Abstract
Transgender people experience distress due to gender incongruence (i.e., a discrepancy between their gender identity and sex assigned at birth). Gender-affirming hormone treatment (GAHT) is a part of gender reassignment treatment. The therapeutic goals of the treatment are to develop the physical characteristics of the affirmed gender as far as possible. Guidelines have been developed for GAHT, which recommend dosage as well as different formulations of oestrogen and testosterone for treatment. Questions arise about the metabolic side effects of hormone treatment. Establishing reference ranges for common analytes in transgender individuals remains a task for laboratory medicine. It has been suggested once GAHT is commenced, the reference ranges for affirmed gender are reported for red blood cells, haemoglobin and haematocrit. For transgender assigned-female-at-birth (AFAB) people, testosterone concentrations are recommended to be within the reference interval established for cisgender men and for transgender assigned-male-at-birth (AMAB) people, estradiol concentrations are within the reference range for cisgender women. Sex-specific reference ranges are available for certain laboratory tests, and these may be organ (e.g., heart)-specific. Transgender-specific reference ranges may be a requirement for such tests. Laboratories may need to make decisions on how to report other tests in the transgender population, e.g., eGFR. Interpretation of further tests (e.g., reproductive hormones) can be individualized depending on clinical information. Electronic medical record systems require fields for gender identity/biological sex at birth so that laboratory results can be flagged appropriately. In this review, we aim to summarise the current position of the role of the laboratory in the clinical care of the transgender individual. Prior to the review, we will summarise the genetics of sex determination, the aetiology of gender incongruence, and the recommendations for GAHT and monitoring for the transgender population.
1. Introduction
Gender incongruency (GI) occurs if the gender identity expressed by the individual and the biological sex of the individual are not consistent with each other. GI may be associated with distress, unease, depression, and low quality of life, which in most cases improve during gender-affirming hormonal treatment (GAHT). In many countries, GAHT is prescribed following an initial assessment by multidisciplinary teams. The teams offer initial psychological support prior to prescribing GAHT [1]. Recent guidelines describe the initial assessment and treatment of GI adolescents and adult individuals. In the scientific literature, transgender people are usually categorised as transgender assigned-male-at-birth (AMAB) people and transgender assigned-female-at-birth (AFAB) people. Guidelines further suggest standard monitoring plans for transgender AFAB and AMAB people treated with GAHT [2,3,4,5]. GAHT can affect laboratory-based diagnostic measurements. Results can be difficult to interpret for physicians due to the absence of published reference intervals.
In this review, we summarise the genetics of sex development, the possible origins of GI, GI treatment, and suggested monitoring following GAHT. This review has a special focus on laboratory interpretation of common tests following GAHT.
2. Method
This narrative review covered several topics, and terms (e.g., gender identity, gender-affirming hormone treatment) were used to identify reviews or articles in PubMed, Medline, Google Scholar, and Web of Science over the past 10 years. From each article/review we extracted further references for studies included in this article.
3. Gender Development
Gender incongruence refers to the discordance between biological sex and gender identity, i.e., children or adults who do not identify with their biological sex. Multiple terms have been used for gender incongruence in the scientific literature, such as transsexualism, transgenderism, gender identity disorder, and gender dysphoria. Minor differences in nuances occur in each term, and the term GI is used as a common term in this review [6]. Prior to examining gender incongruency, this review will first summarise specific genetic signals that ensure sex development in humans. Sex development includes three distinct sequential stages: development of the bipotential gonad; sex determination, gonadal differentiation into testes and ovaries; and sex differentiation, the development of external and internal genitalia, or phenotypic sex. In humans, the bipotential gonads originate at 5 weeks of gestation from the gonadal ridge. The primordial germ cells, precursors to sperm and eggs, actively migrate across the embryo to reach the bipotential gonads [7,8]. Multiple essential genes are involved in bipotential gonadal development. Many of the genes were first reported in animal models. A number of genes (including transcription factors, PBX1, EMX2, and CBX2) [7,9] have been implicated in early gonadal development. Knowledge of factors involved in sex development came from animal models or from case studies in which the genetic or gonadal sex does not equal phenotypical sex, termed disorders of sex development (DSD). As an overall generalisation, factors influencing sex determination are transcriptional regulators, and factors responsible for sex differentiation are secreted hormones and their receptors.
In XY embryos, increasing levels of SF1 (an orphan nuclear receptor) and Wilms Tumour Suppression (WT1) gene, the gonadal development genes, activate the SRY gene expression on the Y chromosome. The SRY gene, the Y chromosomal testis determining gene, initiates the differentiation of Sertoli cells, which develop Leydig cells and germ cells. SRY regulates SOX9 gene expression, which promotes cell differentiation. Several other genes that participate in testicular development are described by Reyes et al. [7]. In males, sex differentiation begins at 7 weeks gestation. Sertoli cells express factors that differentiate and develop Sertoli cells, Leydig cells, and germ cells [10]. Anti-Müllerian hormone (AMH) is one of the earliest cell-specific proteins formed by the Sertoli cells. During male fetal development, AMH provokes the regression of the Müllerian duct, the rudiments of the fallopian tubes, the uterus, and the upper part of the vagina [11]. Human chorionic gonadotrophins (hCG) produced by the placenta in the first trimester of pregnancy and luteinizing hormone (LH) secreted by the pituitary in mid-gestation stimulate Leydig cells to induce testosterone production. Leydig cells respond to hCG/LH, which binds to the LH/hCG receptor and enhances the activity of enzymes that increase testosterone production. Testosterone binds to the androgen receptor in the Wolffian duct to form the male gonaducts. Testosterone is formed into dihydrotestosterone (DHT) by the enzyme 5a-reductase. DHT binds to the androgen receptor with higher affinity than testosterone and drives the differentiation of male external genitalia [12]. The testes are initially located close to the kidneys and, during development, migrate to the lower abdomen and then through the inguinal canal to the scrotum in a hormone-independent process [7].
