Comparative Effects of Neurodynamic Slider and Tensioner Mobilization Techniques on Sympathetic Nervous System Function: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size
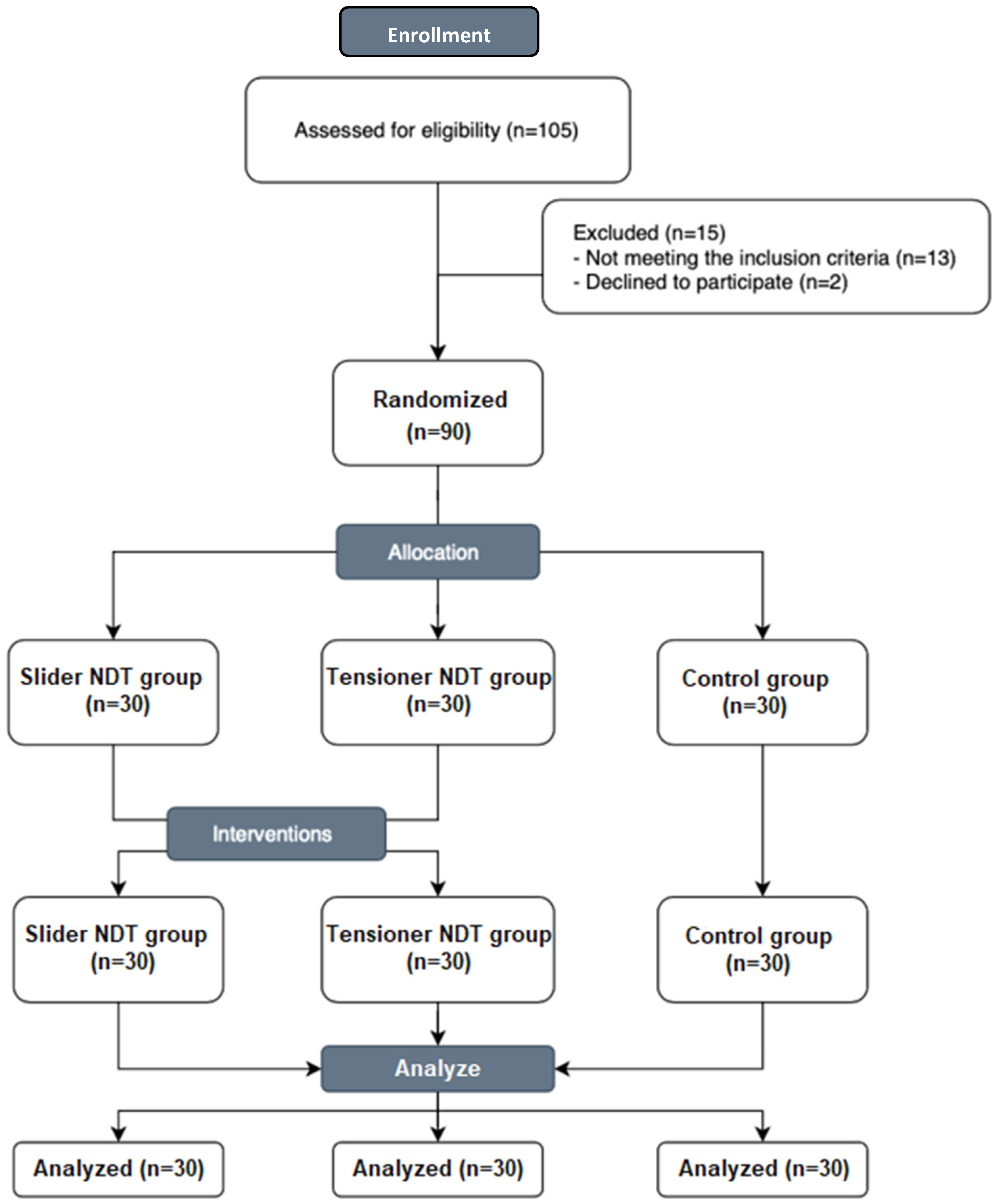
2.3. Participants
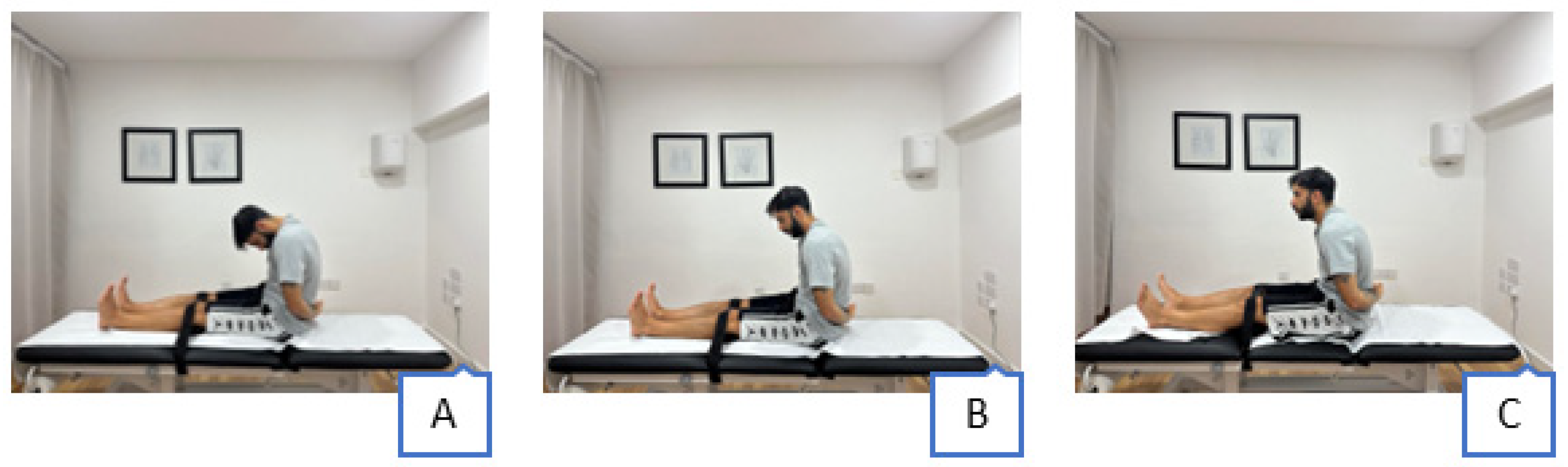
2.4. Interventions
2.5. Randomization and Blinding
2.6. Laboratory Assessments
2.7. Instrumentation and Measurements
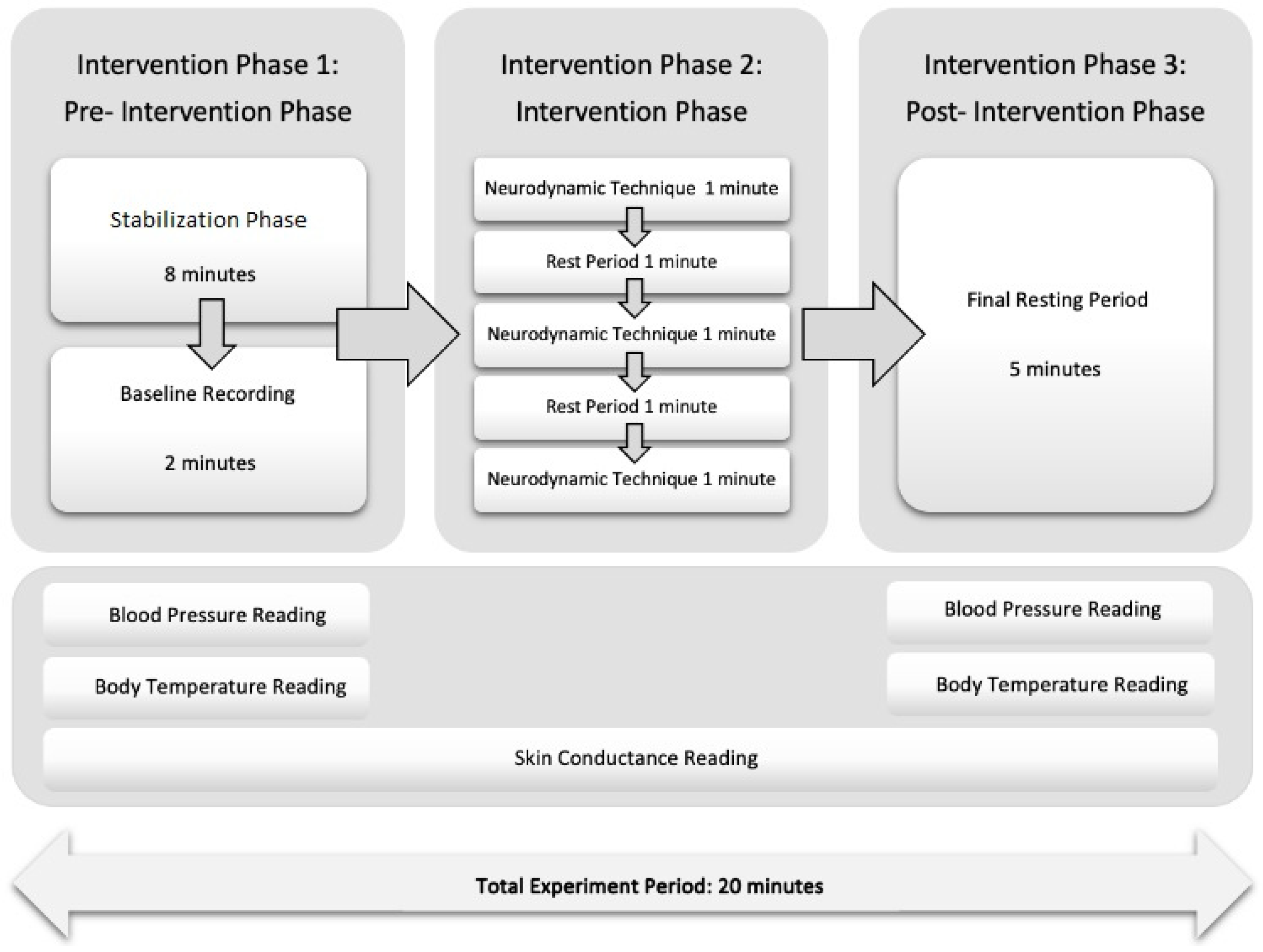
2.8. Procedures
2.9. Statistical Analysis
3. Results
3.1. Baseline-to-Intervention Phase
3.2. Intervention Phase to the Final Resting Phase
3.3. Baseline Phase to the Final Resting Phase
4. Discussion
4.1. Limitations
4.2. Practical Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shacklock, M. Neurodynamics. Physiotherapy 1995, 81, 9–16. [Google Scholar] [CrossRef]
- Coppieters, M.W.; Butler, D.S. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. Man. Ther. 2008, 13, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Nee, R.J.; Butler, D. Management of peripheral neuropathic pain: Integrating neurobiology, neurodynamics, and clinical evidence. Phys. Ther. Sport 2006, 7, 36–49. [Google Scholar] [CrossRef]
- Ellis, R.; Carta, G.; Andrade, R.J.; Coppieters, M.W. Neurodynamics: Is tension contentious? J. Man. Manip. Ther. 2022, 30, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Ahbouch, A.; Qadah, R.M.; Kim, M.; Alrawaili, S.M.; Moustafa, I.M. Impact of the Order of Movement on the Median Nerve Root Function: A Neurophysiological Study with Implications for Neurodynamic Exercise Sequencing. J. Clin. Med. 2024, 13, 913. [Google Scholar] [CrossRef]
- Coppieters, M.W.; Stappaerts, K.H.; Everaert, D.G.; Staes, F.F. Addition of test components during neurodynamic testing: Effect on range of motion and sensory responses. J. Orthop. Sports Phys. Ther. 2001, 31, 226–237. [Google Scholar] [CrossRef]
- Dilley, A.; Lynn, B.; Pang, S.J. Pressure and stretch mechanosensitivity of peripheral nerve fibres following local inflammation of the nerve trunk. Pain 2005, 117, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Bueno, F.R.; Shah, S.B. Implications of tensile loading for the tissue engineering of nerves. Tissue Eng. Part B Rev. 2008, 14, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, M.W.; Alshami, A.M.; Babri, A.S.; Souvlis, T.; Kippers, V.; Hodges, P.W. Strain and excursion of the sciatic, tibial, and plantar nerves during a modified straight leg raising test. J. Orthop. Res. 2006, 24, 1883–1889. [Google Scholar] [CrossRef]
