Patch Augmentation in Arthroscopic Rotator Cuff Surgery—Review of Current Evidence and Newest Trends
Abstract
1. Introduction
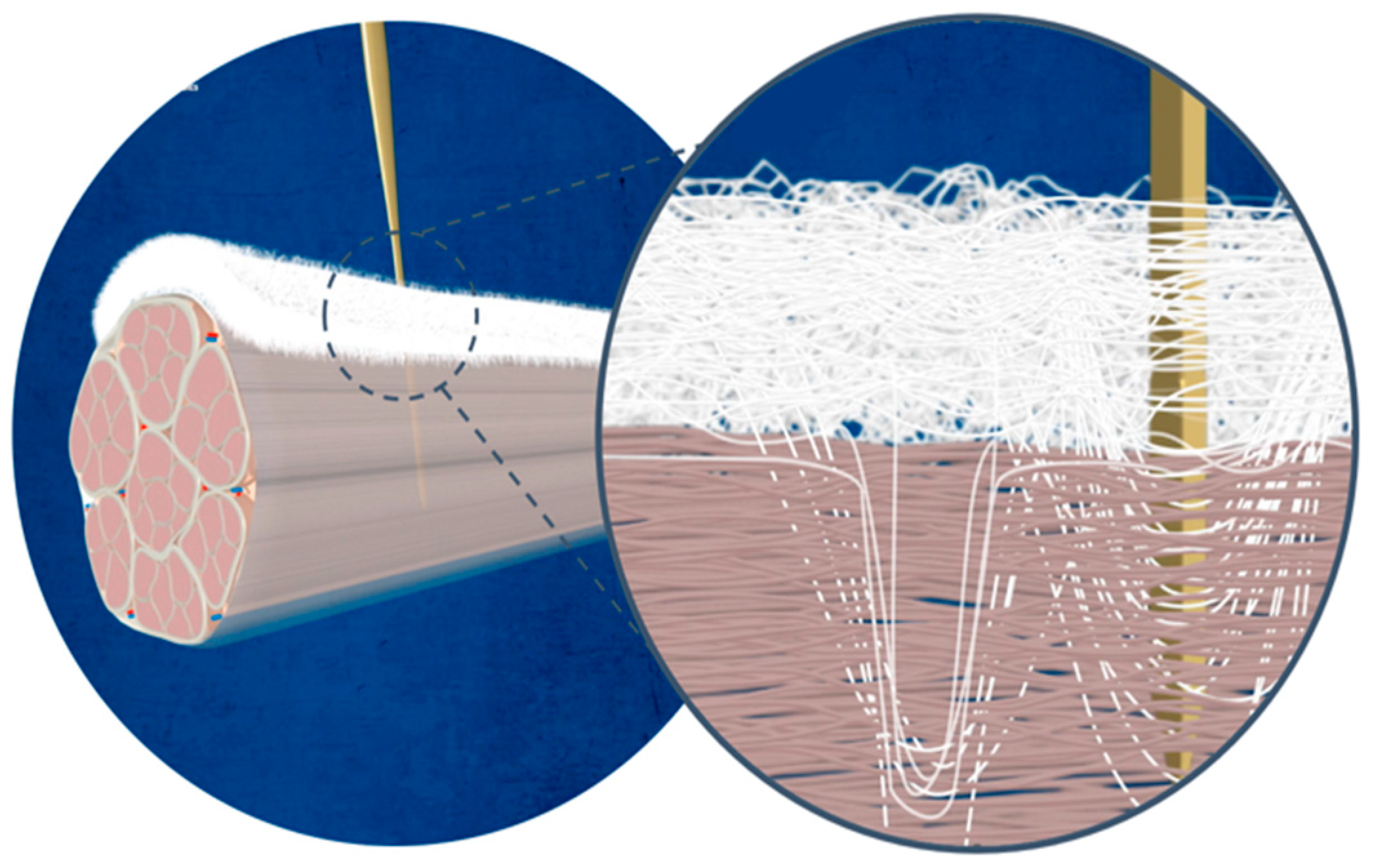
1.1. Rotator Cuff Tears
1.2. Augmentation vs. Interposition
2. Materials and Methods
3. Results
3.1. Different Graft Types
3.1.1. Xenografts
3.1.2. Allograft and Autograft
3.1.3. Synthetic Polymers
3.1.4. The Future of Patch Augmentation
3.1.5. Nylon Patches
3.1.6. Bioresorbable Scaffolds
3.2. PASTA Lesions
3.3. Biological Enhancement of Rotator Cuff Repairs
3.3.1. Mesenchymal Stem Cells (MSCs)
3.3.2. Platelet-Rich Plasma (PRP)
3.3.3. Vitamin D
3.4. Complications
3.5. Cost Effectiveness
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nho, S.J.; Yadav, H.; Shindle, M.K.; MacGillivray, J.D. Rotator cuff degeneration: Etiology and pathogenesis. Am. J. Sports Med. 2008, 36, 987–993. [Google Scholar] [CrossRef]
- DeOrio, J.K.; Cofield, R.H. Results of a second attempt at surgical repair of a failed initial rotator-cuff repair. J. Bone Jt. Surg. (JBJS) 1984, 66, 563–567. Available online: https://journals.lww.com/jbjsjournal/fulltext/1984/66040/results_of_a_second_attempt_at_surgical_repair_of.11.aspx (accessed on 23 March 2024). [CrossRef]
- Guo, S.; Zhu, Y.; Song, G.; Jiang, C. Assessment of Tendon Retraction in Large to Massive Rotator Cuff Tears: A Modified Patte Classification Based on 2 Coronal Sections on Preoperative Magnetic Resonance Imaging with Higher Specificity on Predicting Reparability. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 2822–2830. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Bois, A.J.; Matthewson, G.; Oiwa, S.; More, K.D.; Lo, I.K.Y. The relationship between preoperative Goutallier stage and retear rates following posterosuperior rotator cuff repair: A systematic review. J. Shoulder Elb. Surg. 2023, 32, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, G.; Ipci, F.B.; Gokalp, O.; Egeli, E. The relationship between the duration and the retraction and atrophy grades in traumatic isolated full-thickness supraspinatus tears in young patients. BMC Musculoskelet. Disord. 2024, 25, 535. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.A.; Rodeo, S.A.; Chahla, J.; Ranalletta, M. Current Concepts in Rotator Cuff Repair Techniques: Biomechanical, Functional, and Structural Outcomes. Orthop. J. Sports Med. 2019, 7, 2325967119868674. [Google Scholar] [CrossRef]
- Thorsness, R.; Romeo, A. Massive rotator cuff tears: Trends in surgical management. Orthopedics 2016, 39, 145–151. [Google Scholar] [CrossRef]
- Hein, J.; Reilly, J.M.; Chae, J.; Maerz, T.; Anderson, K. Retear Rates after Arthroscopic Single-Row, Double-Row, and Suture Bridge Rotator Cuff Repair at a Minimum of 1 Year of Imaging Follow-up: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- DFerguson, P.; Lewington, M.R.; Smith, T.D.; Wong, I.H. Graft Utilization in the Augmentation of Large-to-Massive Rotator Cuff Repairs. Am. J. Sports Med. 2016, 44, 2984–2992. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, S.C.; Park, J.H.; Kim, H.G.; Kim, B.T.; Kim, D.Y.; Yoo, J.C. Arthroscopic Incomplete Rotator Cuff Repair with Patch Augmentation Using Acellular Dermal Matrix Allograft. Arthrosc. Tech. 2023, 12, e2203–e2209. [Google Scholar] [CrossRef]