In the absence of the SRY gene, the XX embryo expresses multiple pro-ovarian genes, which include FOXL2, WNT4, and RSPO1. These factors support ovarian differentiation and suppress testis development. FOXL2 maintains granulosa cell differentiation and supports folliculogenesis during development and adulthood. In the female, in the absence of testicular hormones, effective genetic regulation causes Wolffian duct regression and the development of fetal internal genitalia from Müllerian ducts as well as the growth of female external genitalia. The absence of AMH allows the Müllerian ducts to persist and form the fallopian tubes, uterus, and upper third of the vagina. AMH production begins after Müllerian duct differentiation by the counterpart of Sertoli cells, the granulosa cells. AMH is a folliculogenesis regulator and a biomarker of the primordial follicle reserve (Figure 1) [7].
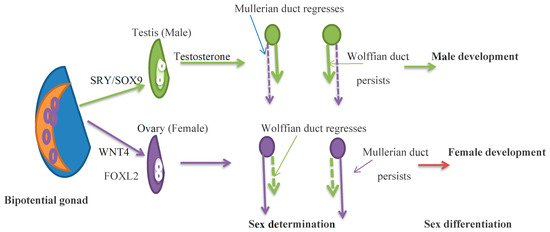
Figure 1.
Germ cells migrate to the gonadal precursor to form the bipotential gonad by 5 weeks of gestation. At 6–8 weeks of gestation, SRY genes, expressed in somatic cells, drive testis determination, and in the absence of the SRY gene, FOXL2 and WNT4 drive ovarian determination. In the XY embryo, testosterone develops the Wolffian duct, and the anti-Müllerian hormone regresses the Müllerian duct. In the XX embryo, the Müllerian duct persists and the Woffian duct regresses. By 12 weeks of gestation, external genitalia are observed. Green shows the development of the male gonad, and purple shows the development of the female gonad.
One of the first hormonal changes in puberty is the pulsatile release of GnRH, which stimulates the release of LH and follicle-stimulating hormone (FSH). LH acts on the theca cells of the ovary to increase estrogen production and the Leydig cells of the testis to increase testosterone. In the female, FSH works on the ovarian follicle to convert oestrogen precursors to oestrogen and within the male Sertoli cells of the testes to form sperm. This results in the formation of the adult male genitalia and in the female development of the breasts. The adrenal gland contributes to the formation of secondary sexual characteristics, particularly the development of pubic and axillary hair, termed pubarche. Tanner staging is an objective classification system that documents the development of secondary sex characteristics in children during puberty [13] (Table 1).

Table 1.
Tanner staging.
Genesis of Gender Incongruence
Despite the increase in gender health research, little is known about the timing of gender identity crystallization and the factors that contribute to the development of a gender identity that is not consistent with the sex determined by chromosomal or biological sex. Factors associated with gender incongruence and the scientific evidence in the literature require further exploration. With the advances in neuroimaging, one suggestion is that differences between male and female human brains may be trivial and population-specific [14]. Ruigrok et al. [15] report on regional brain differences between males and females, which are due to biological and environmental influences. Sex differences in gene expression have been reported in the brain, and sex hormones can further influence brain morphology [16]. Despite decades of research, sex differences in brain function are only partly understood. Gender expression is further likely to be a complex interplay of cultural and environmental factors (psychosocial factors) [17]. Gender identity may be an expression of a complex interplay between biological and environmental pressures.
Publications suggest that the biological origin of transgender identity is based on atypical sexual differentiation of the brain (transgender-specific brain phenotype) [18] or the hormone milieu during intrauterine development. Twin studies suggest a heritable component in transgender identity [19]. Foreman et al. [20] found a significant association between gender incongruence and SRD5A2 and STS alleles, as well as ERα and SULT2A1 genotypes in their cohort of transgender AMAB people. The authors suggest a polygenic basis for the transgender AMAB phenotype. Fernandez et al. [21] suggest that specific genotypic combinations of oestrogen and androgen receptors are associated with the transgender population.
Currently, there is no objective criterion for gender incongruence, and the literature suggests that GI has multiple aetiologies. Understanding the aetiology of GI would help clinicians decide which type of intervention would help in each individual case.
4. Guidelines for GAHT
4.1. Adolescent GI
One suggestion is that most children develop an ability to label their own and others’ genders between 18 and 24 months, and for the majority of adolescents, gender identity agrees with the assigned gender [22]. One review suggests that 1.2–2.7% of children and adolescents and 0.3–0.5% of adults identify as transgender [23]. There is ongoing debate about how children with GI should be treated and how their rights should be respected. The unease with which GI presents in prepubescent children varies; it is often transient and does not continue once puberty begins. Other children with GI exhibit a constant desire to be of the other gender and to match the physical and sexual characteristics of the desired gender. In addition, detransition—or reversing gender transition—can occur in adolescents and young adults [24].
Treatment follows an all-inclusive multidisciplinary clinical and psychosocial assessment of the GI individual, which includes both counselling and support. Treatment includes the following: (1) suppression of puberty by Gonadotropin-Releasing Hormone analogues (GnRHa, or puberty blockers); (2) administration of gender-affirming cross-sex hormones; and (3) gender-affirming surgery [25].