- Lu, B.; Fan, P.; Wang, Y.; Dai, Y.; Xie, J.; Yang, G.; Mo, F.; Xu, Z.; Song, Y.; Liu, J.; et al. Neuronal Electrophysiological Activities Detection of Defense Behaviors Using an Implantable Microelectrode Array in the Dorsal Periaqueductal Gray. Biosensors 2022, 12, 193. [Google Scholar] [CrossRef]
- Burgess, N.E.; Gilbert, K.K.; Sobczak, S.; Sizer, P.S.; Homen, D.; Lierly, M.; Kearns, G.A.; Brismée, J.M. Upper Limb Neurodynamic Mobilization Disperses Intraneural Fluid in Cervical Nerve Roots: A Human Cadaveric Investigation. Musculoskelet. Sci. Pract. 2023, 68, 102876. [Google Scholar] [CrossRef] [PubMed]
- Field, T.; Diego, M.; Hernandez-Reif, M. Depressed mothers’ infants are less responsive to faces and voices. Infant Behav. Dev. 2009, 32, 239–244. [Google Scholar] [CrossRef]
- Diego, M.A.; Field, T.; Hernandez-Reif, M. Vagal activity, gastric motility, and weight gain in massaged preterm neonates. J. Pediatr. 2005, 147, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.K.; Bhougal, S.; Kumar, S. A general class of estimators in stratified random sampling. Commun. Stat. Simul. Comput. 2020, 52, 442–452. [Google Scholar] [CrossRef]
- Herrington, L.; Malloy, S.; Richards, J. The effect of patella taping on vastus medialis oblique and vastus laterialis EMG activity and knee kinematic variables during stair descent. J. Electromyogr. Kinesiol. 2005, 15, 604–607. [Google Scholar] [CrossRef]
- Brown, T.T.; Kuperman, J.M.; Chung, Y.; Erhart, M.; McCabe, C.; Hagler, D.J.; Venkatraman, V.K.; Akshoomoff, N.; Amaral, D.G.; Bloss, C.S. Neuroanatomical assessment of biological maturity. Curr. Biol. 2012, 22, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Beneciuk, J.M.; Bishop, M.D.; George, S.Z. Clinical prediction rules for physical therapy interventions: A systematic review. Phys. Ther. 2009, 89, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Snell, R.S. Clinical Neuroanatomy; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Schrieks, I.C.; van den Berg, R.; Sierksma, A.; Beulens, J.W.; Vaes, W.H.; Hendriks, H.F. Effect of red wine consumption on biomarkers of oxidative stress. Alcohol Alcohol. 2013, 48, 153–159. [Google Scholar] [CrossRef]
- Kupka, J.R.; Sagheb, K.; Al-Nawas, B.; Schiegnitz, E. The Sympathetic Nervous System in Dental Implantology. J. Clin. Med. 2023, 12, 2907. [Google Scholar] [CrossRef]
- Ebersole, K.T.; Cornell, D.J.; Flees, R.J.; Shemelya, C.M.; Noel, S.E. Contribution of the autonomic nervous system to recovery in firefighters. J. Athl. Train. 2020, 55, 1001–1008. [Google Scholar] [CrossRef]
