Metabolic Risk in Patients with a Diminished Ovarian Reserve and Premature Ovarian Insufficiency
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Study Protocol
2.3. Statistics
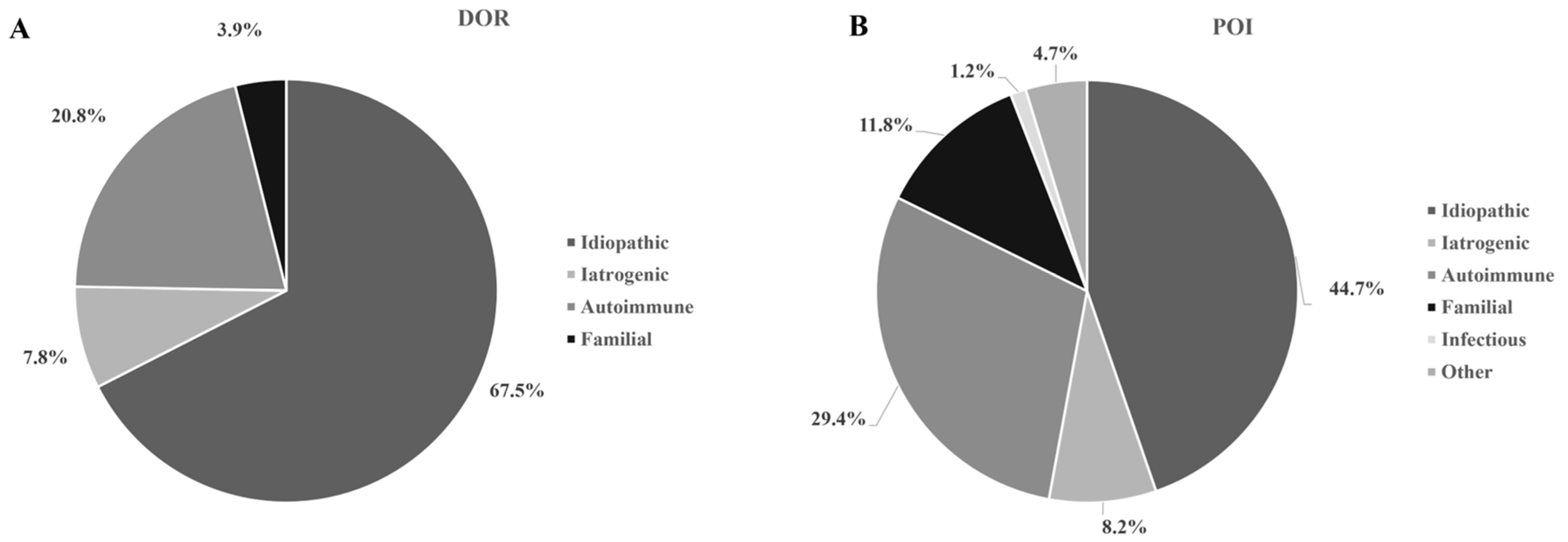
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI; Webber, L.; Davies, M.; Anderson, R.; Bartlett, J.; Braat, D.; Cartwright, B.; Cifkova, R.; de Muinck Keizer-Schrama, S.; Hogervorst, E.; et al. ESHRE Guideline: Management of women with premature ovarian insufficiency. Hum. Reprod. 2016, 31, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.J.; Umair, Z.; Yoon, M.S. Premature Ovarian Insufficiency: Past, Present, and Future. Front. Cell Dev. Biol. 2021, 9, 672890. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Persani, L. Premature ovarian failure. Orphanet J. Rare Dis. 2006, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Wesevich, V.; Kellen, A.N.; Pal, L. Recent advances in understanding primary ovarian insufficiency. F1000Research 2020, 9, 1101. [Google Scholar] [CrossRef]
- Rasool, S.; Shah, D. Fertility with early reduction of ovarian reserve: The last straw that breaks the Camel’s back. Fertil. Res. Pract. 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Chabbert-Buffet, N.; Darai, E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder--a plea for universal definitions. J. Assist. Reprod. Genet. 2015, 32, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Pastore, L.M.; Christianson, M.S.; Stelling, J.; Kearns, W.G.; Segars, J.H. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J. Assist. Reprod. Genet. 2018, 35, 17–23. [Google Scholar] [CrossRef]
- Gleicher, N.; Weghofer, A.; Oktay, K.; Barad, D. Do etiologies of premature ovarian aging (POA) mimic those of premature ovarian failure (POF)? Hum. Reprod. 2009, 24, 2395–2400. [Google Scholar] [CrossRef]
- Welt, C.K. Primary ovarian insufficiency: A more accurate term for premature ovarian failure. Clin. Endocrinol. 2008, 68, 499–509. [Google Scholar] [CrossRef]
- Torrealday, S.; Kodaman, P.; Pal, L. Premature Ovarian Insufficiency—An update on recent advances in understanding and management. F1000Research 2017, 6, 2069. [Google Scholar] [CrossRef]
- Kaplan, J.R.; Manuck, S.B. Ovarian dysfunction, stress, and disease: A primate continuum. ILAR J. 2004, 45, 89–115. [Google Scholar] [CrossRef]
- Gravholt, C.H. Epidemiological, endocrine and metabolic features in Turner syndrome. Eur. J. Endocrinol. 2004, 151, 657–687. [Google Scholar] [CrossRef]
- Podfigurna, A.; Stellmach, A.; Szeliga, A.; Czyzyk, A.; Meczekalski, B. Metabolic Profile of Patients with Premature Ovarian Insufficiency. J. Clin. Med. 2018, 7, 374. [Google Scholar] [CrossRef]