- Cobb, T.E.; Dimock, R.A.; Memon, S.D.; Consigliere, P.; Ajami, S.; Imam, M.; Narvani, A.A. Rotator Cuff Repair with Patch Augmentation: What Do We Know? Arch. Bone Jt. Surg. 2022, 10, 833–846. [Google Scholar] [CrossRef]
- Mihata, T.; McGarry, M.H.; Pirolo, J.M.; Kinoshita, M.; Lee, T.Q. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: A biomechanical cadaveric study. Am. J. Sports Med. 2012, 40, 2248–2255. [Google Scholar] [CrossRef]
- France, E.P.; Paulos, L.E.; Harner, C.D.; Straight, C.B. Biomechanical evaluation of rotator cuff fixation methods. Am. J. Sports Med. 1989, 17, 176–181. [Google Scholar] [CrossRef]
- Nirschl, R.P. Rotator cuff surgery. Instr. Course Lect. 1989, 38, 447–462. [Google Scholar]
- Flury, M.; Rickenbacher, D.; Jung, C.; Schneider, M.M.; Endell, D.; Audigé, L. Porcine Dermis Patch Augmentation of Supraspinatus Tendon Repairs: A Pilot Study Assessing Tendon Integrity and Shoulder Function 2 Years after Arthroscopic Repair in Patients Aged 60 Years or Older. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 24–37. [Google Scholar] [CrossRef]
- Barber, F.A.; Aziz-Jacobo, J. Biomechanical Testing of Commercially Available Soft-Tissue Augmentation Materials. Arthrosc. J. Arthrosc. Relat. Surg. 2009, 25, 1233–1239. [Google Scholar] [CrossRef]
- Thon, S.G.; O’Malley, L.; O’Brien, M.J.; Savoie, F.H. Evaluation of Healing Rates and Safety with a Bioinductive Collagen Patch for Large and Massive Rotator Cuff Tears: 2-Year Safety and Clinical Outcomes. Am. J. Sports Med. 2019, 47, 1901–1908. [Google Scholar] [CrossRef]
- Frazier, L.P.; Quigley, R.A.; Galvin, J.W.; Waterman, B.R.; Brusalis, C.M.; Cole, B.J. Put a Patch on It!: When and How to Perform Soft-Tissue Augmentation in Rotator Cuff Surgery. Oper. Tech. Sports Med. 2023, 31, 150984. [Google Scholar] [CrossRef]
- Adams, J.E.; Zobitz, M.E.; Reach, J.S.; An, K.N.; Steinmann, S.P. Rotator Cuff Repair Using an Acellular Dermal Matrix Graft: An In Vivo Study in a Canine Model. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 700–709. [Google Scholar] [CrossRef]
- Nicholson, G.P.; Breur, G.J.; Van Sickle, D.; Yao, J.Q.; Kim, J.; Blanchard, C.R. Evaluation of a cross-linked acellular porcine dermal patch for rotator cuff repair augmentation in an ovine model. J. Shoulder Elb. Surg. 2007, 16, S184–S190. [Google Scholar] [CrossRef]
- Soler, J.A. Early Complications from the Use of Porcine Dermal Collagen Implants (Permacol) as Bridging Constructs in the Repair of Massive Rotator Cuff Tears. A Report of 4 Cases. 2007. Available online: https://www.researchgate.net/publication/5906969 (accessed on 3 April 2024).
