Evaluating Wideband Tympanometry Absorbance Changes in Cochlear Implant Recipients: Mechanical Insights and Influencing Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Measurement Protocol
2.4. Cochlear Implantation
2.5. Audiological Assessment
2.6. Data Analysis
3. Results
3.1. Study Population
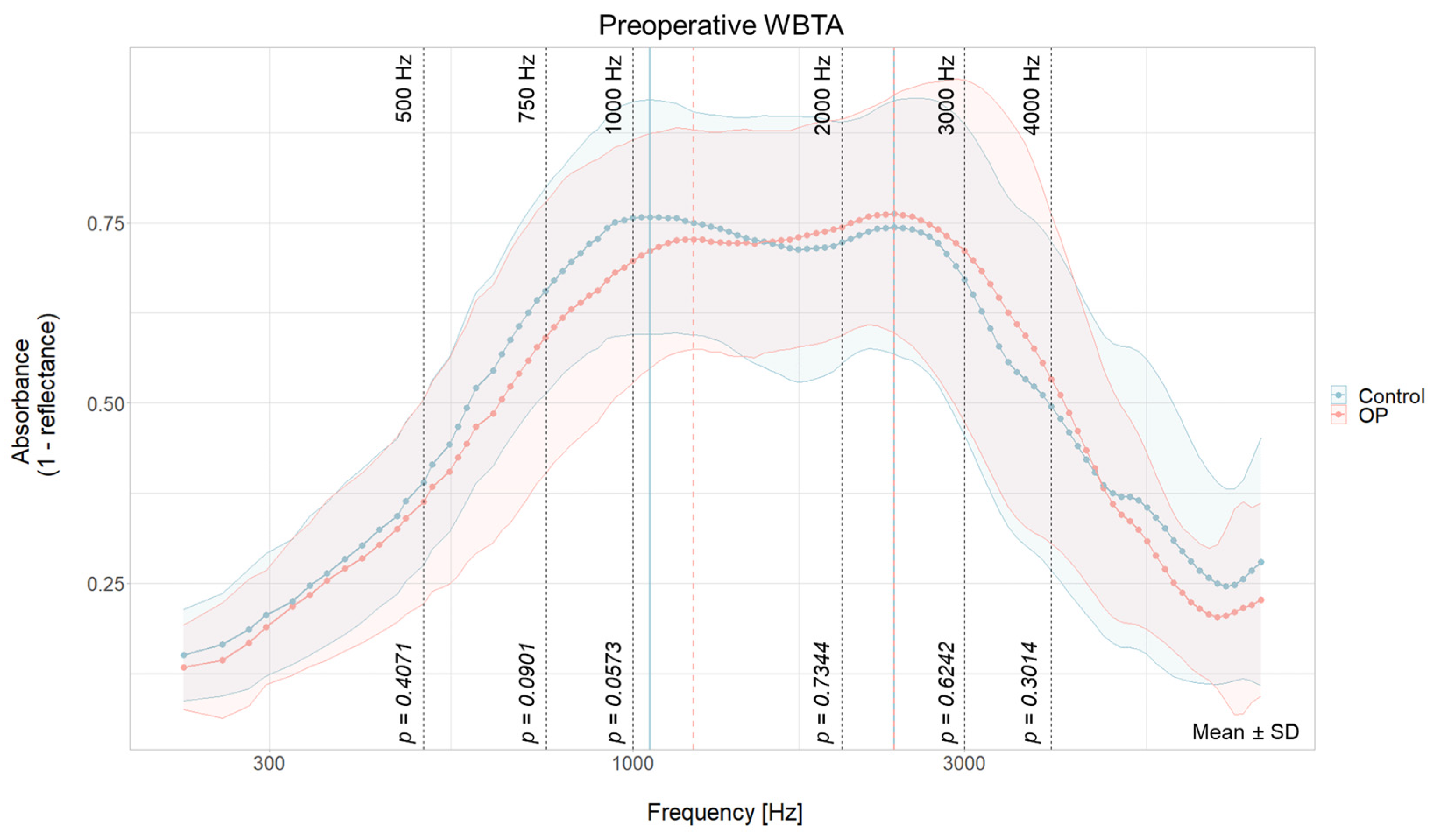
3.2. No Group Differences in Baseline WBTA
3.3. Decreased Absorbance in Lower Frequencies in CI Ear
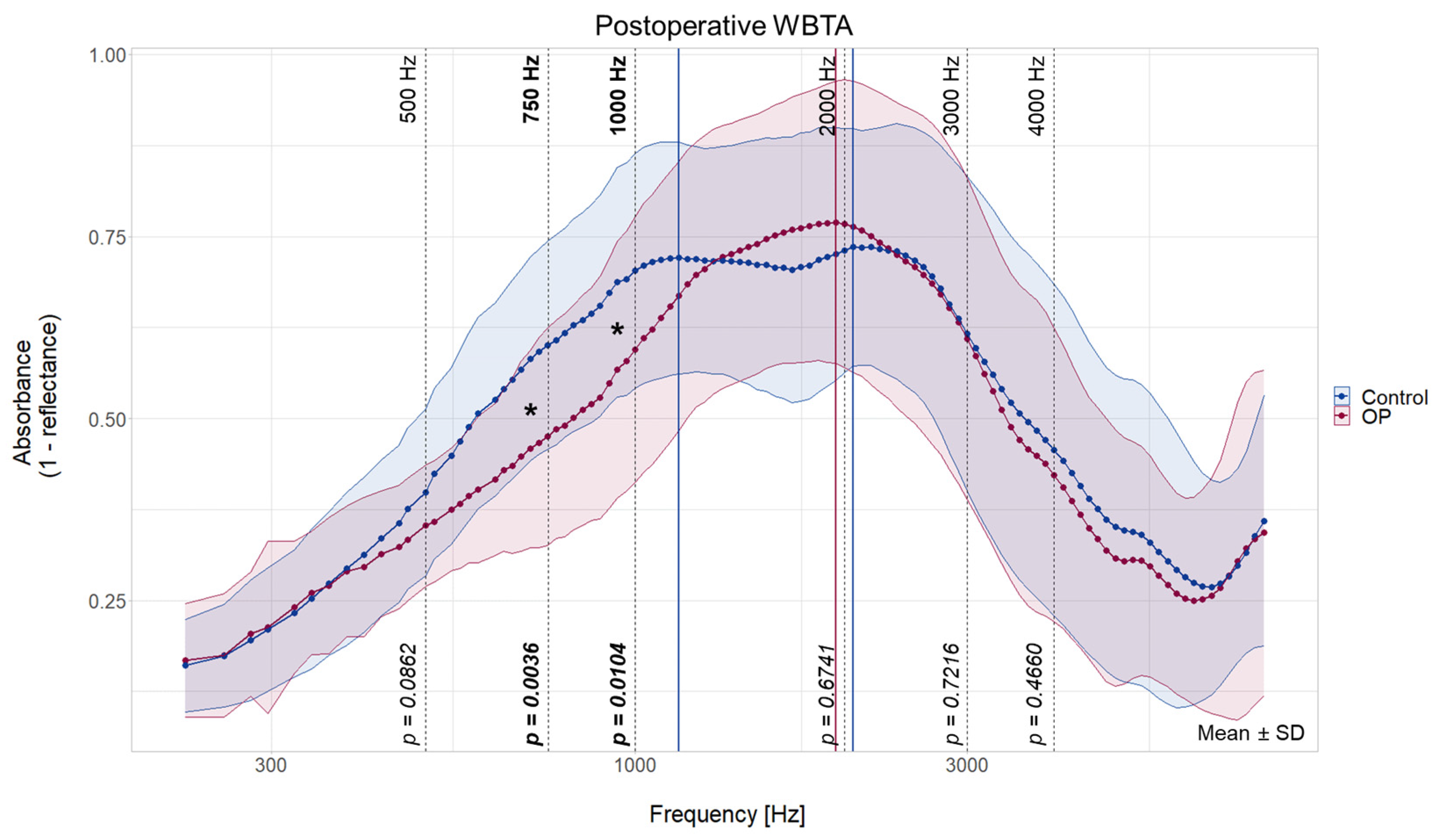
3.4. In-Group Comparison of the WBTA Pre- vs. Postoperatively
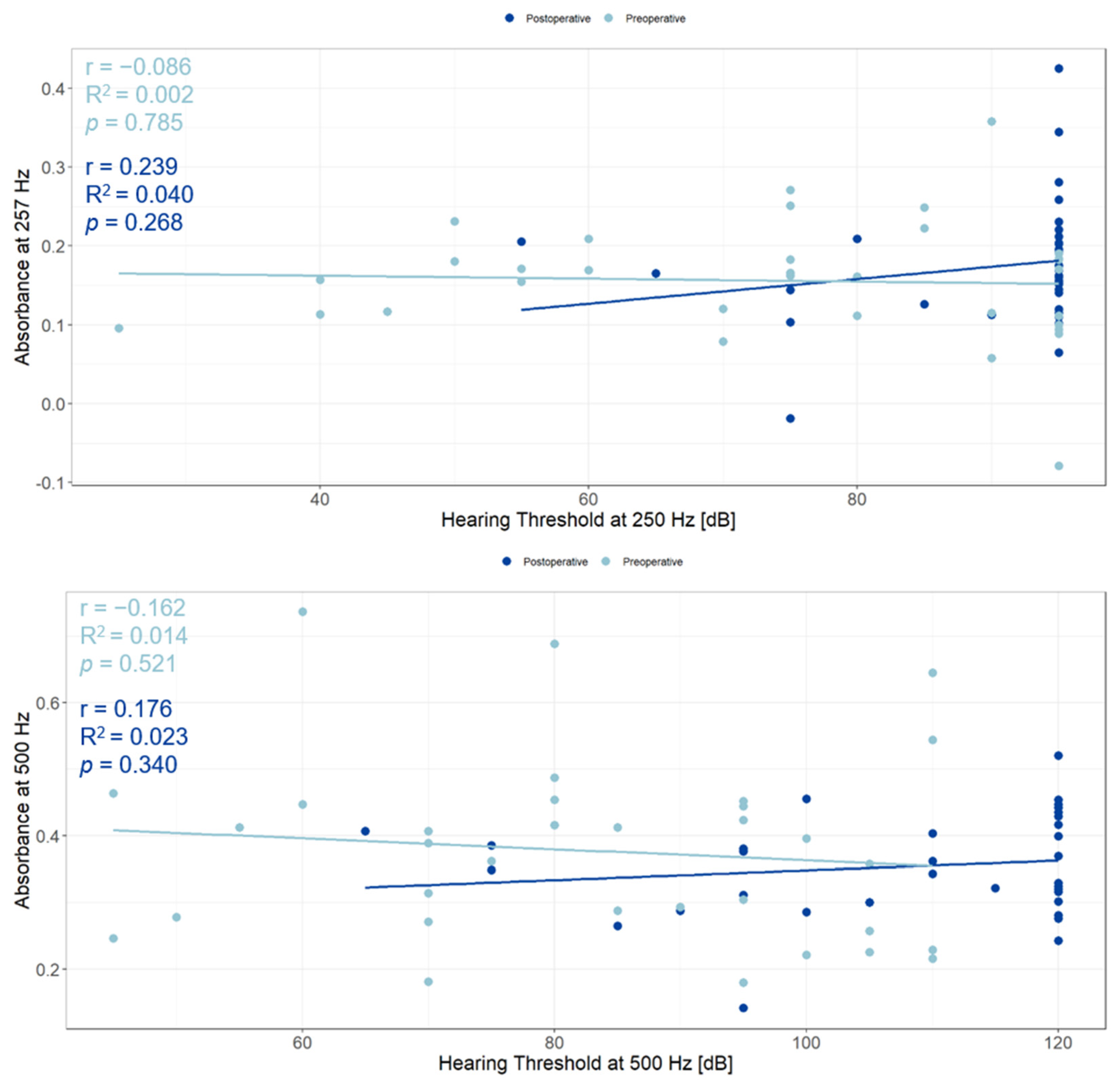
3.5. Relationship between WBTA and Acoustic Hearing
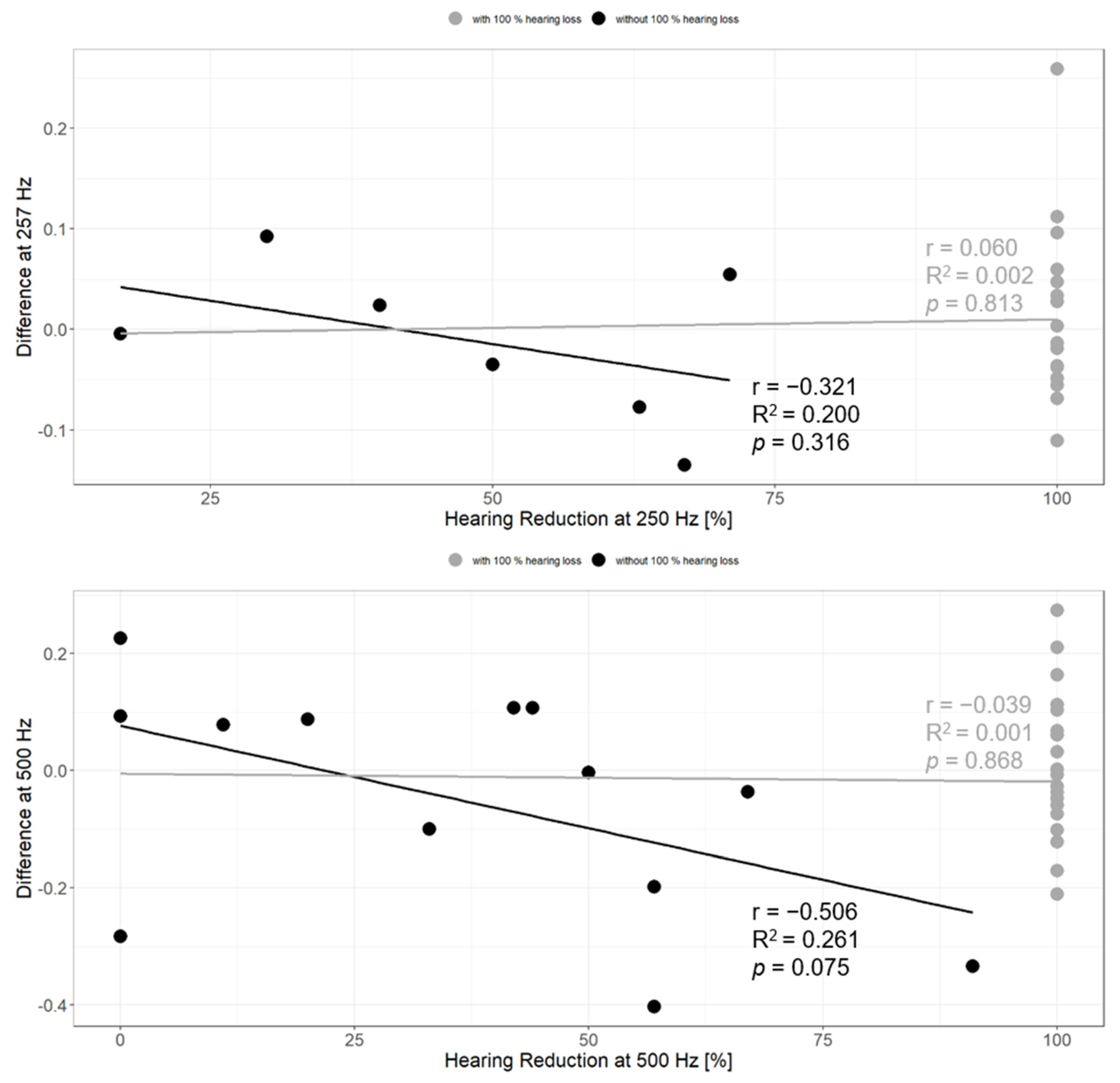
3.6. Contributing Factors
4. Discussion
4.1. Changes in WBTA after Cochlear Implantation
4.2. Influence of WBTA Changes on Residual Hearing
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaefer, S.; Sahwan, M.; Metryka, A.; Kluk, K.; Bruce, I.A. The benefits of preserving residual hearing following cochlear implantation: A systematic review. Int. J. Audiol. 