The Smoky Impact of Nicotinic Acetylcholine Receptors on Testicular Function
Abstract
:1. Introduction
2. Testicular Function and Nicotine
2.1. Structure and Function of nAChRs
2.2. Expression of nAChRs in the Testes
3. Preclinical Models for Studying Nicotine’s Effects on Testicular Function
3.1. Animal Models of Nicotine Exposure
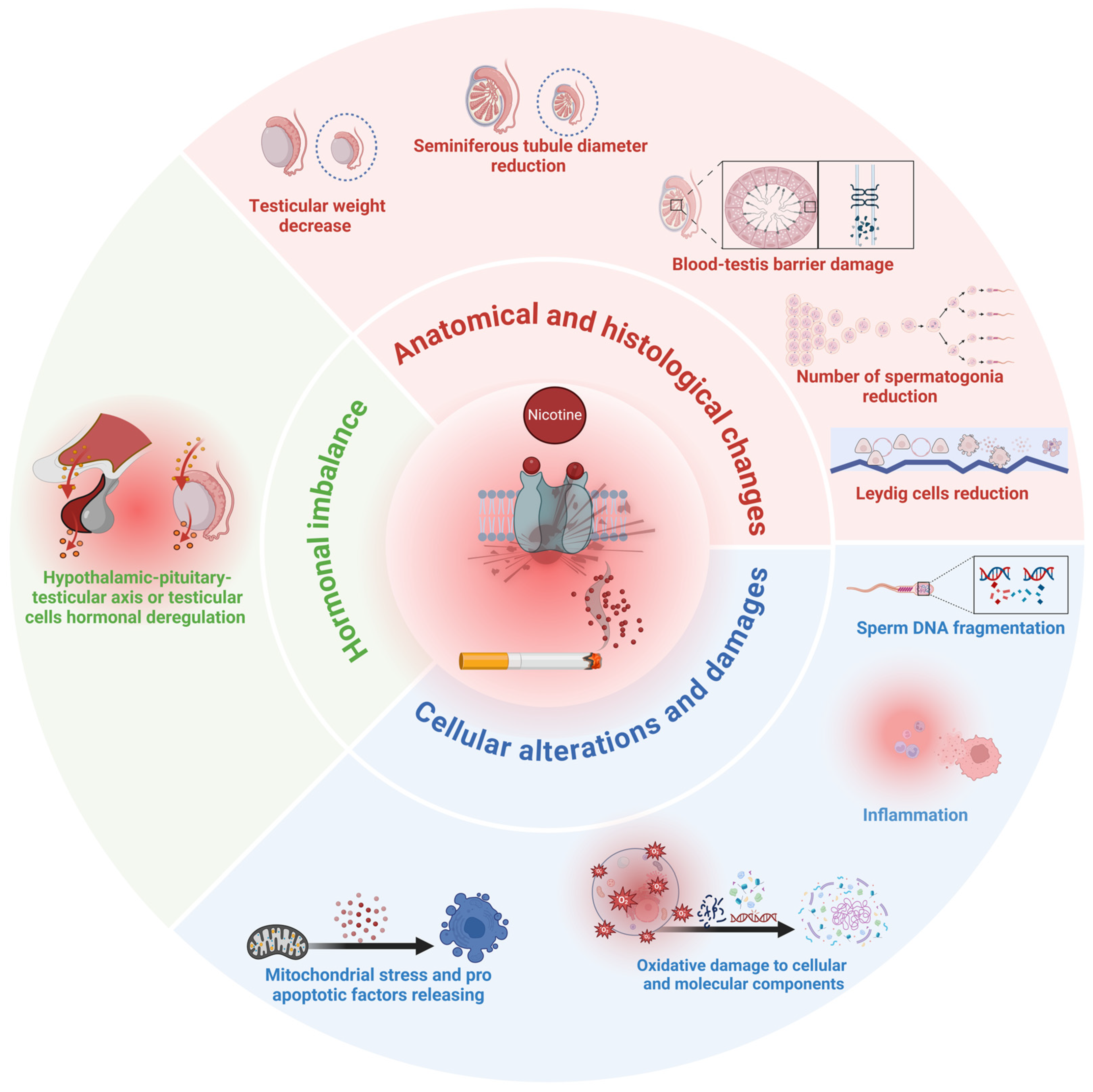
3.2. Mechanisms of Spermatogenesis Impairment from Nicotine Exposure
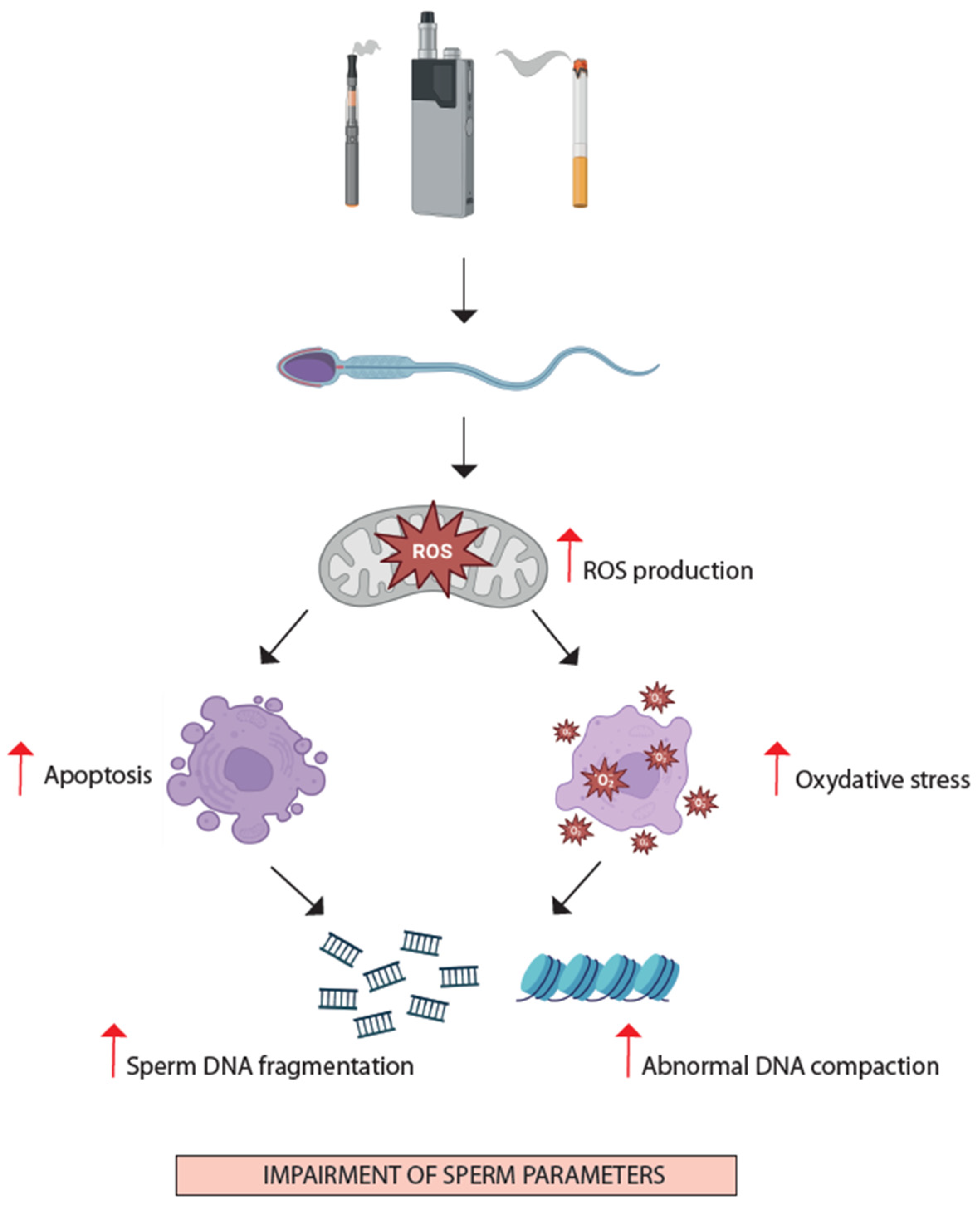
3.2.1. Oxidative Stress and Apoptosis
3.2.2. Hormonal Imbalance
3.2.3. Inflammatory Response
4. Clinical Evidence of Nicotine’s Effects on Testicular Function
4.1. “Conventional” Cigarettes
4.2. New Smoking Habits
4.3. Clinical Data on Smoking Habits and Male Reproductive Health
4.3.1. Semen Quality
4.3.2. Hormone Profile
4.3.3. Sperm Chromatin Integrity and Oxidative Stress
4.3.4. Epigenetic Modifications
5. Future Perspectives
6. Conclusions
- Hormone axis: alterations of blood testosterone and gonadotropin levels.
- Testicular histology: BTB damage, Leydig cell dysfunction, alteration of seminiferous tubules.
- Spermatozoa: increased mitochondrial stress and apoptosis, increased oxidative stress and inflammation, increased SDF.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2025, 4th ed.; WHO Press: Geneva, Switzerland, 2021. [Google Scholar]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette Smoking and Semen Quality: A New Meta-Analysis Examining the Effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 2016, 70, 635–645. [Google Scholar] [CrossRef]
- Lang, G.; Henao, C.; Almstetter, M.; Arndt, D.; Goujon, C.; Maeder, S. Non-Targeted Analytical Comparison of a Heated Tobacco Product Aerosol against Mainstream Cigarette Smoke: Does Heating Tobacco Produce an Inherently Different Set of Aerosol Constituents? Anal. Bioanal. Chem. 2024, 416, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Le Foll, B.; Piper, M.E.; Fowler, C.D.; Tonstad, S.; Bierut, L.; Lu, L.; Jha, P.; Hall, W.D. Tobacco and Nicotine Use. Nat. Rev. Dis. Primers 2022, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Sherwood, N. Systematic Review of Biomarker Findings from Clinical Studies of Electronic Cigarettes and Heated Tobacco Products. Toxicol. Rep. 2021, 8, 282–294. [Google Scholar] [CrossRef]
- Upadhyay, S.; Rahman, M.; Johanson, G.; Palmberg, L.; Ganguly, K. Heated Tobacco Products: Insights into Composition and Toxicity. Toxics 2023, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Cheng, T.; Feng, C.; Walters, M.J. Harmful and Potentially Harmful Constituents in E-Liquids and Aerosols from Electronic Nicotine Delivery Systems (ENDS). Chem. Res. Toxicol. 2024, 37, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- Campolo, F.; Capponi, C.; Tarsitano, M.G.; Tenuta, M.; Pozza, C.; Gianfrilli, D.; Magliocca, F.; Venneri, M.A.; Vicini, E.; Lenzi, A.; et al. CAMP-Specific Phosphodiesterase 8A and 8B Isoforms Are Differentially Expressed in Human Testis and Leydig Cell Tumor. Front. Endocrinol. 2022, 13, 1010924. [Google Scholar] [CrossRef]
- Hermann, B.P.; Cheng, K.; Singh, A.; Roa-De La Cruz, L.; Mutoji, K.N.; Chen, I.C.; Gildersleeve, H.; Lehle, J.D.; Mayo, M.; Westernströer, B.; et al. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep. 2018, 25, 1650–1667.e8. [Google Scholar] [CrossRef]
- Khanehzad, M.; Abbaszadeh, R.; Holakuyee, M.; Modarressi, M.H.; Nourashrafeddin, S.M. FSH Regulates RA Signaling to Commit Spermatogonia into Differentiation Pathway and Meiosis. Reprod. Biol. Endocrinol. 2021, 19, 4. [Google Scholar] [CrossRef]