The European Academy of Pediatrics (EAP) states that ‘the child’s best interests are the primary consideration.’ The provision of puberty blockers and gender-affirming therapy in children under 18 years old is under critical review [24]. There has been controversy around the use of GnRHa to block puberty in peri-pubescent children. GnRHa treatment is reversible. GnRHa treatment gives the adolescent time to reconsider while reducing the development of secondary sexual characteristics. Delay may cause psychological and physical harm, though others have stated that there is no evidence for the latter. Studies suggest that the majority who started GnRHa treatment continued with gender-affirming treatment [26,27]. More studies are needed to describe the effect of transgender hormonal treatment on the skeleton and on brain development during adolescence. For the transgender population, while puberty suppression alone does not affect fertility outcomes, the addition of cross steroids does interfere with reproductive potential [28]. A recent review (Cass review) [29] of the Bell vs Tavistock High Court Case (UK) states that gender-affirming care is not backed by strong evidence on the natural history of GI and the efficacy of treatment alternatives. The following two critical questions need to be answered for pre-pubertal GI patients: (i) is the transition pathway beneficial for the individual? and (ii) is the pathway consistent with the ‘do no harm’ principle [29]? A suggestion is that the decision to treat adolescent GI made by a healthcare professional should be based on individual needs and scientific evidence [30]. It is expected that as experience with puberty suppression in transgender AFAB and AMAB children increases, there will be progress in understanding the best ways to provide endocrine care to transgender GI children, although further studies are needed to investigate adverse events.
4.2. Adult GI
In adult GI, the goals of hormone treatment are to reduce endogenous sex hormone levels and to replace hormones with sex hormone levels consistent with the individual’s gender identity. In transgender AFAB people, several androgen preparations have been used to achieve physiological levels consistent with the individual’s gender identity. Treatment for transgender AMAB people involves either oral or transdermal 17β-estradiol. Other adjunctive therapy is used to reduce endogenous testosterone levels. Progestins with anti-androgen activity, GnRH agonists, and spironolactone are some of the medications available [2]. A summary of recommendations from three different worldwide organisations’ published guidelines is provided in Table 2 [2,3,4,5]. It has been suggested that future guidelines might address the holistic healthcare of transpeople by increasing the evidence base, upgrading the quality of clinical practice guidelines, and increasing the number of health topics considered for the transgender population [31]. Individual goals for non-binary transgender AFAB and AMAB people can be complex, and individualised treatment is suggested. Ideal patient-centered outcomes for GAHT need to be defined for the non-binary population. However, adjusting hormonal treatment to attain some characteristics and not others can be a challenge [5]. Further research is needed to guide individualised hormonal treatment and clinical care in transgender AFAB/AMAB people.

Table 2.
Summary of guidelines on GAHT of transgender AFAB/AMAB persons.
5. Laboratory Tests in Transgender AFAB/AMAB Individuals
Laboratory tests are affected by gender-affirming feminising or masculinising therapy or puberty-suppressing treatment. Laboratory tests are recommended by expert opinion or clinical practice guidelines [2,3,4,5]. Some tests are baseline tests prior to treatment, and others are used for treatment monitoring (Table 3 and Table 4). In addition, transgender individuals may receive laboratory tests for other clinical indications. Laboratory tests likely affected by gender-affirming treatment are those that have sex-specific reference intervals, which may, additionally, be target-organ-based.

Table 3.
Baseline and follow-up protocols during suppression of puberty.

Table 4.
Monitoring of transgender AFAB/AMAB individuals following gender-affirming treatment.
Guidelines concur in that for transgender AMAB people, the suggested estradiol levels are aimed at the adult reference range with suppressed testosterone, and for transgender AFAB people, the suggested testosterone levels are within the adult reference range. Blood tests measure serum estradiol to monitor treatment but cannot monitor synthetic oestrogen use, and clinicians use serum estradiol to monitor treatment. Monitoring frequencies are similar in guidelines developed by WPATH and AusPATH [3,5]. Recommended ranges are used as a guide, and how the patient responds to treatment and associated risk factors that are present may guide GAHT (AusPATH). Other tests suggested are full blood count, electrolytes, renal and liver function tests, glucose, and lipids (AusPATH). Monitoring hormone concentrations as well as physiological changes can be used to optimise gender-affirming therapies and minimise adverse events.
5.1. Red Blood Cell Indices
Several prospective studies have investigated the effect of taking gender-affirming hormones on some analytes. Studies are not always powered to analyse subgroups with differences in medication and dosage and route of hormones or analyte measurement carried out on different analyser platforms. Humble et al. [32] report on transgender people treated with hormone therapy for at least 6 months. In transgender individuals receiving masculinising hormones, when compared to baseline levels, creatinine, red blood cells (RBC), haematocrit, haemoglobin, and testosterone were increased, similar to previous studies, and HDL decreased. In transgender individuals receiving feminising hormones, RBC, haematocrit, haemoglobin, testosterone, and creatinine levels were decreased when compared to baseline levels. SoRelle et al.’s [33] study of transgender individuals on hormone therapy for more than 6 months reported similar changes in RBC, haematocrit, haemoglobin and creatinine. In a small study of transgender AFAB people treated with testosterone, the increase in creatinine and RBC indices was stable for 5 years. In transgender AFAB off GAHT, haemoglobin decreased to the female range in 17 weeks. The study suggests that for RBC indices, reference ranges for a person’s affirmed gender apply once on stable GAHT [34] (Table 5). Other studies have reported similar findings in RBC indices in transgender adolescents following GAHT. The authors did not report other significant laboratory abnormalities in transgender adolescents receiving GAHT [35].
Women and men have different levels of haemoglobin, which is probably the effect of oestrogens and androgens on erythropoiesis [36]. Greene et al. [37] reviewed haematology reference ranges for healthy transgender AMAB/AFAB individuals. The oestrogen-treated cohort had values similar to those of cisgender women, and the testosterone-treated cohort had values similar to those of cisgender men.
5.2. Renal Function
It has been suggested that changes in muscle mass in transgender individuals can contribute to changes in serum creatinine. This raises questions about the calculation of eGFR, which uses sex-based calculations and has implications for the estimation of kidney function. This has consequences for the administration of agents (e.g., intravenous contrast agents) that may impact kidney function, kidney transplant eligibility, or renal failure class allocation. One alternative is to use a more direct measure of GFR estimation such as 24 h urine creatinine clearance [38].