- Verit, F.F.; Akyol, H.; Sakar, M.N. Low antimullerian hormone levels may be associated with cardiovascular risk markers in women with diminished ovarian reserve. Gynecol. Endocrinol. 2016, 32, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, Z.; Fan, Q.; Li, H.; Zhao, L.; Liu, C.; Liang, X. Cholesterol metabolism is decreased in patients with diminished ovarian reserve. Reprod. Biomed. Online 2022, 44, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kahapola Arachchige, K.M.; Wardrop, R.; Lim, E.M.; Stuckey, B.; Hadlow, N. Waiting for an elevated FSH-too late a marker of reduced ovarian reserve? Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 460–464. [Google Scholar] [CrossRef]
- Robeva, R.; Arnaudova, N.; Kirilov, G.; Elenkova, A.; Zacharieva, S. Metanephrine and Normetanephrine Urine Excretion in Patients with PCOS. Acta Med. Bulg. 2022, 49, 5–10. [Google Scholar] [CrossRef]
- Jin, J.; Ruan, X.; Hua, L.; Tian, X.; Li, Y.; Wang, L.; Mueck, A.O. Prevalence of diminished ovarian reserve in Chinese women with polycystic ovary syndrome and sensitive diagnostic parameters. Gynecol. Endocrinol. 2017, 33, 694–697. [Google Scholar] [CrossRef]
- Wang, C.; Di, W.; Gu, Z. Endocrine and glycolipid metabolism characteristics of diminished ovarian reserve in Chinese women with polycystic ovary syndrome. J. Int. Med. Res. 2020, 48, 300060520912982. [Google Scholar] [CrossRef]
- Ahmad, A.K.; Kao, C.N.; Quinn, M.; Lenhart, N.; Rosen, M.; Cedars, M.I.; Huddleston, H. Differential rate in decline in ovarian reserve markers in women with polycystic ovary syndrome compared with control subjects: Results of a longitudinal study. Fertil. Steril. 2018, 109, 526–531. [Google Scholar] [CrossRef]
- Álvarez-Nava, F.; Racines, M.; Witt, J.; Guarderas, J.; Estévez, M.; Lanes, R. Anthropometric variables as cardiovascular risk predictors in a cohort of adult subjects with Turner syndrome. Diabetes Metab. Syndr. Obes. 2019, 12, 1795–1809. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Zhou, T.; Zhao, X.; Wang, Y.; Wang, G.; Gang, X. Glucose Metabolism in Turner Syndrome. Front. Endocrinol. 2019, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Lebenthal, Y.; Levy, S.; Sofrin-Drucker, E.; Nagelberg, N.; Weintrob, N.; Shalitin, S.; De Vries, L.; Tenenbaum, A.; Phillip, M.; Lazar, L. The Natural History of Metabolic Comorbidities in Turner Syndrome from Childhood to Early Adulthood: Comparison between 45,X Monosomy and Other Karyotypes. Front. Endocrinol. 2018, 9, 27. [Google Scholar] [CrossRef]
- Mitsch, C.; Alexandrou, E.; Norris, A.W.; Pinnaro, C.T. Hyperglycemia in Turner syndrome: Impact, mechanisms, and areas for future research. Front. Endocrinol. 2023, 14, 1116889. [Google Scholar] [CrossRef] [PubMed]
- de Kat, A.C.; Verschuren, W.M.; Eijkemans, M.J.; van der Schouw, Y.T.; Broekmans, F.J. The association of low ovarian reserve with cardiovascular disease risk: A cross-sectional population-based study. Hum. Reprod. 2016, 31, 1866–1874. [Google Scholar] [CrossRef]