2021, 60, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Cullen, R.D.; Higgins, C.; Buss, E.; Clark, M.; Pillsbury, H.C.; Buchman, C.A. Cochlear implantation in patients with substantial residual hearing. Laryngoscope 2004, 114, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Snels, C.; IntHout, J.; Mylanus, E.; Huinck, W.; Dhooge, I. Hearing Preservation in Cochlear Implant Surgery: A Meta-Analysis. Otol. Neurotol. 2019, 40, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, N.; Bibas, A.; Jiang, D.; Bamiou, D.E.; Santulli, C.; Jeronimidis, G.; Fitzgerald O’Connor, A. Effect of cochlear implant electrode insertion on middle-ear function as measured by intra-operative laser Doppler vibrometry. J. Laryngol. Otol. 2009, 123, 723–729. [Google Scholar] [CrossRef]
- Greene, N.T.; Mattingly, J.K.; Jenkins, H.A.; Tollin, D.J.; Easter, J.R.; Cass, S.P. Cochlear Implant Electrode Effect on Sound Energy Transfer within the Cochlea During Acoustic Stimulation. Otol. Neurotol. 2015, 36, 1554–1561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huber, A.M.; Hoon, S.J.; Sharouz, B.; Daniel, B.; Albrecht, E. The influence of a cochlear implant electrode on the mechanical function of the inner ear. Otol. Neurotol. 2010, 31, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, N.; Prochazka, L.; Grosse, J.; Dalbert, A.; Tabibi, S.; Chatzimichalis, M.; Dobrev, I.; Kleinjung, T.; Huber, A.; Pfiffner, F. Round Window Reinforcement-Induced Changes in Intracochlear Sound Pressure. Appl. Sci. 2021, 11, 5062. [Google Scholar] [CrossRef]
- Geerardyn, A.; Wils, I.; Putzeys, T.; Fierens, G.; Wouters, J.; Verhaert, N. The impact of round window reinforcement on middle and inner ear mechanics with air and bone conduction stimulation. Hear. Res. 2024, 450, 109049. [Google Scholar] [CrossRef] [PubMed]
- Saoji, A.A.; Shapiro, S.B.; Finley, C.C.; Koka, K.; Cassis, A.M. Changes in Wide-band Tympanometry Absorbance Following Cochlear Implantation. Otol. Neurotol. 2020, 41, e680–e685. [Google Scholar] [CrossRef]