5.3. Liver Enzymes
Studies on the effect of GAHT on transaminases are conflicting. At least two studies report that changes in transaminases are not likely to be of clinical significance [33,39]. A further study suggests that the interpretation of transaminase and alkaline phosphatase levels are affected by gender-affirming testosterone therapy and recommends the use of affirmed gender reference intervals [40].
5.4. Lipids
Mixed results across multiple studies have been reported for total cholesterol, triglycerides (TG), LDL, and HDL in transgender individuals receiving GAHT [41,42,43,44].
5.5. Cardiac Biomarkers
In a further cross-sectional study, similar to healthy cisgender people, transgender AFAB people have higher concentrations of high-sensitivity troponin and lower concentrations of N-terminal pro-brain natriuretic peptide compared with transgender AMAB people [45]. In a small study with a single cut-off value of high-sensitivity troponin I and gender-specific reference ranges, 1.1% of patients would have been reclassified as acute myocardial infarction if the threshold value was based on the gender assigned at birth instead of their affirmed gender identity [46].
5.6. Reproductive Hormones
The distribution of endocrine results for estradiol, SHBG, prolactin, AMH, FSH, LH and testosterone for healthy transgender AMAB people differed from that for cisgender men and cisgender women. Treatment with spironolactone had a significant effect on the distribution levels of these hormones [47]. For transgender AFAB people, the distributions of testosterone and SHBG are similar to those of cisgender men. The distribution of results for estradiol, FSH, LH, progesterone, and prolactin differed from those for cisgender men and women, and AMH and dehydroepiandrosterone (DHEAS) differed from cisgender women [48]. It is suggested that reproductive hormone results should be interpreted in a manner specific to the transgender population.
5.7. Ferritin
Serum ferritin levels are influenced by dietary intake of iron, alcohol intake, chronic liver disease, and inflammatory disorders. Reference ranges for serum ferritin vary according to age and sex. Ferritin reference ranges are lower in premenopausal women compared to postmenopausal women. Female reference ranges are typically lower than those of men. There are no studies on the effect of GAHT on serum ferritin levels [49]. When a diagnosis of iron overload is suspected, and secondary causes are excluded, genetic studies for primary haemochromatosis may be indicated.
5.8. Prostate Specific Antigen
In transgender AMAB people, it is rare for the original prostate to be removed during orchiectomy. There is a risk of prostate cancer as long as the prostate remains in situ. Overall, transgender AMAB people showed a lower risk of prostate cancer compared to cisgender women [50], though it may not be as uncommon as previously supposed [51]. Reports suggest a more aggressive presentation than in cisgender men, with metastatic disease on presentation [52]. Little is known about prostate cancer screening in the transgender AMAB population. Future research avenues are the threshold values for prostate-specific antigen (PSA), which should be considered elevated for those on GAHT.


Table 5.
Impact of GAHT on laboratory tests.
Table 5.
Impact of GAHT on laboratory tests.
| Laboratory Tests | Comments | Reference | ||
|---|---|---|---|---|
| Estradiol treatment | Testosterone treatment | Estradiol GAHT shifts haemoglobin, haematocrit to lower values in line with cisgender women’s reference intervals. Testosterone GAHTshifts reference intervals to higher levels in line with cisgender men’s reference intervals | [32] | |
| RBC | Decrease | Increase | ||
| Hemoglobin | Decrease | Increase | ||
| Hematocrit | Decrease | Increase | ||
| Creatinine | Decrease | Increase | The most reno protective calculated GFR either male/female is suggested; 24h creatinine clearance if indicated | [38] |
| High sensitivity troponin I | Report a reference range that would allow critical results to be appropriately followed; an approach of least harm to the patient is suggested | [45] | ||
| Ferritin | Laboratories use dual reference ranges for cisgender individuals. Interpretation is based on clinical presentation (e.g., pregnancy) in combination with full blood count, liver function test, and markers of inflammation, e.g., CRP. Iron overload: If secondary causes excluded, investigation for primary haemochromatosis gene may be indicated | [49] | ||
| Reproductive hormones | Testosterone, Estradiol | Following stabilisation of treatment with gender-affirming hormones, guidelines suggest treatment goals are physiological levels of the affirmed gender identity cisgender adults. The time of measurement of the hormone is dependent on the method of administration as well as formulation of the GAHT | [2] | |
| Reproductive hormones | LH, FSH, AMH, and DHEAS are variable in a transgender population and are interpreted with clinical information | [47,48] | ||
| PSA | Data for reference ranges in transgender AMAB people and from screening for prostatic cancer is not available | [52] | ||
| Renal function/liver function/lipid profile | Guidelines suggest monitoring of liver function/renal function and lipids during GAHT treatment. Sex-specific reference ranges are not ordinarily stated for the measurements | [44] |
5.9. Laboratory Test Reference Intervals for Transgender Population
The reference interval for clinical laboratory tests is a requirement. They are necessary for the correct interpretation of tests and direct the care of the intended population. GAHT is medically indicated in transgender patients. To help clinically manage transgender patients, reference intervals have to take the effect of treatment on laboratory results into account. A summary of recent advances is given in Table 5. Interpretation may still need to be individualized, especially for individuals on a nonstandard treatment regimen of GAHT, during the initial treatment prior to stabilization of therapy, or with co-existing medical conditions.
One principle [53,54] for the selection of different ranges for patients who have started therapy is the organs and physiological hormones influenced by GAHT. Individuals assigned as male at birth have larger organs, such as heart and muscle, following puberty. The reference range for troponin and creatinine may differ. GAHT can influence erythropoiesis, lipid parameters, and reproductive hormones.