- Valensi, P.; Prévost, G.; Pinto, S.; Halimi, J.-M.; Donal, E. The impact of diabetes on heart failure development: The cardio-renal-metabolic connection. Diabetes Res. Clin. Pract. 2021, 175, 108831. [Google Scholar] [CrossRef] [PubMed]
- Kuther, T. Medical Decision-Making and Minors: Issues of Consent and Assent. Adolescence 2003, 38, 242–258. [Google Scholar]
- Rafiq, S.; Zafar, H.; Gillani, S.A.; Waqas, M.S.; Liaqat, S.; Zia, A.; Rafiq, Y. Effects of Neurodynamic Mobilization on Health-Related Quality of Life and Cervical Deep Flexors Endurance in Patients of Cervical Radiculopathy: A Randomized Trial. BioMed Res. Int. 2022, 2022, 9385459. [Google Scholar] [CrossRef] [PubMed]
- Gharbo, R.S. Autonomic rehabilitation: Adapting to change. Phys. Med. Rehabil. Clin. 2020, 31, 633–648. [Google Scholar] [CrossRef]
- Papacharalambous, C.; Savva, C.; Karagiannis, C.; Giannakou, K. The effectiveness of slider and tensioner neural mobilization techniques in the management of upper quadrant pain: A systematic review of randomized controlled trials. J. Bodyw. Mov. Ther. 2022, 31, 102–112. [Google Scholar] [CrossRef]
- Alharmoodi, B.Y.; Arumugam, A.; Ahbouch, A.; Moustafa, I.M. Comparative effects of tensioning and sliding neural mobilization on peripheral and autonomic nervous system function: A randomized controlled trial. Hong Kong Physiother. J. 2022, 42, 41–53. [Google Scholar] [CrossRef]
- Kim, H.-G.; Jung, J.-H.; Bae, S.-U. Effects of the Three-Direction Movement Control Focus Complex Pain Program and Neurodynamic Focus Complex Pain Program on Pain, Mechanosensitivity, and Body Function in Taekwondo Athletes with Non-Specific Low Back Pain: A Preliminary Study. Healthcare 2024, 12, 422. [Google Scholar] [CrossRef]
- Vicenzino, B.; Collins, D.; Wright, T. Sudomotor Changes Induced by Neural Mobilisation Techniques in Asymptomatic Subjects. J. Man. Manip. Ther. 1994, 2, 66–74. [Google Scholar] [CrossRef]
- Silva, D.R.d.; Osório, R.A.L.; Fernandes, A.B. Influence of neural mobilization in the sympathetic slump position on the behavior of the autonomic nervous system. Res. Biomed. Eng. 2018, 34, 329–336. [Google Scholar] [CrossRef]
- Alshami, A.M.; Alghamdi, M.A.; Abdelsalam, M.S. Effect of Neural Mobilization Exercises in Patients With Low Back-Related Leg Pain With Peripheral Nerve Sensitization: A Prospective, Controlled Trial. J. Chiropr. Med. 2021, 20, 59–69. [Google Scholar] [CrossRef]
- LeBouef, T.; Yaker, Z.; Whited, L. Physiology, Autonomic Nervous System. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538516 (accessed on 1 August 2024).