- Zou, W.; Wang, Z.; Xia, J.; Yang, J. Retinol-binding protein 4 (RBP4) and high sensitivity C-reactive protein (hs-CRP) levels in patients with diminished ovarian reserve (DOR): A cross-sectional study. Reprod. Biol. Endocrinol. 2020, 18, 111. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, L.; Wu, Z.; Li, Y.; Jia, Q.; Cheng, J.C.; Sun, Y.P. A meta-analysis of serum lipid profiles in premature ovarian insufficiency. Reprod. Biomed. Online 2022, 44, 539–547. [Google Scholar] [CrossRef]
- Huang, L.; Wang, H.; Shi, M.; Kong, W.; Jiang, M. Lipid Profile in Patients With Primary Ovarian Insufficiency: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 876775. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lv, Y.; Qi, T.; Luo, Z.; Meng, X.; Ying, Q.; Li, D.; Li, C.; Lan, Y.; Chu, K.; et al. Metabolic profile of women with premature ovarian insufficiency compared with that of age-matched healthy controls. Maturitas 2021, 148, 33–39. [Google Scholar] [CrossRef]
- Ates, S.; Yesil, G.; Sevket, O.; Molla, T.; Yildiz, S. Comparison of metabolic profile and abdominal fat distribution between karyotypically normal women with premature ovarian insufficiency and age matched controls. Maturitas 2014, 79, 306–310. [Google Scholar] [CrossRef]
- Mirinezhad, M.R.; Ghazizadeh, H.; Aghsizadeh, M.; Zamiri Bidary, M.; Naghipour, A.; Hasanzadeh, E.; Yaghooti-Khorasani, M.; Ebrahimi Dabagh, A.; Moghadam, M.R.S.F.; Sheikh Andalibi, N.; et al. The relationship between genetic variants associated with primary ovarian insufficiency and lipid profile in women recruited from MASHAD cohort study. BMC Womens Health. 2022, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Roulot, D.; Degott, C.; Chazouillères, O.; Oberti, F.; Calès, P.; Carbonell, N.; Benferhat, S.; Bresson-Hadni, S.; Valla, D. Vascular involvement of the liver in Turner’s syndrome. Hepatology 2004, 39, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Gravholt, C.H.; Poulsen, H.E.; Ott, P.; Christiansen, J.S.; Vilstrup, H. Quantitative liver functions in Turner syndrome with and without hormone replacement therapy. Eur. J. Endocrinol. 2007, 156, 679–686. [Google Scholar] [CrossRef]
- Koulouri, O.; Ostberg, J.; Conway, G.S. Liver dysfunction in Turner’s syndrome: Prevalence, natural history and effect of exogenous oestrogen. Clin. Endocrinol. 2008, 69, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Fedor, I.; Zold, E.; Barta, Z. Liver Abnormalities in Turner Syndrome: The Importance of Estrogen Replacement. J. Endocr. Soc. 2022, 6, bvac124. [Google Scholar] [CrossRef]
- Robeva, R.; Mladenović, D.; Vesković, M.; Hrnčić, D.; Bjekić-Macut, J.; Stanojlović, O.; Livadas, S.; Yildiz, B.O.; Macut, D. The interplay between metabolic dysregulations and non-alcoholic fatty liver disease in women after menopause. Maturitas 2021, 151, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Weghofer, A.; Kim, A.; Barad, D.H.; Gleicher, N. Age at menarche: A predictor of diminished ovarian function? Fertil. Steril. 2013, 100, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Pasquino, A.M.; Passeri, F.; Pucarelli, I.; Segni, M.; Municchi, G. Spontaneous pubertal development in Turner’s syndrome. Italian Study Group for Turner’s Syndrome. J. Clin. Endocrinol. Metab. 1997, 82, 1810–1813. [Google Scholar]