6. Electronic Medical Record Systems (EMR)
From the laboratory perspective, the appropriate capture of gender information can have several implications, from test ordering to information gathering on the variation of analytes (i.e., to set up analyte reference ranges). The inclusion of gender identity in the EMR and, if the individual chooses to disclose this information, gender identity at birth can be relevant to individual treatment decisions and help in individual care [55]. In the USA, electronic medical records and laboratory information systems have the capacity to capture gender identity information. However, the introduction of the system into medical records can be challenging [56]. As a result of current limitations in the EMR systems, interactions with laboratory services can increase distress to transgender AFAB/AMAB individuals and affect their mental health.
7. GAHT and Other Laboratory Markers
7.1. Risk of Venous Thromboembolism in AMAB People
In cisgender females, treatment with oral contraceptives increased the risk of venous thromboembolism 2–4 fold, whereas the transdermal oestrogen formulation used for hormone replacement treatment does not appear to be associated with a significant venous thromboembolism risk. In a meta-analysis, Totaro et al. [57] suggest that the overall risk of venous thromboembolism in transgender AMAB people undergoing gender affirmation treatment was 2% but was negligible in those <37.5 years. Other studies confirm that the risk of venous thromboembolism during cross-hormone treatment is rare [58], though the risk may be modified by type, dose, route of oestrogen, duration of treatment, increasing age, high BMI, and smoking [59]. Prothrombotic variants, Factor V Leiden, prothrombin G2010A mutation, Protein S deficiency, Protein C deficiency, and antithrombin deficiency can increase the risk of hormone treatment. Previous venous thromboembolism and family history of genetic thrombophilia are considered reasons for thrombophilia screening prior to hormone treatment [2].
7.2. Hyperprolactinemia
Studies report hyperprolactinemia among transgender AMAB people taking both oestrogens and an antiandrogen [60]. The authors found too few cases of prolactinoma in transgender AMAB people on gender-affirming treatment to draw a conclusion. A threshold value for the definition of hyperprolactinemia in transgender AMAB people needs to be established.
7.3. Other Sex Hormone Dependent Tumours
There is little evidence about the effect of GAHT on the development of hormone-dependent cancer among transgender individuals. The evidence for most aspects of breast cancer in transgender AFAB people is inadequate [61,62]. However, one suggestion is that transgender AFAB people carrying a breast cancer mutation should be investigated further. Specific guidelines for breast cancer screening, intended for transgender AFAB people prior to mastectomy, mimic guidelines for cisgender women [61].
7.4. Bone Mineral Density
Sex steroids contribute to bone growth and peak bone mass accumulation during puberty and in adults contribute to the maintenance of bone structure. In transgender AFAB/AMAB adolescents, blocking puberty with gonadotropin-releasing hormone analogues decreases bone mineral density (BMD). Commencement of GAHT at least partially reverses the bone loss associated with pubertal suppression [63]. A review of studies suggests that GAHT in transgender AFAB people does not compromise bone microarchitecture. A summary of several systematic reviews indicates that reports on the effect of GAHT on the bone health of transgender AMAB are inconsistent [64,65]. Some data support the statement that pharmacological oestrogen can increase bone mineral density in transgender AMAB people [66].
The Endocrine Society clinical practice guidelines for gender-incongruent individuals suggest checking bone density in patients who have risk factors for osteoporosis [2], such as hyperparathyroidism or steroid use.
It is not certain as to which database to use for the interpretation of BMD although it is possible to use both male and female databases for reference in the DXA report. The official position of the International Society for Clinical Densitometry (ISCD) is that transgender individuals should use the reference data of the gender conforming to the individual’s gender identity. If the referring provider or the individual requests, a set of male or female Z-scores can be provided to calculate the Z-score against male and female reference data, respectively [67,68]. Algorithms used to predict fracture risk, such as FRAX, use data derived from cisgender cohorts. These algorithms may not be able to correctly calculate fracture risk in the transgender population.
8. GAHT, Vascular Health and Cardiovascular Disease, and Impact of Aging in Transgender Adults
In a systematic review, van Leerdam et al. [69] suggest that GAHT reduces gender dysphoria and body dissatisfaction with a subsequent improvement in psychological well-being and quality of life, though they suggest further studies are indicated. Aggressive modification of cardiovascular risk factors, e.g., optimisation of diabetes, weight, and lipid profile, may be recommended in transgender patients under treatment with GAHT. Case studies suggest thrombotic risk assessment is indicated in at-risk patients [70]. The effects of GAHT on cardiovascular effects are difficult to assess due to the limited number of studies and contradictory outcomes [71]. There is a lack of research on treatment with GAHT during menopause and older age. Shared decision-making for treatment with GAHT in older age to minimise potential adverse effects has been suggested [72].
9. Conclusions
A cascade of complex genetic interactions leads to the formation of male and female phenotypes [73]. The disparity between the sex assigned at birth and the experienced gender or gender identity in GI individuals can cause distress. GI involves multiple aetiologies, and studies suggest the concept that genetic, endocrine, and neuroanatomic as well as a complex interplay of environmental and cultural factors, contribute to GI [2]. In some GI individuals, this distress is so great that they seek medical treatment to cause changes which match their gender identity. Guidelines suggest puberty suppression therapy for transgender AFAB/AMAB adolescents and testosterone and estradiol treatment for young adults who require transition treatment. Several guidelines suggest blood examinations and clinical evaluations should be performed at baseline and following GAHT treatment. A number of the recommended laboratory tests have been shown to be affected by GAHT. Laboratory tests impacted by GAHT are predominantly tests that have sex-specific reference intervals or are based on target organs affected by the biological sex of the individual.
10. Future Directions
A future informatics challenge is to use EMR systems to provide reference intervals and interpretative comments for laboratory tests ordered for transgender AFAB/AMAB individuals receiving GAHT. A study to create a comprehensive data set that can be used for a wide range of purposes and to address current controversies and improve care for GI individuals is a further task in this subject [74].
