Low-Frequency Ventilation May Facilitate Weaning in Acute Respiratory Distress Syndrome Treated with Extracorporeal Membrane Oxygenation: A Randomized Controlled Trial
Abstract
:1. Introduction
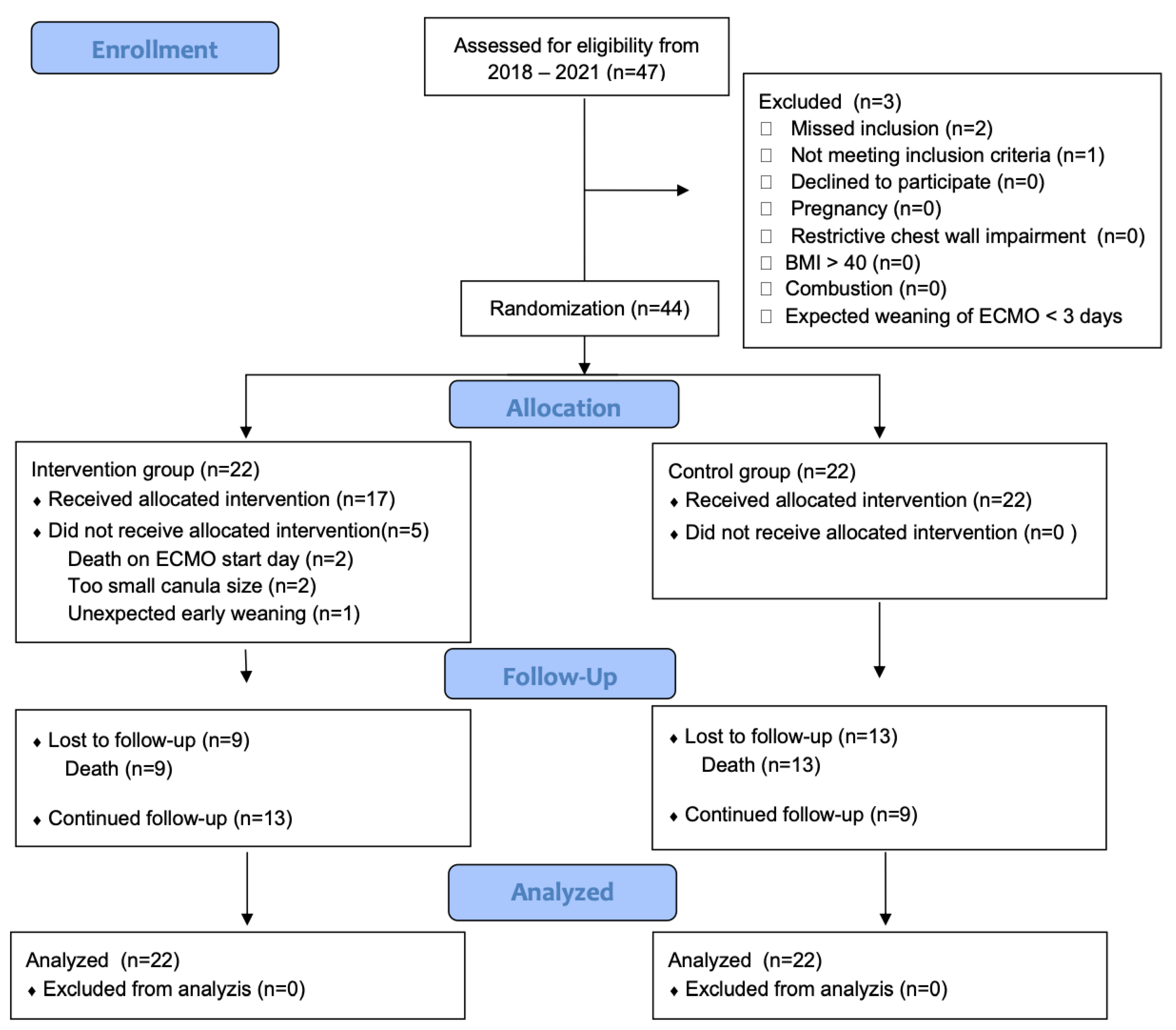
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria and Randomization
2.3. Interventions
- Data sources
- Endpoints/Aims
- Statistical methods
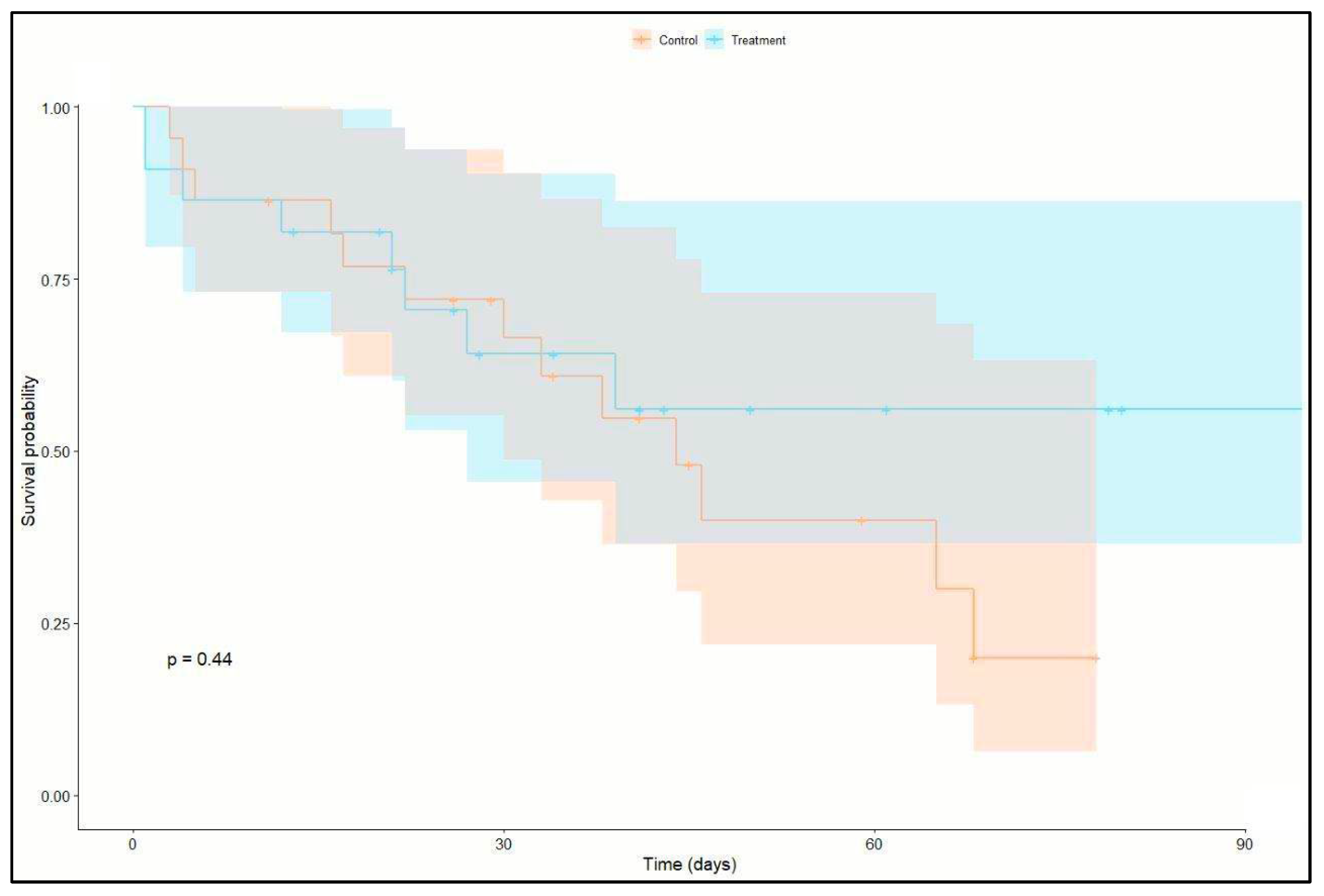
3. Results
- Main findings
- Outcome data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gajkowski, E.F.; Herrera, G.; Hatton, L.; Velia Antonini, M.; Vercaemst, L.; Cooley, E. ELSO Guidelines for Adult and Pediatric Extracorporeal Membrane Oxygenation Circuits. ASAIO J. 2022, 68, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Serpa Neto, A.; Schmidt, M.; Azevedo, L.C.; Bein, T.; Brochard, L.; Beutel, G.; Combes, A.; Costa, E.L.; Hodgson, C.; Lindskov, C.; et al. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: A pooled individual patient data analysis: Mechanical ventilation during ECMO. Intensive Care Med. 2016, 42, 1672–1684. [Google Scholar] [CrossRef]
- Bein, T.; Weber-Carstens, S.; Goldmann, A.; Muller, T.; Staudinger, T.; Brederlau, J.; Muellenbach, R.; Dembinski, R.; Graf, B.M.; Wewalka, M.; et al. Lower tidal volume strategy (approximately 3 mL/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 mL/kg) in severe ARDS: The prospective randomized Xtravent-study. Intensive Care Med. 2013, 39, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Hager, D.N.; Krishnan, J.A.; Hayden, D.L.; Brower, R.G.; Network, A.C.T. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am. J. Respir. Crit. Care Med. 2005, 172, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, N.; De Feo, C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst. Rev. 2013, 2, CD003844. [Google Scholar] [CrossRef]
- Spece, L.J.; Mitchell, K.H.; Caldwell, E.S.; Gundel, S.J.; Jolley, S.E.; Hough, C.L. Low tidal volume ventilation use remains low in patients with acute respiratory distress syndrome at a single center. J. Crit. Care 2018, 44, 72–76. [Google Scholar] [CrossRef]
- Wu, M.Y.; Chang, Y.S.; Huang, C.C.; Wu, T.I.; Lin, P.J. The impacts of baseline ventilator parameters on hospital mortality in acute respiratory distress syndrome treated with venovenous extracorporeal membrane oxygenation: A retrospective cohort study. BMC Pulm. Med. 2017, 17, 181. [Google Scholar] [CrossRef]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef]