- Cagnie, B.; Barbaix, E.; Vinck, E.; D’Herde, K.; Cambier, D. Extrinsic risk factors for compromised blood flow in the vertebral artery: Anatomical observations of the transverse foramina from C3 to C7. Surg. Radiol. Anat. 2005, 27, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chang, M.C. Chronic pain: Structural and functional changes in brain structures and associated negative affective states. Int. J. Mol. Sci. 2019, 20, 3130. [Google Scholar] [CrossRef] [PubMed]
- Eng, J. Sample size estimation: How many individuals should be studied? Radiology 2003, 227, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Pallant, J. SPSS Survival Manual—A Step by Step Guide to Data Analysis Using SPSS for Windows, 3rd ed.; Open University Press: Maidenhead, UK, 2007. [Google Scholar]
- Clar, C.; Tsertsvadze, A.; Court, R.; Hundt, G.L.; Clarke, A.; Sutcliffe, P. Clinical effectiveness of manual therapy for the management of musculoskeletal and non-musculoskeletal conditions: Systematic review and update of UK evidence report. Chiropr. Man. Ther. 2014, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mesa, J.; González-Chica, D.A.; Duquia, R.P.; Bonamigo, R.R.; Bastos, J.L. Sampling: How to select participants in my research study? An. Bras. Dermatol. 2016, 91, 326–330. [Google Scholar] [CrossRef]
- Slater, H.; Vicenzino, B.; Wright, A. ‘Sympathetic slump’: The effects of a novel manual therapy technique on peripheral sympathetic nervous system function. J. Man. Manip. Ther. 1994, 2, 156–162. [Google Scholar] [CrossRef]
- Massie, J.B.; Heller, J.G.; Abitbol, J.-J.; Mcpherson, D.; Garfin, S.R. Postoperative posterior spinal wound infections. Clin. Orthop. Relat. Res. 1992, 284, 99–108. [Google Scholar] [CrossRef]
- Gauriau, C.; Bernard, J.-F. Pain pathways and parabrachial circuits in the rat. Exp. Physiol. 2002, 87, 251–258. [Google Scholar] [CrossRef]
- Perry, J.; Green, A. An investigation into the effects of a unilaterally applied lumbar mobilization technique on peripheral sympathetic nervous system activity in the lower limbs. Man. Ther. 2008, 13, 492–499. [Google Scholar] [CrossRef]
- Rogan, S.; Taeymans, J.; Clarys, P.; Clijsen, R.; Tal-Akabi, A. Feasibility and effectiveness of thoracic spine mobilization on sympathetic/parasympathetic balance in a healthy population-a randomized controlled double-blinded pilot study. Arch. Physiother. 2019, 9, 15. [Google Scholar] [CrossRef]