Funding
The research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Glintborg, D.; T’Sjoen, G.; Ravn, P.; Andersen, M.S. Management of endocrine disease: Optimal feminizing hormone treatment in transgender people. Eur. J. Endocrinol. 2021, 185, R49–R63. [Google Scholar] [CrossRef] [PubMed]
- Hembree, W.C.; Cohen-Kettenis, P.T.; Gooren, L.; Hannema, S.E.; Meyer, W.J.; Murad, M.H.; Rosenthal, S.M.; Safer, J.D.; Tangpricha, V.; T’Sjoen, G.G. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 3869–3903, Erratum in J. Clin. Endocrinol. Metab. 2018, 103, 699. Erratum in J. Clin. Endocrinol. Metab. 2018, 103, 2758–2759. [Google Scholar] [CrossRef] [PubMed]
- Coleman, E.; Radix, A.E.; Bouman, W.P.; Brown, G.R.; de Vries, A.L.C.; Deutsch, M.B.; Ettner, R.; Fraser, L.; Goodman, M.; Green, J.; et al. Standards of Care for the Health of Transgender and Gender Diverse People, Version 8. Int. J. Transgender Health. 2022, 23 (Suppl. 1), S1–S259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://auspath.org.au/wp-content/uploads/2022/05/AusPATH_Informed-Consent-Guidelines_DIGITAL.pdf (accessed on 2 June 2024).
- Cheung, A.S.; Wynne, K.; Erasmus, J.; Murray, S.; Zajac, J.D. Position statement on the hormonal management of adult transgender and gender diverse individuals. Med. J. Aust. 2019, 211, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Beek, F.T.; Cohen-Kettenis, P.T.; Kreukels, B.P.C. Gender incongruence/gender dysphoria and its classification history. Int. Rev. Psychiatry 2016, 28, 5–12. [Google Scholar] [CrossRef]
- Reyes, A.P.; León, N.Y.; Frost, E.R.; Harley, V.R. Genetic control of typical and atypical sex development. Nat. Rev. Urol. 2023, 20, 434–451. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.E.; Lehmann, R. Mechanisms guiding primordial germ cell migration: Strategies from different organisms. Nat. Rev. Mol. Cell Biol. 2010, 11, 37–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostrer, H.; Huang, H.Y.; Masch, R.J.; Shapiro, E. A cellular study of human testis development. Sex. Dev. 2007, 1, 286–292. [Google Scholar] [CrossRef] [PubMed]
- She, Z.Y.; Yang, W.X. Sry and SoxE genes: How they participate in mammalian sex determination and gonadal development? Semin. Cell Dev. Biol. 2017, 63, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Edelsztein, N.Y.; Valeri, C.; Lovaisa, M.M.; Schteingart, H.F.; Rey, R.A. AMH Regulation by Steroids in the Mammalian Testis: Underlying Mechanisms and Clinical Implications. Front. Endocrinol. 2022, 13, 906381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rey, R.A.; Grinspon, R.P. Normal male sexual differentiation and aetiology of disorders of sex development. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, M.; Bokor, B.R. Tanner Stages. [Updated 11 December 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470280/ (accessed on 25 August 2024).
- Eliot, L.; Ahmed, A.; Khan, H.; Patel, J. Dump the “dimorphism”: Comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neurosci. Biobehav. Rev. 2021, 125, 667–697. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, A.N.; Salimi-Khorshidi, G.; Lai, M.C.; Baron-Cohen, S.; Lombardo, M.V.; Tait, R.J.; Suckling, J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014, 39, 34–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lafta, M.S.; Mwinyi, J.; Affatato, O.; Rukh, G.; Dang, J.; Andersson, G.; Schiöth, H.B. Exploring sex differences: Insights into gene expression, neuroanatomy, neurochemistry, cognition, and pathology. Front. Neurosci. 2024, 18, 1340108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fine, R.D.; Olson, K.R.; Gülgöz, S.; Horton, R.; Gelman, S.A. Gender Essentialism Predicts Prejudice against Gender Nonconformity in Two Cultural Contexts. Soc. Dev. 2024, 33, e12720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mueller, S.C.; Guillamon, A.; Zubiaurre-Elorza, L.; Junque, C.; Gomez-Gil, E.; Uribe, C.; Khorashad, B.S.; Khazai, B.; Talaei, A.; Habel, U.; et al. The Neuroanatomy of Transgender Identity: Mega-Analytic Findings From the ENIGMA Transgender Persons Working Group. J. Sex. Med. 2021, 18, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, A.; Weinand, J.D.; Safer, J.D. Evidence supporting the biologic nature of gender identity. Endocr. Pract. 2015, 21, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Foreman, M.; Hare, L.; York, K.; Balakrishnan, K.; Sánchez, F.J.; Harte, F.; Erasmus, J.; Vilain, E.; Harley, V.R. Genetic Link Between Gender Dysphoria and Sex Hormone Signaling. J. Clin. Endocrinol. Metab. 2019, 104, 390–396, Erratum in J. Clin. Endocrinol. Metab. 2020, 105, 393. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; Guillamon, A.; Cortés-Cortés, J.; Gómez-Gil, E.; Jácome, A.; Esteva, I.; Almaraz, M.; Mora, M.; Aranda, G.; Pásaro, E. Molecular basis of Gender Dysphoria: Androgen and estrogen receptor interaction. Psychoneuroendocrinology 2018, 98, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Steensma, T.D.; Kreukels, B.P.; de Vries, A.L.; Cohen-Kettenis, P.T. Gender identity development in adolescence. Horm. Behav. 2013, 64, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Goodman, M.; Adams, N.; Corneil, T.; Hashemi, L.; Kreukels, B.; Motmans, J.; Snyder, R.; Coleman, E. Epidemiological considerations in transgender health: A systematic review with focus on higher quality data. Int. J. Transgender Health. 