- Broman, L.M.; Malfertheiner, M.V.; Montisci, A.; Pappalardo, F. Weaning from veno-venous extracorporeal membrane oxygenation: How I do it. J. Thorac. Dis. 2018, 10 (Suppl. 5), S692–S697. [Google Scholar] [CrossRef]
- Pham, T.; Combes, A.; Roze, H.; Chevret, S.; Mercat, A.; Roch, A.; Mourvillier, B.; Ara-Somohano, C.; Bastien, O.; Zogheib, E.; et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: A cohort study and propensity-matched analysis. Am. J. Respir. Crit. Care Med. 2013, 187, 276–285. [Google Scholar] [CrossRef]
- Del Sorbo, L.; Goffi, A.; Goligher, E.; Fan, E.; Slutsky, A.S. Setting mechanical ventilation in ARDS patients during VV-ECMO: Where are we? Minerva Anestesiol. 2015, 81, 1369–1376. [Google Scholar]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136.e124. [Google Scholar] [CrossRef]
- Lauder, S.N.; Jones, E.; Smart, K.; Bloom, A.; Williams, A.S.; Hindley, J.P.; Ondondo, B.; Taylor, P.R.; Clement, M.; Fielding, C.; et al. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur. J. Immunol. 2013, 43, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.L.; Wang, C.T.; Yang, S.J.; Leu, C.H.; Chen, S.H.; Wu, C.L.; Shiau, A.L. IL-6 ameliorates acute lung injury in influenza virus infection. Sci. Rep. 2017, 7, 43829. [Google Scholar] [CrossRef] [PubMed]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Becher, T.; van der Staay, M.; Schadler, D.; Frerichs, I.; Weiler, N. Calculation of mechanical power for pressure-controlled ventilation. Intensive Care Med. 2019, 45, 1321–1323. [Google Scholar] [CrossRef]
- Moreno, R.P.; Metnitz, P.G.; Almeida, E.; Jordan, B.; Bauer, P.; Campos, R.A.; Iapichino, G.; Edbrooke, D.; Capuzzo, M.; Le Gall, J.R.; et al. SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005, 31, 1345–1355. [Google Scholar] [CrossRef]
- Schmidt, M.; Schellongowski, P.; Patroniti, N.; Taccone, F.S.; Reis Miranda, D.; Reuter, J.; Prodanovic, H.; Pierrot, M.; Dorget, A.; Park, S.; et al. Six-Month Outcome of Immunocompromised Patients with Severe Acute Respiratory Distress Syndrome Rescued by Extracorporeal Membrane Oxygenation. An International Multicenter Retrospective Study. Am. J. Respir. Crit. Care Med. 2018, 197, 1297–1307. [Google Scholar] [CrossRef]