- Scheperle, R.A.; Hajicek, J.J. Wideband Acoustic Immittance in Cochlear Implant Recipients: Reflectance and Stapedial Reflexes. Ear Hear. 2020, 41, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Saki, N.; Shirani, M.; Kardooni, M.; Mirmoemeni, G.; Bayat, A. The effects of cochlear implantation on middle ear function: A prospective study. Int. J. Pediatr. Otorhinolaryngol. 2022, 163, 111368. [Google Scholar] [CrossRef]
- Shahnaz, N.; Bork, K.; Polka, L.; Longridge, N.; Bell, D.; Westerberg, B.D. Energy reflectance and tympanometry in normal and otosclerotic ears. Ear Hear. 2009, 30, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hao, W.; Xu, C.; Ni, D.; Gao, Z.; Shang, Y. A Study of Wideband Energy Reflectance in Patients with Otosclerosis: Data from a Chinese Population. Biomed. Res. Int. 2019, 2019, 2070548. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.E.; Merchant, G.R.; Horton, N.J. Effects of middle-ear disorders on power reflectance measured in cadaveric ear canals. Ear Hear. 2012, 33, 195–208. [Google Scholar] [CrossRef]
- Nakajima, H.H.; Rosowski, J.J.; Shahnaz, N.; Voss, S.E. Assessment of ear disorders using power reflectance. Ear Hear. 2013, 34 (Suppl. S1), 48S–53S. [Google Scholar] [CrossRef]
- Merchant, G.R.; Al-Salim, S.; Tempero, R.M.; Fitzpatrick, D.; Neely, S.T. Improving the Differential Diagnosis of Otitis Media with Effusion Using Wideband Acoustic Immittance. Ear Hear. 2021, 42, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Merchant, G.R.; Röösli, C.; Niesten, M.E.; Hamade, M.A.; Lee, D.J.; McKinnon, M.L.; Ulku, C.H.; Rosowski, J.J.; Merchant, S.N.; Nakajima, H.H. Power reflectance as a screening tool for the diagnosis of superior semicircular canal dehiscence. Otol. Neurotol. 2015, 36, 172–177. [Google Scholar] [CrossRef]
- Kaya, Ş.; Çiçek Çınar, B.; Özbal Batuk, M.; Özgen, B.; Sennaroğlu, G.; Genç, G.A.; Sennaroğlu, L. Wideband tympanometry findings in inner ear malformations. Auris Nasus Larynx 2020, 47, 220–226. [Google Scholar] [CrossRef]
- ISO 8253-1; Acoustics–Audiometric Test Methods–Part 1: Pure-Tone Air and Bone Conduction Audiometry. ISO: Geneva, Switzerland, 2010.