- Gilbert, T.W.; Freund, J.M.; Badylak, S.F. Quantification of DNA in Biologic Scaffold Materials. J. Surg. Res. 2009, 152, 135–139. [Google Scholar] [CrossRef]
- Raeder, R.H.; Badylak, S.F.; Sheehan, C.; Kallakury, B.; Metzger, D.W. Natural anti-galactose 1,3 galactose antibodies delay, but do not prevent the acceptance of extracellular matrix xenografts. Transpl. Immunol. 2002, 10, 15–24. [Google Scholar] [CrossRef]
- Derwin, K.A.; Baker, A.R.; Spragg, R.K.; Leigh, D.R.; Iannotti, J.P. Commercial Extracellular Matrix Scaffolds for Rotator Cuff Tendon Repair Biomechanical, Biochemical, and Cellular Properties. 2006. Available online: https://pubmed.ncbi.nlm.nih.gov/17142417/ (accessed on 3 April 2024).
- Neumann, J.A.; Zgonis, M.H.; Rickert, K.D.; Bradley, K.E.; Kremen, T.J.; Boggess, B.R.; Toth, A.P. Interposition Dermal Matrix Xenografts: A Successful Alternative to Traditional Treatment of Massive Rotator Cuff Tears. Am. J. Sports Med. 2017, 45, 1261–1268. [Google Scholar] [CrossRef]
- Barber, F.A.; Herbert, M.A.; Coons, D.A. Tendon Augmentation Grafts: Biomechanical Failure Loads and Failure Patterns. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 534–538. [Google Scholar] [CrossRef]
- Fini, M.; Bondioli, E.; Castagna, A.; Torricelli, P.; Giavaresi, G.; Rotini, R.; Marinelli, A.; Guerra, E.; Orlandi, C.; Carboni, A.; et al. Decellularized human dermis to treat massive rotator cuff tears: In vitro evaluations. Connect. Tissue Res. 2012, 53, 298–306. [Google Scholar] [CrossRef]
- Barber, F.A.; Burns, J.P.; Deutsch, A.; Labbé, M.R.; Litchfield, R.B. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 8–15. [Google Scholar] [CrossRef]
- Mori, D.; Funakoshi, N.; Yamashita, F.; Wakabayashi, T. Effect of fatty degeneration of the infraspinatus on the efficacy of arthroscopic patch autograft procedure for large to massive rotator cuff tears. Am. J. Sports Med. 2015, 43, 1108–1117. [Google Scholar] [CrossRef]
- Endell, D.; Rüttershoff, K.; Scheibel, M. Biceps Smash Technique: Biceps Tendon Autograft Augmentation for Arthroscopic Rotator Cuff Reconstruction. Arthrosc. Tech. 2023, 12, e383–e386. [Google Scholar] [CrossRef]