- Hogg, R.C.; Raggenbass, M.; Bertrand, D. Nicotinic Acetylcholine Receptors: From Structure to Brain Function. Rev. Physiol. Biochem. Pharmacol. 2003, 147, 1–46. [Google Scholar] [CrossRef]
- Hurst, R.; Rollema, H.; Bertrand, D. Nicotinic Acetylcholine Receptors: From Basic Science to Therapeutics. Pharmacol. Ther. 2013, 137, 22–54. [Google Scholar] [CrossRef]
- Lindstrom, J. Neuronal Nicotinic Acetylcholine Receptors. Ion. Channels 1996, 4, 377–450. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H.; Horiguchi, K. Non-Neuronal Cholinergic System in Regulation of Immune Function with a Focus on α7 NAChRs. Int. Immunopharmacol. 2015, 29, 127–134. [Google Scholar] [CrossRef]
- Maus, A.D.J.; Pereira, E.F.R.; Karachunski, P.I.; Horton, R.M.; Navaneetham, D.; Macklin, K.; Cortes, W.S.; Albuquerque, E.X.; Conti-Fine, B.M. Human and Rodent Bronchial Epithelial Cells Express Functional Nicotinic Acetylcholine Receptors. Mol. Pharmacol. 1998, 54, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Grando, S.A. Biological Functions of Keratinocyte Cholinergic Receptors. J. Investig. Dermatol. Symp. Proc. 1997, 2, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Avellar, M.C.W.; Siu, E.R.; Yasuhara, F.; Maróstica, E.; Porto, C.S. Muscarinic Acetylcholine Receptor Subtypes in the Male Reproductive Tract. J. Mol. Neurosci. 2010, 40, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, S.U.; Eckhardt, I.; Lau, H.; Klein, J.; DeGraaf, Y.C.; Lips, K.S.; Pineau, C.; Gibbins, I.L.; Kummer, W.; Meinhardt, A.; et al. The Cholinergic System in Rat Testis Is of Non-Neuronal Origin. Reproduction 2011, 142, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Ng, S.H.; Luk, A.C.; Suen, H.C.; Qian, Y.; Lee, A.W.T.; Tu, J.; Fung, J.C.L.; Tang, N.L.S.; Feng, B.; et al. Revealing Cellular and Molecular Transitions in Neonatal Germ Cell Differentiation Using Single Cell RNA Sequencing. Development 2019, 146, dev174953. [Google Scholar] [CrossRef]
- Oatley, J.M.; Oatley, M.J.; Avarbock, M.R.; Tobias, J.W.; Brinster, R.L. Colony Stimulating Factor 1 Is an Extrinsic Stimulator of Mouse Spermatogonial Stem Cell Self-Renewal. Development 2009, 136, 1191–1199. [Google Scholar] [CrossRef]
- Ni, C.; Li, Y.; Li, Z.; Tian, L.; Fu, J.; Wu, K.; Wang, Y.; Yao, M.; Ge, R. Cisatracurium Stimulates Testosterone Synthesis in Rat and Mouse Leydig Cells via Nicotinic Acetylcholine Receptor. J. Cell Mol. Med. 2020, 24, 14184–14194. [Google Scholar] [CrossRef]
- Vigueras-Villaseñor, R.M.; Fuentes-Cano, M.A.; Saldaña, M.C.; Espinosa, L.R.; Reynoso-Robles, R.; Rojas, P.; Durán, P.; Rojas-Castañeda, J.C. Fetal and Postnatal Nicotine Exposure Modifies Maturation of Gonocytes to Spermatogonia in Mice. Anal. Cell. Pathol. 2020, 2020, 8892217. [Google Scholar] [CrossRef] [PubMed]
- Favaretto, A.L.; Valença, M.M.; Picanço-Diniz, D.L.W.; Antunes-Rodrigues, J. Inhibitory Role of Cholinergic Agonists on Testosterone Secretion by Purified Rat Leydig Cells. Arch. Int. Physiol. Biochim. Biophys. 1993, 101, 333–335. [Google Scholar] [CrossRef]
- Ge, R.-S.; Dong, Q.; Sottas, C.M.; Chen, H.; Zirkin, B.R.; Hardy, M.P. Gene Expression in Rat Leydig Cells During Development from the Progenitor to Adult Stage: A Cluster Analysis1. Biol. Reprod. 2005, 72, 1405–1415. [Google Scholar] [CrossRef]