- Piekarz, V.; Perry, J. An investigation into the effects of applying a lumbar Maitland mobilization at different frequencies on sympathetic nervous system activity levels in the lower limb. Man. Ther. 2016, 23, 83–89. [Google Scholar] [CrossRef]
- Bueno-Gracia, E.; Fanlo-Mazas, P.; Malo-Urriés, M.; Rodriguez-Mena, D.; Montaner-Cuello, A.; Ciuffreda, G.; Shacklock, M.; Estébanez-de-Miguel, E. Diagnostic Accuracy of the Upper Limb Neurodynamic Test 1 Using Neurodynamic Sequencing in Diagnosis of Carpal Tunnel Syndrome. Musculoskelet. Sci. Pract. 2024, 69, 102897. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.; Straznicky, N.; Eikelis, N.; Esler, M.; Dawood, T.; Masuo, K.; Schlaich, M.; Lambert, G. Gender differences in sympathetic nervous activity: Influence of body mass and blood pressure. J. Hypertens. 2007, 25, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; Gambale, E. Statistics and medicine: The indispensable know-how of the researcher. Transl. Med. 2013, 5, 28. [Google Scholar]
- Sim, J.; Wright, C. Research in Health Care: Concepts, Designs and Methods; Nelson Thornes: Cheltenham, UK, 2000. [Google Scholar]
- Petersen, N.; Vicenzino, B.; Wright, A. The effects of a cervical mobilization technique on sympathetic outflow to the upper limb in normal subjects. Physiother. Theory Pract. 1993, 9, 149–156. [Google Scholar] [CrossRef]
- Chiu, T.; Wright, A. To compare the effects of different rates of application of a cervical mobilization technique on sympathetic outflow to the upper limb in normal subjects. Man. Ther. 1996, 1, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Uematsu, S.; Jankel, W.R.; Edwin, D.H.; Kim, W.; Kozikowski, J.; Rosenbaum, A.; Long, D.M. Quantification of thermal asymmetry: Part 2: Application in low-back pain and sciatica. J. Neurosurg. 1988, 69, 556–561. [Google Scholar] [CrossRef]
- Figner, B.; Murphy, R.O. Using skin conductance in judgment and decision making research. In A Handbook of Process Tracing Methods for Decision Research; Psychology Press: New York, NY, USA, 2011; pp. 163–184. [Google Scholar]
- Elvey, R.L. Physical evaluation of the peripheral nervous system in disorders of pain and dysfunction. J. Hand Ther. 1997, 10, 122–129. [Google Scholar] [CrossRef]
- Cleland, J.; Koppenhaver, S.; Su, J. Netter’s Orthopaedic Clinical Examination: An Evidence-Based Approach; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Hajat, C.; Stein, E. The global burden of multiple chronic conditions: A narrative review. Prev. Med. Rep. 2018, 12, 284–293. [Google Scholar] [CrossRef]