- Skarzynski, H.; van de Heyning, P.; Agrawal, S.; Arauz, S.L.; Atlas, M.; Baumgartner, W.; Caversaccio, M.; de Bodt, M.; Gavilan, J.; Godey, B.; et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol. 2013, 133, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Geerardyn, A.; Zhu, M.; Wu, P.; O’Malley, J.; Nadol, J.B.; Liberman, M.C.; Nakajima, H.H.; Verhaert, N.; Quesnel, A.M. Three-dimensional quantification of fibrosis and ossification after cochlear implantation via virtual re-sectioning: Potential implications for residual hearing. Hear. Res. 2023, 428, 108681. [Google Scholar] [CrossRef]
- Racca, J.M.; Jones, L.L.; Dwyer, R.T.; Ferguson, M.; Sunderhaus, L.; Hood, L.J.; Gifford, R.H. Changes in Acoustic Absorbance Pre- and Post-Cochlear Implantation. Am. J. Audiol. 2022, 31, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.J. Structure and function of the mammalian middle ear. II: Inferring function from structure. J. Anat. 2016, 228, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.E.; Horton, N.J.; Woodbury, R.R.; Sheffield, K.N. Sources of variability in reflectance measurements on normal cadaver ears. Ear Hear. 2008, 29, 651–665. [Google Scholar] [CrossRef] [PubMed]

| Total (N = 38) | No Contralateral CI (N = 31) | With Contralateral CI (N = 7) | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 63.1 (11.8) | 63.6 (12.2) | 61.0 (10.8) |
| Median [Min, Max] | 61.0 [34.0, 82.0] | 64.0 [34.0, 80.0] | 55.0 [53.0, 82.0] |
| Sex | |||
| Female | 22 (57.9%) | 17 (54.8%) | 5 (71.4%) |
| Male | 16 (42.1%) | 14 (45.2%) | 2 (28.6 %) |
| Postop. Time point (days after OP) | |||
| Mean (SD) | 45.6 (18.0) | 45.7 (19.6) | 45.0 (7.68) |
| Median [Min, Max] | 37.0 [27.0, 105] | 36.0 [27.0, 105] | 48.0 [34.0, 54.0] |
| Side of implantation | |||
| Left | 14 (36.8%) | 9 (29.0%) | 5 (71.4%) |
| Rigth | 24 (63.2%) | 22 (71.0%) | 2 (28.6 %) |
| Electrode type | |||
| Precurved | 16 (42.1%) | 14 (45.2%) | 2 (28.6 %) |
| Straight | 22 (57.9%) | 17 (54.8%) | 5 (71.4%) |
| Insertion | |||
| Cochleostomy | 12 (31.6%) | 10 (32.3%) | 2 (28.6 %) |
| RW | 26 (68.4%) | 21 (67.7%) | 5 (71.4%) |
| Control | OP | Difference (Control vs. OP) | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Index | Frequency | Index | Frequency | Index | ||
| Peak 1 | Preoperative | 1059 Hz | 37 | 1224 Hz | 42 | +165 Hz | +5 |
| Postoperative | 1155 Hz | 40 | - | - | - | - | |
| Change | +96 Hz | +3 | - | - | |||
| Peak 2 | Preoperative | 2378 Hz | 65 | 2378 Hz | 65 | 0 | 0 |
| Postoperative | 2058 Hz | 60 | 1943 Hz | 58 | −115 Hz | −2 | |
| Change | −320 Hz | −5 | −435 Hz | +7 | |||
| Linear Mixed Model Fixed Effects: Condition, Sex, Side, Electrodetype, Insertion, Age, Days after Surgery Random Effect: Participant | |||||||
|---|---|---|---|---|---|---|---|
| Frequency | Parameter | Estimate | Std. Error | df | t Value | Pr(<|t|) | |