- Makino, Y.; Hiradate, Y.; Umezu, K.; Hara, K.; Tanemura, K. Expression and Possible Role of Nicotinic Acetylcholine Receptor ε Subunit (Achre) in Mouse Sperm. Biology 2021, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, B.; Burrini, A.G.; Collodel, G.; Falugi, C.; Moretti, E.; Piomboni, P. Localisation of Two Classes of Acetylcholine Receptor-like Molecules in Sperms of Different Animal Species. Zygote 1995, 3, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Bray, C.; Son, J.-H.; Kumar, P.; Meizel, S. Mice Deficient in CHRNA7, a Subunit of the Nicotinic Acetylcholine Receptor, Produce Sperm with Impaired Motility1. Biol. Reprod. 2005, 73, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Meizel, S. Nicotinic Acetylcholine Receptor Subunits and Associated Proteins InHuman Sperm. J. Biol. Chem. 2005, 280, 25928–25935. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Giacone, F.; Iacoviello, L.; Mongioì, L.M.; Volti, G.L.; Barbagallo, I.; Avola, R.; Calogero, A.E. Nicotine Effects and Receptor Expression on Human Spermatozoa: Possible Neuroendocrine Mechanism. Front. Physiol. 2017, 8, 177. [Google Scholar] [CrossRef]
- Bray, C.; Son, J.-H.; Meizel, S. Acetylcholine Causes an Increase of Intracellular Calcium in Human Sperm. MHR Basic. Sci. Reprod. Med. 2005, 11, 881–889. [Google Scholar] [CrossRef]
- Bray, C.; Son, J.-H.; Meizel, S. A Nicotinic Acetylcholine Receptor Is Involved in the Acrosome Reaction of Human Sperm Initiated by Recombinant Human ZP31. Biol. Reprod. 2002, 67, 782–788. [Google Scholar] [CrossRef]
- Ibáñez, C.F.; Pelto-Huikko, M.; Söder, O.; Ritzèn, E.M.; Hersh, L.B.; Hökfelt, T.; Persson, H. Expression of Choline Acetyltransferase MRNA in Spermatogenic Cells Results in an Accumulation of the Enzyme in the Postacrosomal Region of Mature Spermatozoa. Proc. Natl. Acad. Sci. USA 1991, 88, 3676–3680. [Google Scholar] [CrossRef]
- Son, J.-H.; Meizel, S. Evidence Suggesting That the Mouse Sperm Acrosome Reaction Initiated by the Zona Pellucida Involves an α7 Nicotinic Acetylcholine Receptor1. Biol. Reprod. 2003, 68, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Orr-Urtreger, A.; Gö, F.M.; Saeki, M.; Lorenzo, I.; Goldberg, L.; De Biasi, M.; Dani, J.A.; Patrick, J.W.; Beaudet, A.L. Mice Deficient in the 7 Neuronal Nicotinic Acetylcholine Receptor Lack-Bungarotoxin Binding Sites and Hippocampal Fast Nicotinic Currents. J. Neurosci. 2019, 17, 9165–9171. [Google Scholar] [CrossRef]
- Le Foll, B.; Goldberg, S.R. Effects of Nicotine in Experimental Animals and Humans: An Update on Addictive Properties. Handb. Exp. Pharmacol. 2009, 192, 335–367. [Google Scholar] [CrossRef]
- Rodgman, A.; Perfetti, T.A. The Chemical Components of Tobacco and Tobacco Smoke, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9780429146831. [Google Scholar]
- Aydos, K.; Güven, M.C.; Can, B.; Ergün, A. Nicotine Toxicity to the Ultrastructure of the Testis in Rats. BJU Int. 2001, 88, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Kavitharaj, N.K.; Vijayammal, P.L. Nicotine Administration Induced Changes in the Gonadal Functions in Male Rats. Pharmacology 1999, 58, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, R.; Yousef, M.I.; Mantovani, A. Nicotine-Induced Reproductive Toxicity, Oxidative Damage, Histological Changes and Haematotoxicity in Male Rats: The Protective Effects of Green Tea Extract. Exp. Toxicol. Pathol. 2015, 67, 253–259. [Google Scholar] [CrossRef]
- Lagunov, A.; Anzar, M.; Sadeu, J.C.; Khan, M.I.R.; Bruin, J.E.; Woynillowicz, A.K.; Buhr, M.; Holloway, A.C.; Foster, W.G. Effect of in Utero and Lactational Nicotine Exposure on the Male Reproductive Tract in Peripubertal and Adult Rats. Reprod. Toxicol. 2011, 31, 418–423. [Google Scholar] [CrossRef]
- Paccola, C.C.; Miraglia, S.M. Prenatal and Lactation Nicotine Exposure Affects Sertoli Cell and Gonadotropin Levels in Rats. Reproduction 2016, 151, 117–133. [Google Scholar] [CrossRef] [PubMed]