- Hohmann, E. Editorial Commentary: Long Head Biceps Tendon Can Be Used Like a Split Skin Graft: Mesh It and Augment Rotator Cuff Repairs. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 49–50. [Google Scholar] [CrossRef]
- Colbath, G.; Murray, A.; Siatkowski, S.; Pate, T.; Krussig, M.; Pill, S.; Hawkins, R.; Tokish, J.; Mercuri, J. Autograft Long Head Biceps Tendon Can Be Used as a Scaffold for Biologically Augmenting Rotator Cuff Repairs. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 38–48. [Google Scholar] [CrossRef]
- Rhee, S.M.; Oh, J.H. Bridging graft in irreparable massive rotator cuff tears: Autogenic biceps graft versus allogenic dermal patch graft. Clin. Orthop. Surg. (CIOS) 2017, 9, 497–505. [Google Scholar] [CrossRef]
- Park, J.H.; Park, K.T.; Kim, S.C.; Bukhary, H.A.; Lee, S.M.; Yoo, J.C. Arthroscopic biceps augmentation does not improve clinical outcomes during incomplete repair of large to massive rotator cuff tears. Bone Jt. J. 2022, 104-B, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, R.J.; Knapik, D.M.; Akkus, O. Biologic and synthetic grafts in the reconstruction of large to massive rotator cuff tears. J. Am. Acad. Orthop. Surg. 2016, 24, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Holland, C.; Thompson, M.S.; Vollrath, F.; Carr, A.J. Tensile and shear mechanical properties of rotator cuff repair patches. J. Shoulder Elb. Surg. 2012, 21, 1168–1176. [Google Scholar] [CrossRef]
- Ranebo, M.C.; Hallgren, H.C.B.; Norlin, R.; Adolfsson, L.E. Long-term clinical and radiographic outcome of rotator cuff repair with a synthetic interposition graft: A consecutive case series with 17 to 20 years of follow-up. J. Shoulder Elb. Surg. 2018, 27, 1622–1628. [Google Scholar] [CrossRef]
- Smolen, D.; Haffner, N.; Mittermayr, R.; Hess, F.; Sternberg, C.; Leuzinger, J. Application of a new polyester patch in arthroscopic massive rotator cuff repair—A prospective cohort study. J. Shoulder Elb. Surg. 2020, 29, e11–e21. [Google Scholar] [CrossRef]