2020, 21, 125–137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brierley, J.; Larcher, V.; Hadjipanayis, A.A.; Grossman, Z. European Academy of Paediatrics statement on the clinical management of children and adolescents with gender dysphoria. Front. Pediatr. 2024, 12, 1298884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Claahsen-van der Grinten, H.; Verhaak, C.; Steensma, T.; Middelberg, T.; Roeffen, J.; Klink, D. Gender incongruence and gender dysphoria in childhood and adolescence-current insights in diagnostics, management, and follow-up. Eur. J. Pediatr. 2021, 180, 1349–1357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brik, T.; Vrouenraets, L.J.J.J.; de Vries, M.C.; Hannema, S.E. Trajectories of Adolescents Treated with Gonadotropin-Releasing Hormone Analogues for Gender Dysphoria. Arch. Sex. Behav. 2020, 49, 2611–2618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steininger, J.; Knaus, S.; Kaufmann, U.; Ott, J.; Riedl, S. Treatment trajectories of gender incongruent Austrian youth seeking gender-affirming hormone therapy. Front. Endocrinol. 2024, 15, 1258495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panagiotakopoulos, L. Transgender medicine—Puberty suppression. Rev. Endocr. Metab. Disord. 2018, 19, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Halasz, G.; Amos, A. Gender dysphoria: Reconsidering ethical and iatrogenic factors in clinical practice. Australas. Psychiatry 2024, 32, 26–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calcaterra, V.; Tornese, G.; Zuccotti, G.; Staiano, A.; Cherubini, V.; Gaudino, R.; Fazzi, E.M.; Barbi, E.; Chiarelli, F.; Corsello, G.; et al. Adolescent gender dysphoria management: Position paper from the Italian Academy of Pediatrics, the Italian Society of Pediatrics, the Italian Society for Pediatric Endocrinology and Diabetes, the Italian Society of Adolescent Medicine and the Italian Society of Child and Adolescent Neuropsychiatry. Ital. J. Pediatr. 2024, 50, 73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dahlen, S.; Connolly, D.; Arif, I.; Junejo, M.H.; Bewley, S.; Meads, C. International clinical practice guidelines for gender minority/trans people: Systematic review and quality assessment. BMJ Open 2021, 11, e048943. [Google Scholar] [CrossRef]
- Humble, R.M.; Imborek, K.L.; Nisly, N.; Greene, D.N.; Krasowski, M.D. Common Hormone Therapies Used to Care for Transgender Patients Influence Laboratory Results. J. Appl. Lab. Med. 2019, 3, 799–814. [Google Scholar] [CrossRef] [PubMed]
- SoRelle, J.A.; Jiao, R.; Gao, E.; Veazey, J.; Frame, I.; Quinn, A.M.; Day, P.; Pagels, P.; Gimpel, N.; Patel, K. Impact of Hormone Therapy on Laboratory Values in Transgender Patients. Clin. Chem. 2019, 65, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.N.; Jiao, R.; Day, P.; Pagels, P.; Gimpel, N.; SoRelle, J.A. Dynamic Impact of Hormone Therapy on Laboratory Values in Transgender Patients over Time. J. Appl. Lab. Med. 2021, 6, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Millington, K.; Lee, J.Y.; Olson-Kennedy, J.; Garofalo, R.; Rosenthal, S.M.; Chan, Y.M. Laboratory Changes During Gender-Affirming Hormone Therapy in Transgender Adolescents. Pediatrics 2024, 153, e2023064380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murphy, W.G. The sex difference in haemoglobin levels in adults—Mechanisms, causes, and consequences. Blood Rev. 2014, 28, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.N.; McPherson, G.W.; Rongitsch, J.; Imborek, K.L.; Schmidt, R.L.; Humble, R.M.; Nisly, N.; Dole, N.J.; Dane, S.K.; Frerichs, J.; et al. Hematology reference intervals for transgender adults on stable hormone therapy. Clin. Chim. Acta. 2019, 492, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Humble, R.M.; Greene, D.N.; Schmidt, R.L.; Winston McPherson, G.; Rongitsch, J.; Imborek, K.L.; Nisly, N.; Dole, N.J.; Dane, S.K.; Frerichs, J.; et al. Reference Intervals for Clinical Chemistry Analytes for Transgender Men and Women on Stable Hormone Therapy. J. Appl. Lab. Med. 2022, 7, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, L.; Zhang, Q.; Getahun, D.; Jasuja, G.K.; McCracken, C.; Pisegna, J.; Roblin, D.; Silverberg, M.J.; Tangpricha, V.; Vupputuri, S.; et al. Longitudinal Changes in Liver Enzyme Levels Among Transgender People Receiving Gender Affirming Hormone Therapy. J. Sex. Med. 2021, 18, 1662–1675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krasowski, M.D.; Hines, N.G.; Imborek, K.L.; Greene, D.N. Impact of sex used for assignment of reference intervals in a population of patients taking gender-affirming hormones. J. Clin. Transl. Endocrinol. 2024, 36, 100350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wierckx, K.; Van Caenegem, E.; Schreiner, T.; Haraldsen, I.; Fisher, A.D.; Toye, K.; Kaufman, J.M.; T’Sjoen, G. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: Results from the European network for the investigation of gender incongruence. J. Sex. Med. 2014, 11, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, M.B.; Bhakri, V.; Kubicek, K. Effects of cross-sex hormone treatment on transgender women and men. Obstet. Gynecol. 2015, 125, 605–610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandez, J.D.; Tannock, L.R. Metabolic effects of hormone therapy in transgender patients. Endocr. Pract. 