- La Maestra, S.; De Flora, S.; Micale, R.T. Effect of Cigarette Smoke on DNA Damage, Oxidative Stress, and Morphological Alterations in Mouse Testis and Spermatozoa. Int. J. Hyg. Environ. Health 2015, 218, 117–122. [Google Scholar] [CrossRef]
- He, L.; You, S.; Gong, H.; Zhang, J.; Wang, L.; Zhang, C.; Huang, Y.; Zhong, C.; Zou, Y. Cigarette Smoke Induces Rat Testicular Injury via Mitochondrial Apoptotic Pathway. Mol. Reprod. Dev. 2017, 84, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, J.; Yang, L.; Li, X.; Hua, R.; Xu, D.; Jiang, Z.; Li, Q. The Role of Ferroptosis Mediated by Bmal1/Nrf2 in Nicotine -Induce Injury of BTB Integrity. Free Radic. Biol. Med. 2023, 200, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Güven, M.C.; Can, B.; Ergün, A.; Saran, Y.; Aydos, K. Ultrastructural Effects of Cigarette Smoke on Rat Testis. Eur. Urol. 1999, 36, 645–649. [Google Scholar] [CrossRef]
- Marinucci, L.; Balloni, S.; Bellucci, C.; Lilli, C.; Stabile, A.M.; Calvitti, M.; Aglietti, M.C.; Gambelunghe, A.; Muzi, G.; Rende, M.; et al. Effects of Nicotine on Porcine Pre-Pupertal Sertoli Cells: An In Vitro Study. Toxicol. Vitro 2020, 67, 104882. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhan, C.; Xu, W.; Wang, Z.; Nie, D.; Zhao, X.; Zhang, D.; Gu, Y.; Wang, L.; Chen, Z.; et al. Nicotine Elevates Sperm Motility and Induces Pfn1 Promoter Hypomethylation in Mouse Testis. Andrology 2015, 3, 967–978. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, W.; Nie, D.; Zhang, D.; Dai, J.; Zhao, X.; Zhang, M.; Wang, Z.; Chen, Z.; Qiao, Z. Nicotine Induces Nme2-Mediated Apoptosis in Mouse Testes. Biochem. Biophys. Res. Commun. 2016, 472, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Isoyama, E.; Sofikitis, N.; Miyagawa, I. Effects of Smoking on Testicular Function and Fertilizing Potential in Rats. Urol. Res. 1998, 26, 45–48. [Google Scholar] [CrossRef]
- Oyeyipo, I.; Raji, Y.; Bolarinwa, A. Nicotine Alters Male Reproductive Hormones in Male Albino Rats: The Role of Cessation. J. Hum. Reprod. Sci. 2013, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Aruldhas, M.M.; Subramanian, S.; Sekar, P.; Vengatesh, G.; Chandrahasan, G.; Govindarajulu, P.; Akbarsha, M.A. Chronic Chromium Exposure-Induced Changes in Testicular Histoarchitecture Are Associated with Oxidative Stress: Study in a Non-Human Primate (Macaca Radiata Geoffroy). Hum. Reprod. 2005, 20, 2801–2813. [Google Scholar] [CrossRef]
- Seema, P.; Swathy, S.S.; Indira, M. Protective Effect of Selenium on Nicotine-Induced Testicular Toxicity in Rats. Biol. Trace Elem. Res. 2007, 120, 212–218. [Google Scholar] [CrossRef]
- Erat, M.; Ciftci, M.; Gumustekin, K.; Gul, M. Effects of Nicotine and Vitamin E on Glutathione Reductase Activity in Some Rat Tissues in Vivo and in Vitro. Eur. J. Pharmacol. 2007, 554, 92–97. [Google Scholar] [CrossRef]
- Mohammadghasemi, F.; Khanaki, K.; Moravati, H.; Faghani, M. The Amelioration of Nicotine-Induced Reproductive Impairment in Male Mouse by Sambucus ebulus L. Fruit. Extract. Anat. Cell Biol. 2021, 54, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Oyeyipo, I.; Raji, Y.; Bolarinwa, A. Antioxidant Profile Changes in Reproductive Tissues of Rats Treated with Nicotine. J. Hum. Reprod. Sci. 2014, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Jana, K.; Samanta, P.K.; De, D.K. Nicotine Diminishes Testicular Gametogenesis, Steroidogenesis, and Steroidogenic Acute Regulatory Protein Expression in Adult Albino Rats: Possible Influence on Pituitary Gonadotropins and Alteration of Testicular Antioxidant Status. Toxicol. Sci. 2010, 116, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Sarasin, A. Adrenal-Mediated Rather than Direct Effects of Nicotine as a Basis of Altered Sex Steroid Synthesis in Fetal and Neonatal Rat. Reprod. Toxicol. 