- Haslacher, H.; Gerner, M.; Hofer, P.; Jurkowitsch, A.; Hainfellner, J.; Kain, R.; Wagner, O.F.; Perkmann, T. Usage Data and Scientific Impact of the Prospectively Established Fluid Bioresources at the Hospital-Based MedUni Wien Biobank. Biopreserv. Biobank 2018, 16, 477–482. [Google Scholar] [CrossRef]
- Contentin, L.; Ehrmann, S.; Giraudeau, B. Heterogeneity in the definition of mechanical ventilation duration and ventilator-free days. Am. J. Respir. Crit. Care Med. 2014, 189, 998–1002. [Google Scholar] [CrossRef]
- Araos, J.; Alegria, L.; Garcia, P.; Cruces, P.; Soto, D.; Erranz, B.; Amthauer, M.; Salomon, T.; Medina, T.; Rodriguez, F.; et al. Near-Apneic Ventilation Decreases Lung Injury and Fibroproliferation in an Acute Respiratory Distress Syndrome Model with Extracorporeal Membrane Oxygenation. Am. J. Respir. Crit. Care Med. 2019, 199, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, L.; Goffi, A.; Tomlinson, G.; Pettenuzzo, T.; Facchin, F.; Vendramin, A.; Goligher, E.C.; Cypel, M.; Slutsky, A.S.; Keshavjee, S.; et al. Effect of Driving Pressure Change During Extracorporeal Membrane Oxygenation in Adults with Acute Respiratory Distress Syndrome: A Randomized Crossover Physiologic Study. Crit. Care Med. 2020, 48, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Rozencwajg, S.; Guihot, A.; Franchineau, G.; Lescroat, M.; Brechot, N.; Hekimian, G.; Lebreton, G.; Autran, B.; Luyt, C.E.; Combes, A.; et al. Ultra-Protective Ventilation Reduces Biotrauma in Patients on Venovenous Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. Crit. Care Med. 2019, 47, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Guervilly, C.; Fournier, T.; Chommeloux, J.; Arnaud, L.; Pinglis, C.; Baumstarck, K.; Boucekine, M.; Valera, S.; Sanz, C.; Adda, M.; et al. Ultra-lung-protective ventilation and biotrauma in severe ARDS patients on veno-venous extracorporeal membrane oxygenation: A randomized controlled study. Crit. Care 2022, 26, 383. [Google Scholar] [CrossRef] [PubMed]
- Kallet, R.H.; Campbell, A.R.; Dicker, R.A.; Katz, J.A.; Mackersie, R.C. Effects of tidal volume on work of breathing during lung-protective ventilation in patients with acute lung injury and acute respiratory distress syndrome. Crit. Care Med. 2006, 34, 8–14. [Google Scholar] [CrossRef]