- Semmons, J. The role of specialist physiotherapy in a pain management clinic–traditional and novel approaches. Anaesth. Intensive Care Med. 2022, 23, 405–408. [Google Scholar] [CrossRef]
- Schmelz, M. Translating nociceptive processing into human pain models. Exp. Brain Res. 2009, 196, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Thacker, M.; Clark, A.; Marchand, F.; McMahon, S. Pathophysiology of Peripheral Neuropathic Pain: Immune Cells and Molecules. Anesth. Analg. J. 2007, 105, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, C.; McCarthy, T. The effect of neural stretching technique on sympathetic outflow to the lower limbs. J. Orthop. Sports Phys. Ther. 1992, 16, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Quarti-Trevano, F.; Seravalle, G.; Dell’Oro, R.; Vanoli, J.; Perseghin, G.; Mancia, G. Sympathetic Neural Mechanisms Underlying Attended and Unattended Blood Pressure Measurement. Hypertension 2021, 78, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.; Durall, C.; Scott, S. Effects of Slump Long Sitting on Peripheral Sudomotor and Vasomotor Function: A Pilot Study. J. Man. Manip. Ther. 2002, 10, 67–75. [Google Scholar] [CrossRef]
- Joshi, K.C.; Eapen, C.; Kumar, S.P. Normal sensory and range of motion (ROM) responses during Thoracic Slump Test (ST) in asymptomatic subjects. J. Man. Manip. Ther. 2013, 21, 24–32. [Google Scholar] [CrossRef]
- Chu, J.; Allen, D.D.; Pawlowsky, S.; Smoot, B. Peripheral response to cervical or thoracic spinal manual therapy: An evidence-based review with meta analysis. J. Man. Manip. Ther. 2014, 22, 220–229. [Google Scholar] [CrossRef]
- Coppieters, M.W.; Alshami, A.M. Longitudinal excursion and strain in the median nerve during novel nerve gliding exercises for carpal tunnel syndrome. J. Orthop. Res. 2007, 25, 972–980. [Google Scholar] [CrossRef]
- Paraskevopoulos, E.; Koumantakis, G.; Papandreou, M. The effectiveness of neuromobilization in patients with cervical radiculopathy: A systematic review with meta-analysis. J. Sport Rehabil. 2022, 32, 325–334. [Google Scholar] [CrossRef]
- Paraskevopoulos, E.; Karanasios, S.; Gioftsos, G.; Tatsios, P.; Koumantakis, G.; Papandreou, M. The effectiveness of neuromobilization exercises in carpal tunnel syndrome: Systematic review and meta-analysis. Physiother. Theory Pract. 2022, 28, 2037–2076. [Google Scholar] [CrossRef]
- Ogata, K.; Naito, M. Blood flow of peripheral nerve effects of dissection stretching and compression. J. Hand Surg. Br. Eur. Vol. 1986, 11, 10–14. [Google Scholar]
- Zaheer, S.A.; Ahmed, Z. Neurodynamic Techniques in the Treatment of Mild-to-Moderate Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 4888. [Google Scholar] [CrossRef] [PubMed]