2003, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, W.; Wu, J.; Zhang, D.; Abou-Shakra, A.; Di, L.; Wang, Z.; Wang, L.; Yang, F.; Qiao, Z. Nicotine Induced Autophagy of Leydig Cells Rather than Apoptosis Is the Major Reason of the Decrease of Serum Testosterone. Int. J. Biochem. Cell Biol. 2018, 100, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, W.; Zhang, D.; Dai, J.; Cao, Y.; Xie, Y.; Wang, L.; Qiao, Z.; Qiao, Z. Nicotine Inhibits Murine Leydig Cell Differentiation and Maturation via Regulating Hedgehog Signal Pathway. Biochem. Biophys. Res. Commun. 2019, 510, 1–7. [Google Scholar] [CrossRef]
- Guo, X.; Wang, H.; Wu, X.; Chen, X.; Chen, Y.; Guo, J.; Li, X.; Lian, Q.; Ge, R.-S. Nicotine Affects Rat Leydig Cell Function in Vivo and Vitro via Down-Regulating Some Key Steroidogenic Enzyme Expressions. Food Chem. Toxicol. 2017, 110, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.; Barbieri, R.L.; Friedman, A.J. Nicotine and Cotinine Inhibit Rat Testis Androgen Biosynthesis in Vitro. J. Steroid Biochem. 1989, 33, 627–630. [Google Scholar] [CrossRef]
- Bose, M.; Debnath, D.; Chen, Y.; Bose, H.S. Folding, Activity and Import of Steroidogenic Acute Regulatory Protein into Mitochondria Changed by Nicotine Exposure. J. Mol. Endocrinol. 2007, 39, 67–79. [Google Scholar] [CrossRef]
- Mohsenzadeh, Y.; Rahmani, A.; Cheraghi, J.; Pyrani, M.; Asadollahi, K. Prenatal Exposure to Nicotine in Pregnant Rat Increased Inflammatory Marker in Newborn Rat. Mediat. Inflamm. 2014, 2014, 274048. [Google Scholar] [CrossRef]
- Ukwenya, V. Testosterone Propionate Ameliorates Oxidatve Stress and Inflammation in Nicotine-Induced Testicular Toxicity. J. Exp. Clin. Anat. 2019, 18, 74. [Google Scholar] [CrossRef]
- Kushwaha, S.; Jena, G. Effects of Nicotine on the Testicular Toxicity of Streptozotocin-Induced Diabetic Rat. Hum. Exp. Toxicol. 2014, 33, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.W.; Zhang, Z.; Wang, H.R.; Luo, L.; Xu, B. Associations between Tobacco Inhalation and Semen Parameters in Men with Primary and Secondary Infertility: A Cross-Sectional Study. Front. Endocrinol. 2024, 15, 1396793. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Sansone, A.; Pallotti, F.; Ferlin, A.; Pivonello, R.; Isidori, A.M.; Maggi, M.; Jannini, E.A. People Smoke for Nicotine, but Lose Sexual and Reproductive Health for Tar: A Narrative Review on the Effect of Cigarette Smoking on Male Sexuality and Reproduction. J. Endocrinol. Investig. 2020, 43, 1391–1408. [Google Scholar] [CrossRef]
- Montjean, D.; Godin Pagé, M.H.; Bélanger, M.C.; Benkhalifa, M.; Miron, P. An Overview of E-Cigarette Impact on Reproductive Health. Life 2023, 13, 827. [Google Scholar] [CrossRef]
- Pallotti, F.; Pelloni, M.; Colangelo, S.; Gianfrilli, D.; Lenzi, A.; Lombardo, F.; Paoli, D. Environmental Impact on Semen Quality and Male Fertility. In Environmental Endocrinology and Endocrine Disruptors: Endocrine and Endocrine-Targeted Actions and Related Human Diseases; Pivonello, R., Diamanti-Kandarakis, E., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 239–269. ISBN 978-3-030-39044-0. [Google Scholar]
- Bazid, H.A.S.; Attia, A.M.; Yousef, A.M.; Fawal, A.N.; Mostafa, M.I. Evaluating the Serum and Seminal Plasma Levels of Zinc and Cadmium in Smokers and Their Relation to the Semen Parameters. Biol. Trace Elem. Res. 2022, 200, 1002–1009. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Li, S. Relationship between Cadmium Content in Semen and Male Infertility: A Meta-Analysis. Environ. Sci. Pollut. Res. 2019, 26, 1947–1953. [Google Scholar] [CrossRef]