- Kallet, R.H.; Corral, W.; Silverman, H.J.; Luce, J.M. Implementation of a low tidal volume ventilation protocol for patients with acute lung injury or acute respiratory distress syndrome. Respir. Care 2001, 46, 1024–1037. [Google Scholar]
- Holanda, M.A.; Vasconcelos, R.D.S.; Ferreira, J.C.; Pinheiro, B.V. Patient-ventilator asynchrony. J. Bras. Pneumol. 2018, 44, 321–333. [Google Scholar] [CrossRef]
- Sameed, M.; Meng, Z.; Marciniak, E.T. EOLIA trial: The future of extracorporeal membrane oxygenation in acute respiratory distress syndrome therapy? Breathe 2019, 15, 244–246. [Google Scholar] [CrossRef]
- Tonna, J.E.; Abrams, D.; Brodie, D.; Greenwood, J.C.; Rubio Mateo-Sidron, J.A.; Usman, A.; Fan, E. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021, 67, 601–610. [Google Scholar] [CrossRef]
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Combes, A.; Agerstrand, C.; Annich, G.; Diaz, R.; Fan, E.; Hryniewicz, K.; Lorusso, R.; et al. Extracorporeal membrane oxygenation for COVID-19: Evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet 2021, 398, 1230–1238. [Google Scholar] [CrossRef]
- Abrams, D.; Schmidt, M.; Pham, T.; Beitler, J.R.; Fan, E.; Goligher, E.C.; McNamee, J.J.; Patroniti, N.; Wilcox, M.E.; Combes, A.; et al. Mechanical Ventilation for Acute Respiratory Distress Syndrome during Extracorporeal Life Support. Research and Practice. Am. J. Respir. Crit. Care Med. 2020, 201, 514–525. [Google Scholar] [CrossRef]
- Hermann, M.; Laxar, D.; Krall, C.; Hafner, C.; Herzog, O.; Kimberger, O.; Koenig, S.; Kraft, F.; Maleczek, M.; Markstaller, K.; et al. Duration of invasive mechanical ventilation prior to extracorporeal membrane oxygenation is not associated with survival in acute respiratory distress syndrome caused by coronavirus disease 2019. Ann. Intensive Care 2022, 12, 6. [Google Scholar] [CrossRef]
- Retamal, J.; Damiani, L.F.; Basoalto, R.; Benites, M.H.; Bruhn, A.; Larsson, A.; Bugedo, G. Physiological and inflammatory consequences of high and low respiratory rate in acute respiratory distress syndrome. Acta Anaesthesiol. Scand. 