- Devereaux, P.; Yusuf, S. The evolution of the randomized controlled trial and its role in evidence-based decision making. J. Intern. Med. 2003, 254, 105–113. [Google Scholar] [CrossRef]
- Pellicciari, L.; Paci, M.; Geri, T.; Piscitelli, D.; Baccini, M. Mobilization of the contralateral limb in Slump position: Effects on knee extension in healthy adult subjects. Acta Biomed. Atenei Parm. 2019, 90, 245. [Google Scholar] [CrossRef]
- Ran, T.; Ke, S.; Li, J.; Lyu, M.; Zhou, Y.; Zhang, R.; Song, X.; Wang, M. Relieved Low Back Pain after Total Hip Arthroplasty in Patients with Both Hip Osteoarthritis and Lumbar Degenerative Disease. Orthop. Surg. J. 2021, 13, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, M.W.; Hough, A.; Dilley, A. Different nerve-gliding exercises induce different magnitudes of median nerve longitudinal excursion: An in vivo study using dynamic ultrasound imaging. J. Orthop. Sports Phys. Ther. 2009, 39, 164–171. [Google Scholar] [CrossRef]
- Santos, F.; Silva, J.; Giardini, A.; Rocha, P.; Achermann, A.; Alves, A.; Britto, L.; Chacur, M. Neural mobilization reverses behavioral and cellular changes that characterize neuropathic pain in rats. Mol. Pain 2012, 8, 57. [Google Scholar] [CrossRef]
- Herman, S.; Abend, N.; Bleck, T.; Chapman, K.; Drislane, F.; Emerson, R.; Gerard, E.; Hahn, C.; Husain, A.; Kaplan, O.; et al. Consensus Statement on Continuous EEG in Critically Ill Adults and Children, Part I: Indications. J. Clin. Neurophysiol. 2015, 32, 87–95. [Google Scholar] [CrossRef]
- Shacklock, M. Clinical Neurodynamics: A New System of Neuromusculoskeletal Treatment; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
| Variable | Slider Technique Group (n a = 30) | Tensioner Technique Group (n = 30) | Control Group (n = 30) | p-Value |
|---|---|---|---|---|
| Sex (Male/Female) | 53%/47% | 53%/47% | 47%/53% | - |
| Age (Years) | 21.70 ± 2.63 | 26.30 ± 3.17 | 27.00 ± 3.16 | 0.11 |
| Weight (kg b) | 73.20 ± 7.92 | 73.10 ± 7.97 | 73.80 ± 7.95 | 0.29 |
| Height (cm c) | 173.80 ± 1.79 | 173.10 ± 1.78 | 174.40 ± 1.79 | 0.59 |
| BMI d (kg/cm2 e) | 23.70 ± 2.51 | 23.80 ± 2.54 | 24.00 ± 2.53 | 0.35 |
| Room temperature (°C f) | 24.70 ± 2.51 | 24.60 ± 2.49 | 24.60 ± 2.51 | 0.51 |
| Side | Outcome Measures | Slider Group | Tensioner Group | Control Group | p-Value |
|---|---|---|---|---|---|
| Left Leg | Percentage change in skin conductance (μMhos a) | 42.31 ± 38.27 | 57.33 ± 28.64 | 10.03 ± 11.17 bc | <0.001 |