- Giulioni, C.; Maurizi, V.; De Stefano, V.; Polisini, G.; Teoh, J.Y.-C.; Milanese, G.; Galosi, A.B.; Castellani, D. The Influence of Lead Exposure on Male Semen Parameters: A Systematic Review and Meta-Analysis. Reprod. Toxicol. 2023, 118, 108387. [Google Scholar] [CrossRef]
- He, Y.; Zou, L.; Luo, W.; Yi, Z.; Yang, P.; Yu, S.; Liu, N.; Ji, J.; Guo, Y.; Liu, P.; et al. Heavy Metal Exposure, Oxidative Stress and Semen Quality: Exploring Associations and Mediation Effects in Reproductive-Aged Men. Chemosphere 2020, 244, 125498. [Google Scholar] [CrossRef]
- Sun, J.; Yu, G.; Zhang, Y.; Liu, X.; Du, C.; Wang, L.; Li, Z.; Wang, C. Heavy Metal Level in Human Semen with Different Fertility: A Meta-Analysis. Biol. Trace Elem. Res. 2017, 176, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Altieri, I.; Gandini, L.; Lenzi, A.; Pichini, S.; Rosa, M.; Zuccaro, P.; Dondero, F. Nicotine, Cotinine, and Trans-3-Hydroxycotine Levels in Seminal Plasma of Smokers: Effects on Sperm Parameters. Ther. Drug Monit. 1993, 15, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Dill, M.; Deconinck, E.; Barhdadi, S. Method Development and Validation of an Aerosol Sampling Technique for the Analysis of Nicotine in Electronic Cigarette Aerosols. Molecules 2024, 29, 3487. [Google Scholar] [CrossRef]
- Bentley, M.C.; Almstetter, M.; Arndt, D.; Knorr, A.; Martin, E.; Pospisil, P.; Maeder, S. Comprehensive Chemical Characterization of the Aerosol Generated by a Heated Tobacco Product by Untargeted Screening. Anal. Bioanal. Chem. 2020, 412, 2675–2685. [Google Scholar] [CrossRef]
- Kuehl, P.J.; McDonald, J.D.; Weber, D.T.; Khlystov, A.; Nystoriak, M.A.; Conklin, D.J. Composition of Aerosols from Thermal Degradation of Flavors Used in ENDS and Tobacco Products. Inhal. Toxicol. 2022, 34, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Scherer, G.; Mütze, J.; Pluym, N.; Scherer, M. Assessment of Nicotine Delivery and Uptake in Users of Various Tobacco/Nicotine Products. Curr. Res. Toxicol. 2022, 3, 100067. [Google Scholar] [CrossRef]
- Christen, S.E.; Hermann, L.; Bekka, E.; Vonwyl, C.; Hammann, F.; van der Velpen, V.; Eap, C.B.; Benowitz, N.L.; Haschke, M.; Liakoni, E. Pharmacokinetics and Pharmacodynamics of Inhaled Nicotine Salt and Free-Base Using an E-Cigarette: A Randomized Crossover Study. Nicotine Tob. Res. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gandini, L.; Lombardo, F.; Lenzi, A.; Culasso, F.; Pacifici, R.; Zuccaro, P.; Dondero, F. The In-Vitro Effects of Nicotine and Cotinine on Sperm Motility. Hum. Reprod. 1997, 12, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.; Polosa, R.; Perdichizzi, A.; Guarino, F.; La Vignera, S.; Scarfia, A.; Fratantonio, E.; Condorelli, R.; Bonanno, O.; Barone, N.; et al. Cigarette Smoke Extract Immobilizes Human Spermatozoa and Induces Sperm Apoptosis. Reprod. Biomed. Online 2009, 19, 564–571. [Google Scholar] [CrossRef]
- Kanetkar, S.R.; Mohite, S.; Kadam, R.S.; Gupta, N.; Vadhel, C.R. Effect of Tobacco Use on Semen in Infertile Male. J. Pharm. Bioallied Sci. 2024, 6, S412–S414. [Google Scholar] [CrossRef]
- Borges, E., Jr.; Braga, D.P.d.A.F.; Provenza, R.R.; Figueira, R.d.C.S.; Iaconelli, A., Jr.; Setti, A.S. Paternal Lifestyle Factors in Relation to Semen Quality and In Vitro Reproductive Outcomes. Andrologia 2018, 50, e13090. [Google Scholar] [CrossRef] [PubMed]