2021, 65, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Amado-Rodriguez, L.; Del Busto, C.; Lopez-Alonso, I.; Parra, D.; Mayordomo-Colunga, J.; Arias-Guillen, M.; Albillos-Almaraz, R.; Martin-Vicente, P.; Lopez-Martinez, C.; Huidobro, C.; et al. Biotrauma during ultra-low tidal volume ventilation and venoarterial extracorporeal membrane oxygenation in cardiogenic shock: A randomized crossover clinical trial. Ann. Intensive Care 2021, 11, 132. [Google Scholar] [CrossRef]
- Datzmann, T.; Trager, K. Extracorporeal membrane oxygenation and cytokine adsorption. J. Thorac. Dis. 2018, 10 (Suppl. 5), S653–S660. [Google Scholar] [CrossRef] [PubMed]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A.; Network, N.A. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchie, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Borrmann, M.; Brandes, F.; Kirchner, B.; Klein, M.; Billaud, J.N.; Reithmair, M.; Rehm, M.; Schelling, G.; Pfaffl, M.W.; Meidert, A.S. Extensive blood transcriptome analysis reveals cellular signaling networks activated by circulating glycocalyx components reflecting vascular injury in COVID-19. Front. Immunol. 2023, 14, 1129766. [Google Scholar] [CrossRef]
- Sinha, P.; Calfee, C.S.; Cherian, S.; Brealey, D.; Cutler, S.; King, C.; Killick, C.; Richards, O.; Cheema, Y.; Bailey, C.; et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: A prospective observational study. Lancet Respir. Med. 2020, 8, 1209–1218. [Google Scholar] [CrossRef]
- Schmidt, M.; Pham, T.; Arcadipane, A.; Agerstrand, C.; Ohshimo, S.; Pellegrino, V.; Vuylsteke, A.; Guervilly, C.; McGuinness, S.; Pierard, S.; et al. Mechanical Ventilation Management during Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. An International Multicenter Prospective Cohort. Am. J. Respir. Crit. Care Med. 2019, 200, 1002–1012. [Google Scholar] [CrossRef]


| Control Group | Treatment Group | |
|---|---|---|
| Duration | 72 h from inclusion 1 | |
| Ventilation Mode | Conventional settings 2 | Pressure controlled ventilation |
| Respiratory Rate | 12–25 per minute | 4–5 per minute |
| PEEP | ≥10 cm H2O | 14–16 cm H2O 3 |
| Peak Pressure | <30 cm H2O | 23–25 cm H2O 4 |
| Tidal Volume | <6 mL/kg PBW | 4 mL/kg PBW |
| I:E | 1:1–1:2 | 1:5 |
| All Patients | Control | Treatment | p Value | ||
|---|---|---|---|---|---|
| Age, mean (SD)–years | 56(±12) | 56(±10) | 56(±14) | 0.912 | |
| Sex, Male, no. (%) | 31(70) | 16(73) | 15(68) | 1 | |