| Right Leg | Percentage change in skin conductance (μMhos a) | 40.45 ± 26.78 | 38.67 ± 23.16 | 4.93 ± 14.08 bc | <0.001 |
| Side | Outcome Measures | Slider Group | Tensioner Group | Control Group | p-Value |
|---|---|---|---|---|---|
| Left Leg | Percentage change in skin conductance (μMhos a) | −34.21 ± 23.77 | −39.18 ± 20.08 | −10.57 ± 14.14 bc | <0.001 |
| Right Leg | Percentage change in skin conductance (μMhos a) | −38.62 ± 22.53 | −26.58 ± 27.56 | −10.02 ± 15.65 bc | <0.001 |
| Percentage change in Systolic Blood Pressure (mmHg d) | 4.55 ± 0.11 | 4.55 ± 0.11 | 4.55 ± 0.11 | 0.95 | |
| Percentage change in Diastolic Blood Pressure (mmHg d) | 4.65 ± 0.14 | 4.65 ± 0.14 | 4.65 ± 0.14 | 0.06 | |
| Percentage change in Body Temperature (°C e) | 36.92 ± 0.39 | 36.99 ± 0.36 | 36.81 ± 0.43 bc | <0.001 | |
| Side | Outcome Measures | Slider Group | Tensioner Group | Control Group | p-Value |
|---|---|---|---|---|---|
| Left Leg | Percentage change in skin conductance (μMhos a) | 30.77 ± 10.49 | 42.89 ± 11.83 | 11.37 ± 2.35 bc | <0.001 |
| Right Leg | Percentage change in skin conductance (μMhos a) | 36.09 ± 7.94 | −30.44 ± 14.87 | 9.6 ± 0.4 bc | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papacharalambous, C.; Savva, C.; Karagiannis, C.; Paraskevopoulos, E.; Pamboris, G.M. Comparative Effects of Neurodynamic Slider and Tensioner Mobilization Techniques on Sympathetic Nervous System Function: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 5098. https://doi.org/10.3390/jcm13175098
Papacharalambous C, Savva C, Karagiannis C, Paraskevopoulos E, Pamboris GM. Comparative Effects of Neurodynamic Slider and Tensioner Mobilization Techniques on Sympathetic Nervous System Function: A Randomized Controlled Trial. Journal of Clinical Medicine. 2024; 13(17):5098. https://doi.org/10.3390/jcm13175098
Chicago/Turabian StylePapacharalambous, Charalambos, Christos Savva, Christos Karagiannis, Eleftherios Paraskevopoulos, and George M. Pamboris. 2024. "Comparative Effects of Neurodynamic Slider and Tensioner Mobilization Techniques on Sympathetic Nervous System Function: A Randomized Controlled Trial" Journal of Clinical Medicine 13, no. 17: 5098. https://doi.org/10.3390/jcm13175098
APA StylePapacharalambous, C., Savva, C., Karagiannis, C., Paraskevopoulos, E., & Pamboris, G. M. (2024). Comparative Effects of Neurodynamic Slider and Tensioner Mobilization Techniques on Sympathetic Nervous System Function: A Randomized Controlled Trial. Journal of Clinical Medicine, 13(17), 5098. https://doi.org/10.3390/jcm13175098