- Meyer, D.C.; Bachmann, E.; Darwiche, S.; Moehl, A.; von Rechenberg, B.; Gerber, C.; Snedeker, J.G. Rotator Cuff Repair and Overlay Augmentation by Direct Interlocking of a Nonwoven Polyethylene Terephthalate Patch Into the Tendon: Evaluation in an Ovine Model. Am. J. Sports Med. 2023, 51, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, M.D.; Dietrich, M.; Andronic, O.; Nikolic, N.; Grueninger, P. Arthroscopic repair of posterosuperior rotator cuff tears with bioabsorbable patch augmentation: A magnetic resonance–controlled case series with 1-year follow-up. JBJS Int. 2020, 4, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, A.; Haynes, W.; Landao-Bassonga, E.; Lee, C.; Ruan, R.; Breidahl, W.; Heidari, B.S.; Mitchell, C.A.; Zheng, M. A bio-inductive collagen scaffold that supports human primary tendon-derived cell growth for rotator cuff repair. J. Orthop. Translat. 2021, 31, 91–101. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Zhang, C.; Jin, R.L.; Shen, T.; Gu, P.C.; Lin, X.J.; Chen, J.D. Arthroscopic Rotator Cuff Repair with Graft Augmentation of 3-Dimensional Biological Collagen for Moderate to Large Tears: A Randomized Controlled Study. Am. J. Sports Med. 2018, 46, 1424–1431. [Google Scholar] [CrossRef]
- Ellman, H. Diagnosis and Treatment of Incomplete Rotator Cuff Tears. Clin. Orthop. Relat. Res. 1990, 254, 64–74. Available online: http://europepmc.org/abstract/MED/2182260 (accessed on 26 June 2024). [CrossRef]
- Plachel, F.; Korn, G.; Traweger, A.; Ortmaier, R.; Resch, H.; Moroder, P. Long-term results after arthroscopic treatment of symptomatic Ellman grade 2 PASTA lesions. J. Shoulder Elb. Surg. 2019, 28, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Woodmass, J.M.; Bois, A.J.; Boorman, R.S.; Thornton, G.M.; Lo, I.K.Y. Arthroscopic Repair of Articular Surface Partial-Thickness Rotator Cuff Tears: Transtendon Technique versus Repair after Completion of the Tear—A Meta-Analysis. Advances in Orthopedics 2016; Hindawi Publishing Corporation: Nasr, Egypt, 2016. [Google Scholar] [CrossRef]