- Elenkova, A.; Atanasova, I.; Kirilov, G.; Natchev, E.; Ivanova, R.; Kovatcheva, R.; Vandeva, S.; Tcharaktchiev, D.; Zacharieva, S. Autoimmune hypothyroidism is three times more frequent in female prolactinoma patients compared to healthy women: Data from a cross-sectional case-control study. Endocrine 2017, 57, 486–493. [Google Scholar] [CrossRef]
- El-Mansoury, M.; Bryman, I.; Berntorp, K.; Hanson, C.; Wilhelmsen, L.; Landin-Wilhelmsen, K. Hypothyroidism is common in Turner syndrome: Results of a five-year follow-up. J. Clin. Endocrinol. Metab. 2005, 90, 2131–2135. [Google Scholar] [CrossRef]
- Mortensen, K.H.; Cleemann, L.; Hjerrild, B.E.; Nexo, E.; Locht, H.; Jeppesen, E.M.; Gravholt, C.H. Increased prevalence of autoimmunity in Turner syndrome--influence of age. Clin. Exp. Immunol. 2009, 156, 205–210. [Google Scholar] [CrossRef]
- Grossi, A.; Crinò, A.; Luciano, R.; Lombardo, A.; Cappa, M.; Fierabracci, A. Endocrine autoimmunity in Turner syndrome. Ital. J. Pediatr. 2013, 39, 79. [Google Scholar] [CrossRef] [PubMed]
- Bakalov, V.K.; Gutin, L.; Cheng, C.M.; Zhou, J.; Sheth, P.; Shah, K.; Arepalli, S.; Vanderhoof, V.; Nelson, L.M.; Bondy, C.A. Autoimmune disorders in women with Turner syndrome and women with karyotypically normal primary ovarian insufficiency. J. Autoimmun. 2012, 38, 315–321. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kitahara, Y.; Osuka, S.; Tsukui, Y.; Kobayashi, M.; Iwase, A. Effect of hypothyroidism and thyroid autoimmunity on the ovarian reserve: A systematic review and meta-analysis. Reprod. Med. Biol. 2021, 21, e12427. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, N.P.; Sakkas, E.; Vaiarelli, A.; Poppe, K.; Camus, M.; Tournaye, H. Thyroid autoimmunity, hypothyroidism and ovarian reserve: A cross-sectional study of 5000 women based on age-specific AMH values. Hum. Reprod. 2015, 30, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, R.L.; Meherji, P.K.; Kadam, S.S.; Gupta, S.K.; Nandedkar, T.D. Circulating auto-antibodies against the zona pellucida and thyroid microsomal antigen in women with premature ovarian failure. J. Reprod. Immunol. 2005, 66, 53–67. [Google Scholar] [CrossRef]
- Esteves, S.C.; Roque, M.; Bedoschi, G.M.; Conforti, A.; Humaidan, P.; Alviggi, C. Defining Low Prognosis Patients Undergoing Assisted Reproductive Technology: POSEIDON Criteria-The Why. Front. Endocrinol. 2018, 9, 461. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef]