| BMI, mean (SD)–kg/m2 | 30(±9) | 30(±11) | 29(±6) | 0.738 | |
| COVID-19, no. (%) | 26(59) | 12(55) | 14(64) | 0.759 | |
| SAPS III, mean (SD) | 64(±14) | 60(±14) | 67(±13) | 0.074 | |
| Tracheostomy, no. (%) | 30(68) | 18(82) | 12(55) | 0.106 | |
| COMORBIDITIES | All patients | Control | Treatment | p value | |
| Arterial hypertension, no. (%) | 19(43) | 9(41) | 10(45) | 0.851 | |
| Chronic heart disease, no. (%) | 6(14) | 2(9) | 4(18) | 0.659 | |
| Obesity, no. (%) | 5(11) | 3(14) | 2(9) | 0.690 | |
| Diabetes, no. (%) | 8(18) | 3(14) | 5(23) | 0.794 | |
| Chronic respiratory disease, no. (%) | 8(18) | 3(14) | 5(23) | 0.672 | |
| Chronic kidney disease, no. (%) | 18(41) | 10(23) | 8(18) | 0.759 | |
| Immunosuppression, no. (%) | 6(14) | 4(18) | 2(9) | 0.763 | |
| VENTILATION PRE-ECMO | All patients | Control | Treatment | p value | |
| PEEP, mean (SD)–cm H2O | 12(±3) | 11(±3) | 12(±3) | 0.316 | |
| TV, mean (SD)–ml | 355(±141) | 401(±144) | 309(±126) | 0.030 | |
| TV, mean (SD)–ml/kg PBW | 5.4(±2.1) | 6.2(±2.2) | 4.6(±1.9) | 0.013 | |
| Respiratory rate, mean (SD)–/min | 18(±5) | 20(±4) | 16(±6) | 0.021 | |
| Peak pressure, mean (SD)–cm H2O | 29(±4) | 31(±3) | 27(±4) | 0.003 | |
| Driving pressure, mean (SD)–cm H2O | 17(±5) | 19(±3) | 15(±5) | 0.001 | |
| MP, median (IQR)–J/min | 18.6(9.6,28.9), n = 43 | 26.9(18.6,33.2), n = 21 | 10.7(8.5,19.9), n = 22 | 0.002 | |
| BASELINE VALUES PRE-ECMO | All patients | Control | Treatment | p value | |
| pre-ECMO IMV, median (IQR)–days | 6(1,10) | 2.5(1,8) | 7.5 (5.2,10) | 0.015 | |
| PaO2/FiO2, mean (SD) | 96(±62) | 82(±50) | 109(±71) | 0.324 | |
| PaO2, mean (SD)–mmHg | 73(±20), n = 40 | 73(± 19), n = 19 | 72(±21), n = 21 | 0.875 | |
| PaCO2, mean (SD)–mmHg | 71(±27), n = 40 | 68(±19), n = 19 | 73(±33), n = 21 | 0.521 | |
| pH, mean (SD)–mmHg | 7.29(±0.13), n = 40 | 7.31(±0.10), n = 19 | 7.27(±0.15), n = 21 | 0.403 | |
| BE, mean (SD)–mmol/L | 6(±6), n = 40 | 6(±6), n = 19 | 5(±7), n = 21 | 0.553 | |
| NMBA, no. (%) | 20(45%) | 11(50%) | 9(41%) | 0.762 | |
| ECMO CONFIGURATION MEAN DURING 72 h on ECMO | All Patients | Control | Treatment | p Value |
|---|---|---|---|---|
| Venoarterial, no. (%) | 6(14%) | 1(5%) | 5(23%) | 0.188 |
| Venovenous, no. (%) | 38(86%) | 21(95%) | 17(77%) | 0.188 |