- Holmboe, S.A.; Priskorn, L.; Jensen, T.K.; Skakkebaek, N.E.; Andersson, A.-M.; Jørgensen, N. Use of E-Cigarettes Associated with Lower Sperm Counts in a Cross-Sectional Study of Young Men from the General Population. Hum. Reprod. 2020, 35, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Corona, G.; Vitale, P.; Maseroli, E.; Rossi, M.; Fino, M.G.; Maggi, M. Current Smoking Is Associated with Lower Seminal Vesicles and Ejaculate Volume, despite Higher Testosterone Levels, in Male Subjects of Infertile Couples. Hum. Reprod. 2015, 30, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Thakur, J.; Choudhari, S.G. Effectiveness of Healthcare Interventions on Smoking Cessation in Adolescents in Low- and Middle-Income Countries: A Narrative Review. Cureus 2024, 16, e54051. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, F.; Sheldon, E.; Sharma, J.; Canturk, K.M.; Otu, H.H.; Nawshad, A. Nicotine Exposure During Pregnancy Results in Persistent Midline Epithelial Seam With Improper Palatal Fusion. Nicotine Tob. Res. 2016, 18, 604–612. [Google Scholar] [CrossRef] [PubMed]
- İlhan, S.Ö.; Fincan, G.S.Ö.; Okçay, Y.; Koç, D.S.; Aşkin, C.İ.; Kibar, A.K.; Vural, İ.M.; Sarioğlu, Y. Enhancing Effect of Nicotine on Electrical Field Stimulation Elicited Contractile Responses in Isolated Rabbit Bladder Straight Muscle; the Role of Cannabinoid and Vanilloid Receptors. Turk. J. Med. Sci. 2022, 52, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Leung, J.Y.Y.; Lin, S.L.; Mary Schooling, C. Cigarette Smoking and Testosterone in Men and Women: A Systematic Review and Meta-Analysis of Observational Studies. Prev. Med. 2016, 85, 1–10. [Google Scholar] [CrossRef]
- Shah, S.S.; Shah, M.; Habib, S.H.; Shah, F.A.; Malik, M.O. Correlation of Plasma Kisspeptin with Total Testosterone Levels in Smokeless Tobacco and Smoking Tobacco Users in a Healthy Cohort: A Cross-Sectional Study. Andrologia 2019, 51, e13409. [Google Scholar] [CrossRef]
- Kimblad, A.; Ollvik, G.; Lindh, C.H.; Axelsson, J. Decreased Sperm Counts in Swedish Users of Oral Tobacco. Andrology 2022, 10, 1181–1188. [Google Scholar] [CrossRef]
- Ferlin, A.; Calogero, A.E.; Krausz, C.; Lombardo, F.; Paoli, D.; Rago, R.; Scarica, C.; Simoni, M.; Foresta, C.; Rochira, V.; et al. Management of Male Factor Infertility: Position Statement from the Italian Society of Andrology and Sexual Medicine (SIAMS): Endorsing Organization: Italian Society of Embryology, Reproduction, and Research (SIERR). J. Endocrinol. Investig. 2022, 45, 1085–1113. [Google Scholar] [CrossRef]
- Zhao, C.; Xie, Y.; Zhou, X.; Zhang, Q.; Wang, N. The Effect of Different Tobacco Tar Levels on DNA Damage in Cigarette Smoking Subjects. Toxicol. Res. 2020, 9, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Morgil, G.K.; Çok, İ. Evaluation and Comparison of DNA Alkylation and Oxidative Damage in E-Cigarette and Heated Tobacco Users. Toxicol. Mech. Methods 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki-Ching, S.; Williams, M.; Hua, M.; Li, J.; Bates, S.M.; Robinson, A.N.; Lyons, T.W.; Goniewicz, M.L.; Talbot, P. Correlation between Biomarkers of Exposure, Effect and Potential Harm in the Urine of Electronic Cigarette Users. BMJ Open Respir. Res. 2020, 7, e000452. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-M.; Chia, S.-E.; Ni, Z.-Y.; New, A.-L.; Lee, B.-L.; Ong, C.-N. Detection of Oxidative Dna Damage in Human Sperm and the Association with Cigarette Smoking. Reprod. Toxicol. 1997, 11, 675–680. [Google Scholar] [CrossRef]
- Conflitti, A.C.; Cicolani, G.; Buonacquisto, A.; Pallotti, F.; Faja, F.; Bianchini, S.; Blaconà, G.; Bruno, S.M.; Linari, A.; Lucarelli, M.; et al. Sperm DNA Fragmentation and Sperm-Borne MiRNAs: Molecular Biomarkers of Embryo Development? Int. J. Mol. Sci. 2023, 24, 7. [Google Scholar] [CrossRef]