- Rossi, L.A.; Atala, N.A.; Bertona, A.; Bongiovanni, S.; Tanoira, I.; Maignon, G.; Ranalletta, M. Long-Term Outcomes after In Situ Arthroscopic Repair of Partial Rotator Cuff Tears. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 698–702. [Google Scholar] [CrossRef]
- Felsch, Q.; Mai, V.; Durchholz, H.; Flury, M.; Lenz, M.; Capellen, C.; Audigé, L. Complications within 6 Months after Arthroscopic Rotator Cuff Repair: Registry-Based Evaluation According to a Core Event Set and Severity Grading. J. Arthrosc. Relat. Surg. 2021, 37, 50–58. [Google Scholar] [CrossRef]
- Bushnell, B.D.; Bishai, S.K.; Krupp, R.J.; McMillan, S.; Schofield, B.A.; Trenhaile, S.W.; McIntyre, L.F. Treatment of Partial-Thickness Rotator Cuff Tears with a Resorbable Bioinductive Bovine Collagen Implant: 1-Year Results From a Prospective Multicenter Registry. Orthop. J. Sports Med. 2021, 9, 23259671211027850. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, S.; Kuss, M.; Kong, Y.; Shi, W.; Streubel, P.N.; Li, T.; Duan, B. 3D printing of multilayered scaffolds for rotator cuff tendon regeneration. Bioact. Mater. 2020, 5, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal stem cells empowering tendon regenerative therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef] [PubMed]
- Rohman, M.L.; Snow, M. Use of biologics in rotator cuff disorders: Current concept review. J. Clin. Orthop. Trauma 2021, 19, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Hurley, E.T.; Fat, D.L.; Moran, C.J.; Mullett, H. The Efficacy of Platelet-Rich Plasma and Platelet-Rich Fibrin in Arthroscopic Rotator Cuff Repair: A Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2019, 47, 753–761. [Google Scholar] [CrossRef]
- Dougherty, K.A.; Dilisio, M.F.; Agrawal, D.K. Vitamin D and the immunomodulation of rotator cuff injury. J. Inflamm. Res. 2016, 9, 123–131. [Google Scholar] [CrossRef]
- Rosen, M.R.; Lakehomer, H.; Kasik, C.S.; Stephenson, K. Suture and anchors may be retained during treatment of deep infection after rotator cuff repair: A systematic review. J. ISAKOS 2019, 4, 108–112. [Google Scholar] [CrossRef]