| ECMO blood flow, mean (SD)-l/min | 3.2(±0.7) | 3.1(±0.6) | 3.3(±0.8) | 0.373 |
| ECMO sweep gas flow, mean (SD)-l/min | 3.9(±1.2) | 3.6(±1.3) | 4.3(±1.1) | 0.066 |
| OUTCOME | All Patients | Control | Treatment | p Value |
|---|---|---|---|---|
| Ventilator-free days until day 28, mean (SD)–days | 4.2(±5.8) | 3(±5.5) | 5.4(±6) | 0.117 |
| ICU LOS, median (IQR)–days | 30(17,45) | 34(18,46) | 27(15,43) | 0.526 |
| ECMO duration, median (IQR)–days | 13(7,27) | 16(8,30) | 12(6,23) | 0.372 |
| ICU mortality, no. (%) | 21(48) | 13(59) | 8(36) | 0.227 |
| 28-day mortality, no. (%) | 12(27) | 5(23) | 7(32) | 0.735 |
| 90-day mortality, no. (%) | 17(39) | 10(45) | 7(32) | 0.536 |
| pre-ECMO IMV, mean (SD)–days vs. ICU death | 8(±8) | 8(±6) | 8(±10) | 0.997 |
| CAUSE OF DEATH | All patients | Control | Treatment | p value |
| Multiorgan failure, no. (%) | 7(16) | 4(18) | 3(14) | 1 |
| Intracranial bleeding, no. (%) | 2(5) | 2(9) | 0(0) | 0.57 |
| Heart failure, no. (%) | 2(5) | 1(5) | 1(5) | 0.864 |
| COVID-19 ARDS, no. (%) | 3(7) | 1(5) | 2(9) | 0.784 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermann, M.; König, S.; Laxar, D.; Krall, C.; Kraft, F.; Krenn, K.; Baumgartner, C.; Tretter, V.; Maleczek, M.; Hermann, A.; et al. Low-Frequency Ventilation May Facilitate Weaning in Acute Respiratory Distress Syndrome Treated with Extracorporeal Membrane Oxygenation: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 5094. https://doi.org/10.3390/jcm13175094
Hermann M, König S, Laxar D, Krall C, Kraft F, Krenn K, Baumgartner C, Tretter V, Maleczek M, Hermann A, et al. Low-Frequency Ventilation May Facilitate Weaning in Acute Respiratory Distress Syndrome Treated with Extracorporeal Membrane Oxygenation: A Randomized Controlled Trial. Journal of Clinical Medicine. 2024; 13(17):5094. https://doi.org/10.3390/jcm13175094
Chicago/Turabian StyleHermann, Martina, Sebastian König, Daniel Laxar, Christoph Krall, Felix Kraft, Katharina Krenn, Clemens Baumgartner, Verena Tretter, Mathias Maleczek, Alexander Hermann, and et al. 2024. "Low-Frequency Ventilation May Facilitate Weaning in Acute Respiratory Distress Syndrome Treated with Extracorporeal Membrane Oxygenation: A Randomized Controlled Trial" Journal of Clinical Medicine 13, no. 17: 5094. https://doi.org/10.3390/jcm13175094