- Weghofer, A.; Brill, H.; Feichtinger, R.; Barad, D.; Gleicher, N. Does autoimmunity play a role in the pathophysiology of premature ovarian ageing? Reprod. Biomed. Online 2008, 16, 830–834. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Andersen, N.H.; Christin-Maitre, S.; Davis, S.M.; Duijnhouwer, A.; Gawlik, A.; Maciel-Guerra, A.T.; Gutmark-Little, I.; Fleischer, K.; Hong, D.; et al. Clinical practice guidelines for the care of girls and women with Turner syndrome. Eur. J. Endocrinol. 2024, 190, G53–G151. [Google Scholar]

| Healthy Women n = 28 | Women with DOR n = 77 | Women with POI n = 85 | Women with TS n = 36 | p1 | p2 | p3 | p4 | p5 | p6 | p7 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 28.32 ± 4.38 [29.00] | 30.22 ± 6.10 [31.00] | 30.14 ± 6.59 [31.00] | 24.44 ± 6.66 [23.00] | 0.089 | 0.084 | 0.003 | 0.941 | <0.001 | <0.001 | <0.001 |
| Height (cm) | 167.32 ± 7.02 [168.00] | 166.41 ± 5.79 [165.00] | 163.89 ± 7.39 [164.50] | 148.72 ± 6.90 [148.50] | 0.469 | 0.044 | <0.001 | 0.060 | <0.001 | <0.001 | <0.001 |
| BMI (kg/m2) | 21.95 ± 4.49 [20.83] | 23.28 ± 5.49 [22.18] | 22.70 ± 4.77 [21.62] | 24.94 ± 5.35 [23.99] | 0.250 | 0.386 | 0.005 | 0.808 | 0.054 | 0.023 | 0.047 |
| Smoking * | 21.4% | 22.1% | 29.4% | 8.3% | 1.00 | 0.473 | 0.163 | 0.370 | 0.111 | 0.017 | 0.090 |
| Systolic BP (mmHg) | 103.57 ± 10.08 [100.00] | 111.56 ± 11.60 [110.00] | 113.14 ± 15.56 [110.00] | 117.64 ± 18.80 [117.50] | 0.002 | <0.001 | <0.001 | 0.720 | 0.162 | 0.264 | 0.001 |
| Diastolic BP (mmHg) | 67.86 ± 7.13 [70.00] | 74.50 ± 8.23 [72.50] | 74.59 ± 11.48 [70.00] | 74.29 ± 9.73 [70.00] | 0.001 | 0.004 | 0.006 | 0.744 | 0.952 | 0.805 | 0.008 |
| Menarche (years) | 12.68 ± 1.22 [12.50] | 12.67 ± 1.45 [13.00] | 13.61 ± 1.66 [14.00] | 14.91 ± 1.51 [14.00] | 0.840 | 0.005 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 |
| AITD | 10.7% | 20.8% | 29.4% | 52.8% | 0.389 | 0.075 | <0.001 | 0.277 | <0.001 | 0.022 | <0.001 |
| TSH (µIU/mL) | 2.21 ± 1.06 [2.00] | 2.16 ± 1.06 [1.90] | 2.53 ± 2.04 [2.10] | 7.78 ± 17.92 [3.00] | 0.941 | 0.657 | 0.001 | 0.530 | <0.001 | <0.001 | <0.001 |
| Testosterone (nmol/L) | 1.28 ± 0.59 [1.05] | 1.81 ± 1.02 [1.60] | 2.01 ± 1.52 [1.60] | 1.47 ± 0.76 [1.45] | 0.020 | 0.025 | 0.517 | 0.947 | 0.320 | 0.393 | 0.084 |
| LH (IU/L) | 3.87 ± 1.91 [3.30] | 6.61 ± 5.45 [5.21] | 29.43 ± 17.98 [25.30] | 19.51 ± 16.45 [16.45] | 0.005 | <0.001 | <0.001 | <0.001 | <0.001 | 0.005 | <0.001 |
| FSH (IU/L) | 6.61 ± 1.83 [6.90] | 13.83 ± 4.59 [12.60] | 72.73 ± 38.75 [69.15] | 60.60 ± 39.37 [64.00] | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.104 | <0.001 |
| Healthy Women n = 28 | Women with DOR n = 77 | Women with POI n = 85 | Women with TS n = 36 | p1 | p2 | p3 | p4 | p5 | p6 | p7 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose (mmol/L) | 4.96 ± 0.37 [5.00] | 5.12 ± 0.41 [5.17] | 5.31 ± 1.09 [5.13] | 5.30 ± 1.57 [5.10] | 0.056 | 0.043 | 0.563 | 0.796 | 0.533 | 0.350 | 0.205 |