- Albers, S.; Ono, Y.; Kirchner, F.; Fal, M.F.; Kircher, J. Midterm outcomes of autologous bridging of rotator cuff tears with an autologous tendon patch (TEAR patch). J. Shoulder Elb. Surg. 2024, 33, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kang, Y.; Zhao, W.; Jiang, J.; Jiang, Y.; Zhao, B.; Jiao, M.; Yuan, B.; Zhao, J.; Ma, B. Evaluation of patches for rotator cuff repair: A systematic review and meta-analysis based on animal studies. Bioact. Mater. 2022, 10, 474–491. [Google Scholar] [CrossRef]
- D’Ambrosi, R.; Ragone, V.; Comaschi, G.; Usuelli, F.G.; Ursino, N. Retears and complication rates after arthroscopic rotator cuff repair with scaffolds: A systematic review. Cell Tissue Bank. 2019, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yeazell, S.; Lutz, A.; Bohon, H.; Shanley, E.; Thigpen, C.A.; Kissenberth, M.J.; Pill, S.G. Increased stiffness and reoperation rate in partial rotator cuff repairs treated with a bovine patch: A propensity-matched trial. J. Shoulder Elb. Surg. 2022, 31, S131–S135. [Google Scholar] [CrossRef]
- Saithna, A. Editorial Commentary: Bioinductive Collagen Implants Reduce Rotator Cuff Retear, yet Cost-Effectiveness and Improvement in Clinical Outcomes Are Unclear. Arthrosc. J. Arthrosc. Relat. Surg. 2024, 40, 1774–1776. [Google Scholar] [CrossRef]
- Cook, J.A.; Baldwin, M.; Cooper, C.; Nagra, N.S.; Crocker, J.C.; Glaze, M.; Greenall, G.; Rangan, A.; Kottam, L.; Rees, J.L.; et al. Findings from the patch augmented rotator cuff surgery (PARCS) feasibility study. Pilot Feasibility Stud. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Rognoni, C.; Nherera, L.M.; Garofalo, R.; Guerra, E.; Longo, U.G.; Taverna, E.; Tarricone, R. Economic Evaluation of a Bioinductive Implant for the Repair of Rotator Cuff Tears Compared with Standard Surgery in Italy. Adv. Ther. 2023, 40, 5271–5284. [Google Scholar] [CrossRef]
- McIntyre, L.F.; Nherera, L.M.; Schlegel, T.F. Resorbable Bioinductive Collagen Implant Is Cost Effective in the Treatment of Rotator Cuff Tears. Arthrosc. Sports Med. Rehabil. 2023, 5, e367–e374. [Google Scholar] [CrossRef]