- Paoli, D.; Pallotti, F.; Lenzi, A.; Lombardo, F. Fatherhood and Sperm DNA Damage in Testicular Cancer Patients. Front. Endocrinol. 2018, 9, 506. [Google Scholar] [CrossRef]
- Szabó, A.; Váncsa, S.; Hegyi, P.; Váradi, A.; Forintos, A.; Filipov, T.; Ács, J.; Ács, N.; Szarvas, T.; Nyirády, P.; et al. Lifestyle-, Environmental-, and Additional Health Factors Associated with an Increased Sperm DNA Fragmentation: A Systematic Review and Meta-Analysis. Reprod. Biol. Endocrinol. 2023, 21, 5. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; El Shahawy, O.; Skosana, B.T.; Boillat, T.; Loney, T.; du Plessis, S.S. The Mutagenic Effect of Tobacco Smoke on Male Fertility. Environ. Sci. Pollut. Res. 2022, 29, 62055–62066. [Google Scholar] [CrossRef]
- Hammadeh, M.E.; Hamad, M.F.; Montenarh, M.; Fischer-Hammadeh, C. Protamine Contents and P1/P2 Ratio in Human Spermatozoa from Smokers and Non-Smokers. Hum. Reprod. 2010, 25, 2708–2720. [Google Scholar] [CrossRef]
- Colacurci, N.; De Leo, V.; Ruvolo, G.; Piomboni, P.; Caprio, F.; Pivonello, R.; Papaleo, E.; La Verde, E.; Depalo, R.; Lispi, M.; et al. Recombinant FSH Improves Sperm DNA Damage in Male Infertility: A Phase II Clinical Trial. Front. Endocrinol. 2018, 9, 383. [Google Scholar] [CrossRef]
- Åsenius, F.; Danson, A.F.; Marzi, S.J. DNA Methylation in Human Sperm: A Systematic Review. Hum. Reprod. Update 2020, 26, 841–873. [Google Scholar] [CrossRef] [PubMed]
- Laqqan, M.M.; Yassin, M.M. Cigarette Heavy Smoking Alters DNA Methylation Patterns and Gene Transcription Levels in Humans Spermatozoa. Environ. Sci. Pollut. Res. 2022, 29, 26835–26849. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, E.L.; Amoako, A.A.; Konje, J.C.; Gant, T.W.; Marczylo, T.H. Smoking Induces Differential MiRNA Expression in Human Spermatozoa: A Potential Transgenerational Epigenetic Concern? Epigenetics 2012, 7, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.F.; Oyovwi, M.O.; Olatinwo, G.; Phillips, A.O. Unfolding the Complexity of Epigenetics in Male Reproductive Aging: A Review of Therapeutic Implications. Mol. Biol. Rep. 2024, 51, 881. [Google Scholar] [CrossRef] [PubMed]
- Manetti, D.; Dei, S.; Arias, H.R.; Braconi, L.; Gabellini, A.; Teodori, E.; Romanelli, M.N. Recent Advances in the Discovery of Nicotinic Acetylcholine Receptor Allosteric Modulators. Molecules 2023, 28, 1270. [Google Scholar] [CrossRef] [PubMed]
- Ghewade, P.; Vagha, S.; Ghewade, B.; Gadkari, P. Role of Dietary Antioxidant Supplements in Male Infertility: A Review. Cureus 2024, 16, e61951. [Google Scholar] [CrossRef]
- Hajiasgharzadeh, K.; Naghipour, B.; Shahabi, P.; Dastmalchi, N.; Alipour, M.R. The Role of microRNAs in Nicotine Signaling. EXCLI J. 2023, 22, 433–450. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbagallo, F.; Assenza, M.R.; Torrisi, F.; Buonacquisto, A.; Pallotti, F. The Smoky Impact of Nicotinic Acetylcholine Receptors on Testicular Function. J. Clin. Med. 2024, 13, 5097. https://doi.org/10.3390/jcm13175097
Barbagallo F, Assenza MR, Torrisi F, Buonacquisto A, Pallotti F. The Smoky Impact of Nicotinic Acetylcholine Receptors on Testicular Function. Journal of Clinical Medicine. 2024; 13(17):5097. https://doi.org/10.3390/jcm13175097
Chicago/Turabian StyleBarbagallo, Federica, Maria Rita Assenza, Filippo Torrisi, Alessandra Buonacquisto, and Francesco Pallotti. 2024. "The Smoky Impact of Nicotinic Acetylcholine Receptors on Testicular Function" Journal of Clinical Medicine 13, no. 17: 5097. https://doi.org/10.3390/jcm13175097




