doi.org/10.3390/jcm13175047
Sokolska JM, Károlyi M, Hiestand DR, Gastl M, Weber L, Sokolski M, Kosmala W, Alkadhi H, Gruner C, Manka R. Myocardial Fibrosis Quantification Methods by Cardiovascular Magnetic Resonance Imaging in Patients with Fabry Disease. Journal of Clinical Medicine. 2024; 13(17):5047. https://doi.org/10.3390/jcm13175047
Chicago/Turabian StyleSokolska, Justyna M., Mihály Károlyi, Dana R. Hiestand, Mareike Gastl, Lucas Weber, Mateusz Sokolski, Wojciech Kosmala, Hatem Alkadhi, Christiane Gruner, and Robert Manka. 2024. "Myocardial Fibrosis Quantification Methods by Cardiovascular Magnetic Resonance Imaging in Patients with Fabry Disease" Journal of Clinical Medicine 13, no. 17: 5047. https://doi.org/10.3390/jcm13175047