- Quigley, R.; Verma, N.; Evuarherhe, A.; Cole, B.J. Rotator Cuff Repair with Graft Augmentation Improves Function, Decreases Revisions, and Is Cost-Effective. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 2166–2174. [Google Scholar] [CrossRef]

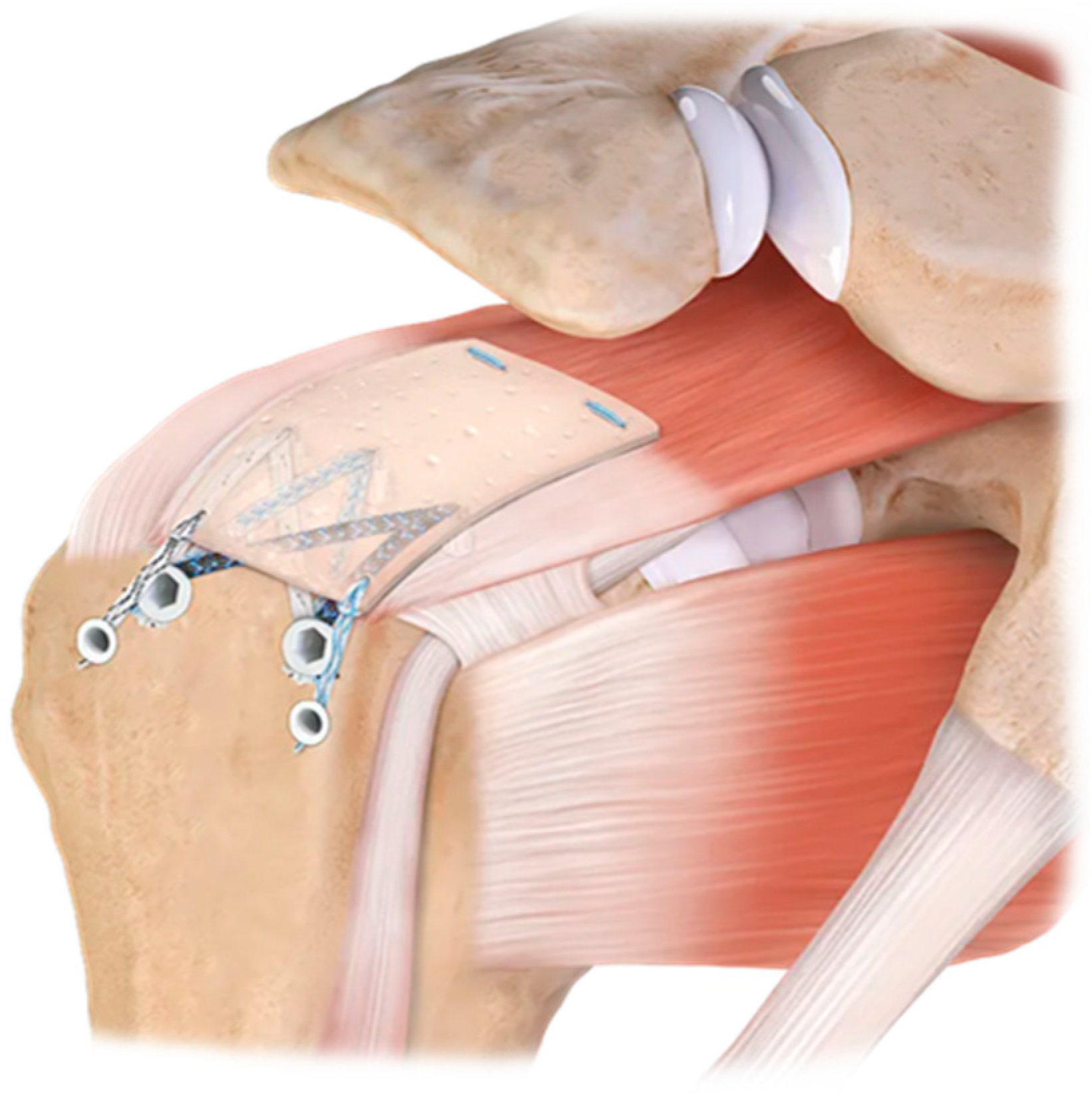

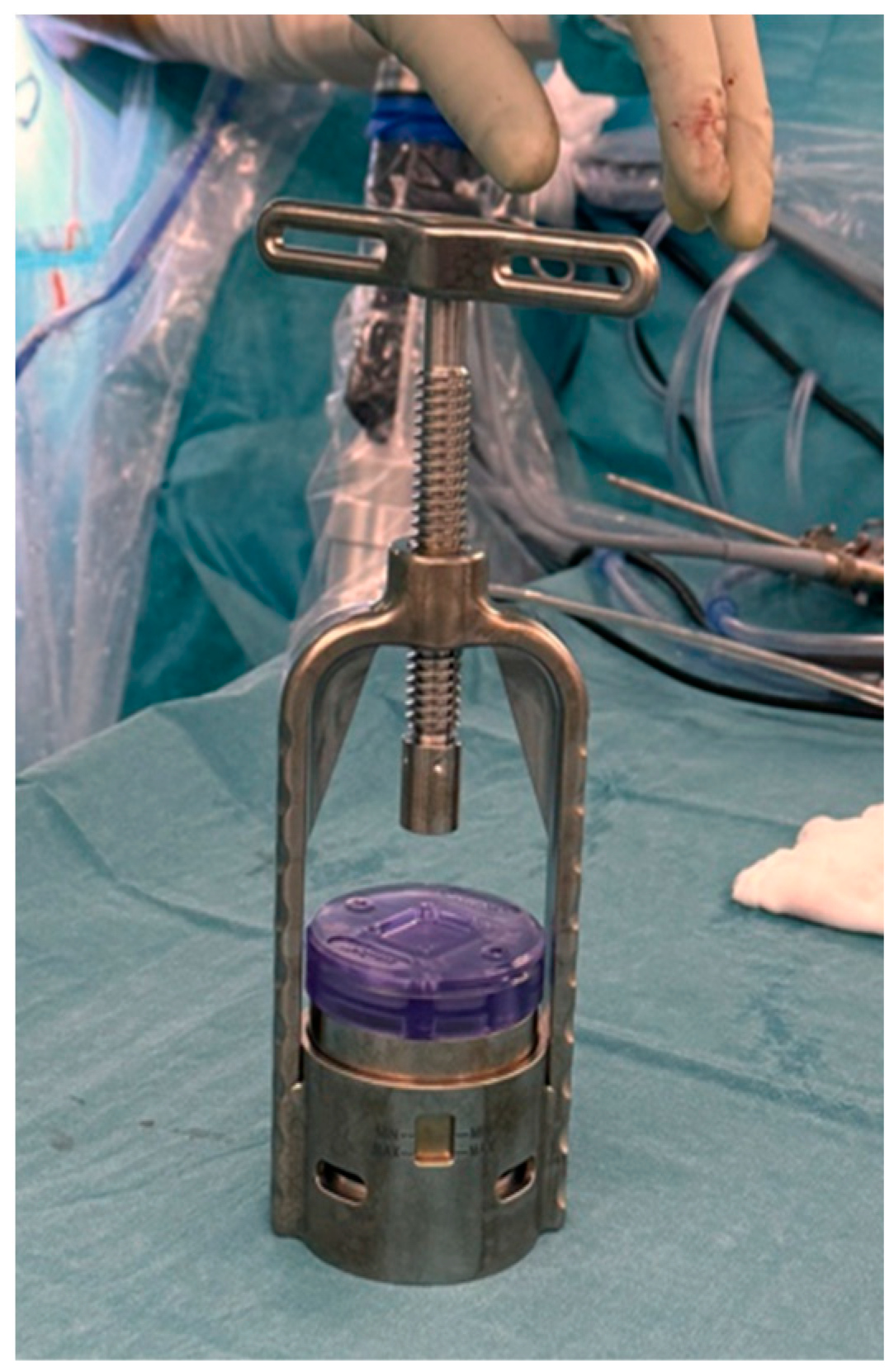
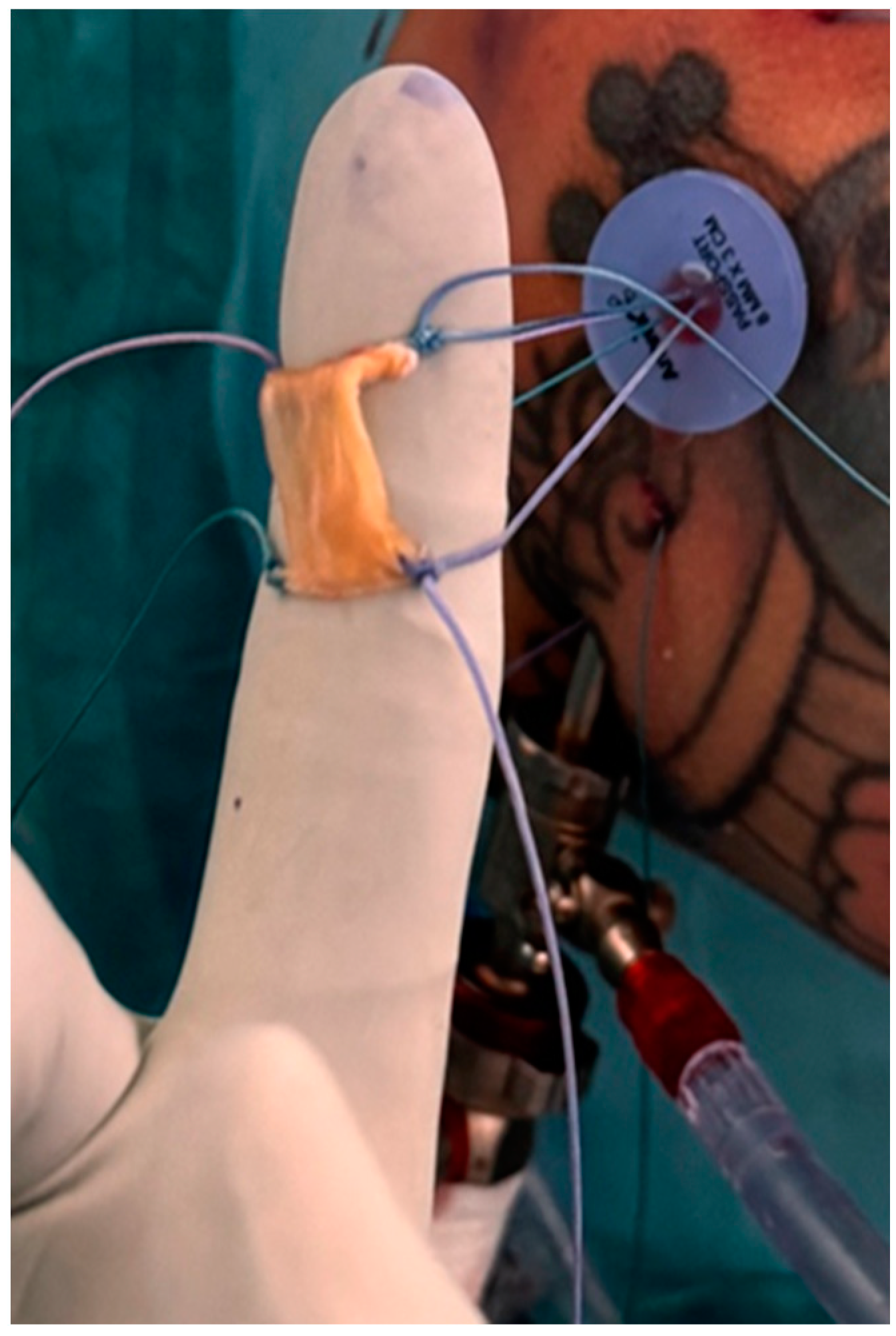
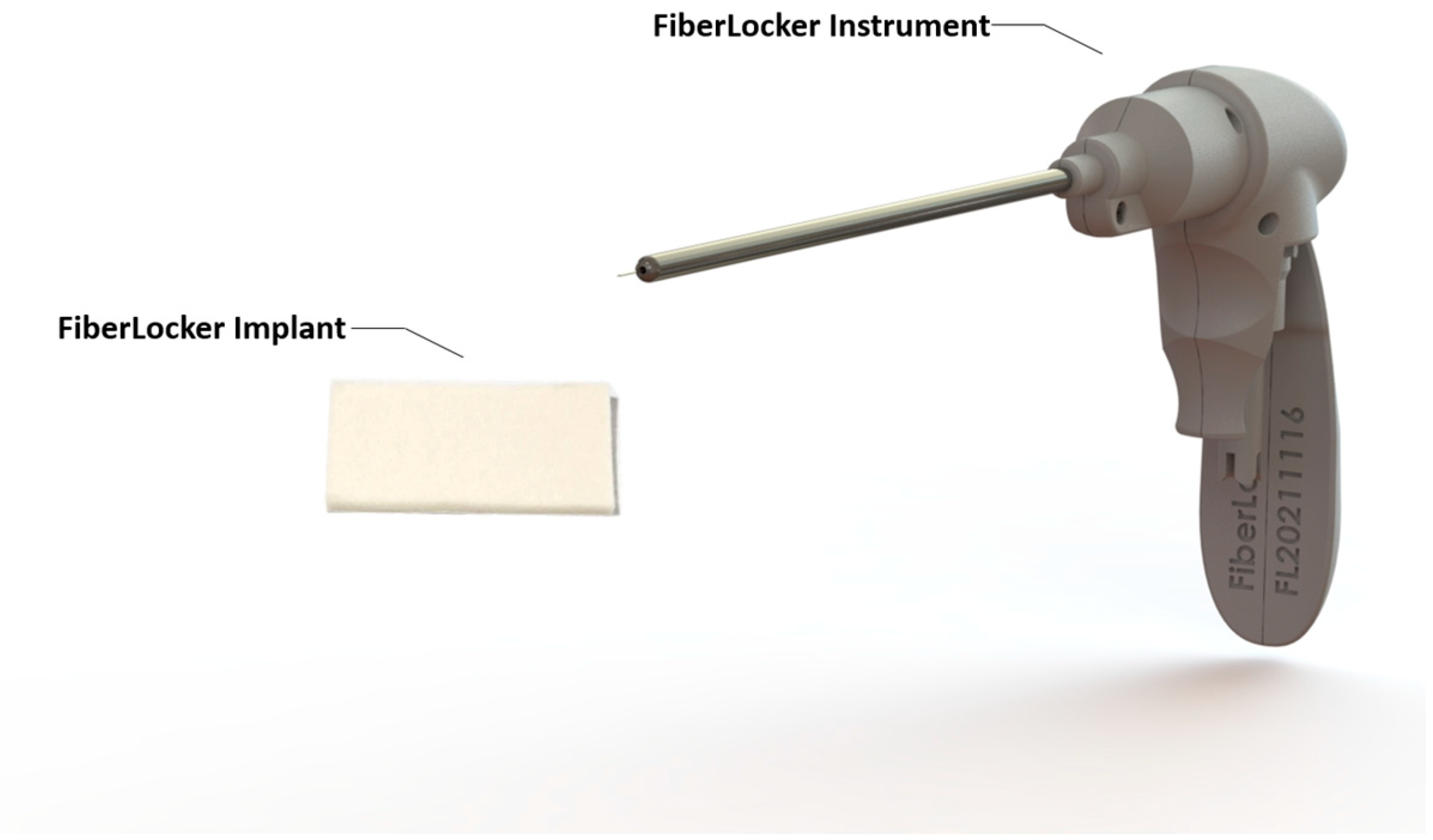
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Randomized controlled trials (RCTs), cohort studies, case-control studies, case series, and systematic reviews/meta-analyses | Animal studies, in vitro studies |
| Patients diagnosed with rotator cuff tears who underwent surgical repair | Studies missing information about rotator cuff augmentation or techniques other than patch augmentation |
| Studies that assess outcomes such as graft incorporation, mechanical integrity, postoperative pain, range of motion, muscle strength, and retear rates | Studies that do not report on clinical or mechanical outcomes relevant to graft performance |
| Studies examining graft-related complications like infections, tissue rejection, and adverse events | Studies involving patients with comorbidities that significantly affect rotator cuff healing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, M.; Dirkx, G.K.; Rosso, C. Patch Augmentation in Arthroscopic Rotator Cuff Surgery—Review of Current Evidence and Newest Trends. J. Clin. Med. 2024, 13, 5066. https://doi.org/10.3390/jcm13175066
Russo M, Dirkx GK, Rosso C. Patch Augmentation in Arthroscopic Rotator Cuff Surgery—Review of Current Evidence and Newest Trends. Journal of Clinical Medicine. 2024; 13(17):5066. https://doi.org/10.3390/jcm13175066
Chicago/Turabian StyleRusso, Maximilian, Gert Karl Dirkx, and Claudio Rosso. 2024. "Patch Augmentation in Arthroscopic Rotator Cuff Surgery—Review of Current Evidence and Newest Trends" Journal of Clinical Medicine 13, no. 17: 5066. https://doi.org/10.3390/jcm13175066
APA StyleRusso, M., Dirkx, G. K., & Rosso, C. (2024). Patch Augmentation in Arthroscopic Rotator Cuff Surgery—Review of Current Evidence and Newest Trends. Journal of Clinical Medicine, 13(17), 5066. https://doi.org/10.3390/jcm13175066