| 500 Hz | Intercept | 0.2759 | 0.07664 | 33.70 | 3.601 | 0.00101 | ** |
| OP postoperative | −0.04377 | 0.02434 | 103.0 | −1.798 | 0.07508 | ∙ | |
| RW Insertion | −0.02683 | 0.04557 | 31.50 | −0.583 | 0.56016 | ns | |
| 750 Hz | Intercept | 0.4479 | 0.1199 | 32.87 | 3.737 | 0.000708 | *** |
| OP postoperative | −0.119974 | 0.033677 | 99.131489 | −3.562 | 0.000567 | *** | |
| RW Insertion | −0.126833 | 0.073565 | 30.028891 | −1.724 | 0.094973 | ∙ | |
| 1000 Hz | Intercept | 0.506528 | 0.13221 | 32.844087 | 3.831 | 0.000545 | *** |
| OP postoperative | −0.105709 | 0.033218 | 98.365972 | −3.182 | 0.001956 | ** | |
| RW Insertion | −0.170700 | 0.082006 | 30.346332 | −2.082 | 0.045915 | * | |
| 2000 Hz | Intercept | 0.5653909 | 0.1494511 | 32.2508813 | 3.783 | 0.000636 | *** |
| OP postoperative | 0.0277305- | 0.0367911 | 99.5998430 | 0.754 | 0.45279 | ns | |
| RW Insertion | −0.0731738 | 0.0897201 | 30.6410767 | −0.816 | 0.42104 | ns | |
| 3000 Hz | Intercept | 0.4990750 | 0.1971817 | 32.2091739 | 2.531 | 0.0165 | * |
| OP postoperative | −0.0177946 | 0.0419342 | 98.5756814 | −0.424 | 0.67224 | ns | |
| RW Insertion | 0.0187486 | 0.1166916 | 30.7764810 | 0.161 | 0.87340 | ns | |
| 4000 Hz | Intercept | 0.3789150 | 0.1912172 | 33.0597188 | 1.982 | 0.0559 | ∙ |
| OP postoperative | −0.0325083 | 0.0391552 | 98.9464383 | −0.830 | 0.4084 | ns | |
| RW Insertion | −0.0789805 | 0.1147044 | 31.6396881 | −0.689 | 0.4961 | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertschinger, R.; von Mitzlaff, C.; Geys, M.; Kunut, A.; Dobrev, I.; Veraguth, D.; Röösli, C.; Huber, A.; Dalbert, A. Evaluating Wideband Tympanometry Absorbance Changes in Cochlear Implant Recipients: Mechanical Insights and Influencing Parameters. J. Clin. Med. 2024, 13, 5128. https://doi.org/10.3390/jcm13175128
Bertschinger R, von Mitzlaff C, Geys M, Kunut A, Dobrev I, Veraguth D, Röösli C, Huber A, Dalbert A. Evaluating Wideband Tympanometry Absorbance Changes in Cochlear Implant Recipients: Mechanical Insights and Influencing Parameters. Journal of Clinical Medicine. 2024; 13(17):5128. https://doi.org/10.3390/jcm13175128
Chicago/Turabian StyleBertschinger, Rahel, Christian von Mitzlaff, Marlies Geys, Ahmet Kunut, Ivo Dobrev, Dorothe Veraguth, Christof Röösli, Alexander Huber, and Adrian Dalbert. 2024. "Evaluating Wideband Tympanometry Absorbance Changes in Cochlear Implant Recipients: Mechanical Insights and Influencing Parameters" Journal of Clinical Medicine 13, no. 17: 5128. https://doi.org/10.3390/jcm13175128
APA StyleBertschinger, R., von Mitzlaff, C., Geys, M., Kunut, A., Dobrev, I., Veraguth, D., Röösli, C., Huber, A., & Dalbert, A. (2024). Evaluating Wideband Tympanometry Absorbance Changes in Cochlear Implant Recipients: Mechanical Insights and Influencing Parameters. Journal of Clinical Medicine, 13(17), 5128. https://doi.org/10.3390/jcm13175128