| Insulin (µIU/mL) | 6.10 ± 4.29 [5.05] | 10.99 ± 7.64 [9.10] | 11.58 ± 6.21 [9.60] | 9.89 ± 5.83 [9.50] | <0.001 | <0.001 | 0.018 | 0.271 | 0.878 | 0.366 | <0.001 |
| HOMA-IR | 1.37 ± 1.06 [1.19] | 2.47 ± 1.82 [2.02] | 2.69 ± 1.56 [2.32] | 2.15 ± 1.49 [1.74] | <0.001 | <0.001 | 0.062 | 0.176 | 0.651 | 0.186 | <0.001 |
| Cholesterol (mmol/L) | 4.16 ± 0.75 [3.92] | 5.05 ± 0.99 [5.02] | 4.84 ± 1.21 [4.50] | 4.98 ± 1.11 [4.75] | <0.001 | 0.006 | <0.001 | 0.171 | 0.550 | 0.506 | <0.001 |
| HDL-cholesterol (mmol/L) | 1.58 ± 0.28 [1.60] | 1.57 ± 0.42 [1.54] | 1.64 ± 0.61 [1.48] | 1.66 ± 0.39 [1.69] | 0.717 | 0.707 | 0.403 | 0.987 | 0.329 | 0.421 | 0.768 |
| LDL-cholesterol (mmol/L) | 2.31 ± 0.61 [2.26] | 2.99 ± 0.89 [2.94] | 2.67 ± 1.03 [2.39] | 2.84 ± 0.96 [2.66] | <0.001 | 0.192 | 0.020 | 0.035 | 0.500 | 0.354 | 0.009 |
| Triglycerides (mmol/L) | 0.60 ± 0.43 [0.48] | 0.90 ± 0.48 [0.73] | 0.99 ± 0.83 [0.79] | 1.07 ± 0.79 [0.83] | <0.001 | <0.001 | <0.001 | 0.760 | 0.278 | 0.410 | <0.001 |
| ALAT (mmol/L) | 11.06 ± 5.05 [10.45] | 14.83 ± 6.25 [13.00] | 19.19 ± 13.77 [15.50] | 29.21 ± 28.21 [16.55] | 0.003 | <0.001 | <0.001 | 0.174 | 0.013 | 0.155 | <0.001 |
| ASAT (mmol/L) | 15.49 ± 3.92 [14.35] | 15.88 ± 3.76 [15.40] | 19.34 ± 6.02 [17.70] | 27.25 ± 21.61 [19.00] | 0.455 | <0.001 | <0.001 | <0.001 | <0.001 | 0.024 | <0.001 |
| GGT (mmol/L) | 10.87 ± 3.75 [9.90] | 16.33 ± 9.09 [13.00] | 17.48 ± 9.91 [14.70] | 57.34 ± 74.78 [27.20] | 0.013 | <0.001 | <0.001 | 0.315 | 0.004 | 0.011 | <0.001 |
| Creatinine (mmol/L) | 59.11 ± 7.45 [58.00] | 57.83 ± 9.87 [56.00] | 68.13 ± 99.58 [56.00] | 51.16 ± 10.95 [49.00] | 0.296 | 0.216 | 0.002 | 0.748 | 0.004 | 0.007 | 0.007 |
| Uric acid (mmol/L) | 261.22 ± 59.24 [265.00] | 254.40 ± 73.66 [241.00] | 273.47 ± 76.19 [262.00] | 279.37 ± 71.15 [289.50] | 0.731 | 0.706 | 0.482 | 0.343 | 0.192 | 0.625 | 0.631 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robeva, R.; Elenkova, A.; Kirilov, G.; Zacharieva, S. Metabolic Risk in Patients with a Diminished Ovarian Reserve and Premature Ovarian Insufficiency. J. Clin. Med. 2024, 13, 5105. https://doi.org/10.3390/jcm13175105
Robeva R, Elenkova A, Kirilov G, Zacharieva S. Metabolic Risk in Patients with a Diminished Ovarian Reserve and Premature Ovarian Insufficiency. Journal of Clinical Medicine. 2024; 13(17):5105. https://doi.org/10.3390/jcm13175105
Chicago/Turabian StyleRobeva, Ralitsa, Atanaska Elenkova, Georgi Kirilov, and Sabina Zacharieva. 2024. "Metabolic Risk in Patients with a Diminished Ovarian Reserve and Premature Ovarian Insufficiency" Journal of Clinical Medicine 13, no. 17: 5105. https://doi.org/10.3390/jcm13175105
APA StyleRobeva, R., Elenkova, A., Kirilov, G., & Zacharieva, S. (2024). Metabolic Risk in Patients with a Diminished Ovarian Reserve and Premature Ovarian Insufficiency. Journal of Clinical Medicine, 13(17), 5105. https://doi.org/10.3390/jcm13175105