2016, 22, 383–388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waters, J.; Linsenmeyer, W. The impact of gender-affirming hormone therapy on nutrition-relevant biochemical measures. Front. Nutr. 2024, 11, 1339311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greene, D.N.; Schmidt, R.L.; Christenson, R.H.; Rongitsch, J.; Imborek, K.L.; Rebuck, H.; Lorey, T.S.; Saenger, A.K.; Krasowski, M.D. Distribution of High-Sensitivity Cardiac Troponin and N-Terminal Pro-Brain Natriuretic Peptide in Healthy Transgender People. JAMA Cardiol. 2022, 7, 1170–1174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Taur, A.; Chen, A.; Wu, Y.L.; Lee, M.S. Sex-Specific Cardiac Troponin Thresholds in Transgender Patients With Suspected Acute Coronary Syndrome. JAMA Netw. Open. 2023, 6, e2337345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greene, D.N.; Schmidt, R.L.; Winston McPherson, G.; Rongitsch, J.; Imborek, K.L.; Dickerson, J.A.; Drees, J.C.; Humble, R.M.; Nisly, N.; Dole, N.J.; et al. Reproductive Endocrinology Reference Intervals for Transgender Women on Stable Hormone Therapy. J. Appl. Lab. Med. 2021, 6, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.N.; Schmidt, R.L.; Winston-McPherson, G.; Rongitsch, J.; Imborek, K.L.; Dickerson, J.A.; Drees, J.C.; Humble, R.M.; Nisly, N.; Dole, N.J.; et al. Reproductive Endocrinology Reference Intervals for Transgender Men on Stable Hormone Therapy. J. Appl. Lab. Med. 2021, 6, 41–50. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, E.J.; Rossi, E.; Beilby, J.P.; Trinder, D.; Olynyk, J.K. Factors that affect serum levels of ferritin in Australian adults and implications for follow-up. Clin. Gastroenterol. Hepatol. 2014, 12, 101–108.e4. [Google Scholar] [CrossRef] [PubMed]
- de Nie, I.; de Blok, C.J.M.; van der Sluis, T.M.; Barbé, E.; Pigot, G.L.S.; Wiepjes, C.M.; Nota, N.M.; van Mello, N.M.; Valkenburg, N.E.; Huirne, J.; et al. Prostate Cancer Incidence under Androgen Deprivation: Nationwide Cohort Study in Trans Women Receiving Hormone Treatment. J. Clin. Endocrinol. Metab. 2020, 105, e3293–e3299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loria, M.; Gilbert, D.; Tabernacki, T.; Maravillas, M.A.; McNamara, M.; Gupta, S.; Mishra, K. Incidence of prostate cancer in transgender women in the US: A large database analysis. Prostate Cancer Prostatic Dis. 2024; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Crowley, F.; Mihalopoulos, M.; Gaglani, S.; Tewari, A.K.; Tsao, C.K.; Djordjevic, M.; Kyprianou, N.; Purohit, R.S.; Lundon, D.J. Prostate cancer in transgender women: Considerations for screening, diagnosis and management. Br. J. Cancer. 2023, 128, 177–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irwig, M.S. Which reference range should we use for transgender and gender diverse patients? J. Clin. Endocrinol. Metab. 2021, 106, e1479–e1480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheung, A.S.; Lim, H.Y.; Cook, T.; Zwickl, S.; Ginger, A.; Chiang, C.; Zajac, J.D. Approach to Interpreting Common Laboratory Pathology Tests in Transgender Individuals. J. Clin. Endocrinol. Metab. 2021, 106, 893–901. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cahill, S.R.; Baker, K.; Deutsch, M.B.; Keatley, J.; Makadon, H.J. Inclusion of Sexual Orientation and Gender Identity in Stage 3 Meaningful Use Guidelines: A Huge Step Forward for LGBT Health. LGBT Health 2016, 3, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Lyon, M.E.; Luu, H.S. Providing Inclusive Care for Transgender Patients: Capturing Sex and Gender in the Electronic Medical Record. J. Appl. Lab. Med. 2021, 6, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Totaro, M.; Palazzi, S.; Castellini, C.; Parisi, A.; D’Amato, F.; Tienforti, D.; Baroni, M.G.; Francavilla, S.; Barbonetti, A. Risk of Venous Thromboembolism in Transgender People Undergoing Hormone Feminizing Therapy: A Prevalence Meta-Analysis and Meta-Regression Study. Front. Endocrinol. 2021, 12, 741866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ott, J.; Kaufmann, U.; Bentz, E.K.; Huber, J.C.; Tempfer, C.B. Incidence of thrombophilia and venous thrombosis in transsexuals under cross-sex hormone therapy. Fertil. Steril. 2010, 93, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Dix, C.; Moloney, M.; Tran, H.A.; McFadyen, J.D. Venous Thromboembolism and Estrogen-Containing Gender-Affirming Hormone Therapy. Thromb. Haemost. 2024, 124, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.M.; Baker, K.E.; Sharma, R.; Dukhanin, V.; McArthur, K.; Robinson, K.A. Effects of antiandrogens on prolactin levels among transgender women on estrogen therapy: A systematic review. Int. J. Transgender Health 2020, 21, 391–402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wahlström, E.; Audisio, R.A.; Selvaggi, G. Aspects to consider regarding breast cancer risk in trans men: A systematic review and risk management approach. PLoS ONE 2024, 19, e0299333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pamulapati, S.; Conroy, M.; Cortina, C.; Harding, E.; Kamaraju, S. Systematic Review on Gender-Affirming Testosterone Therapy and the Risk of Breast Cancer: A Challenge for Physicians Treating Patients from Transgender and Gender-Diverse Populations. Arch. Sex. Behav. 2024, 53, 1969–1980. [Google Scholar] [CrossRef] [PubMed]
- Schagen, S.E.E.; Wouters, F.M.; Cohen-Kettenis, P.T.; Gooren, L.J.; Hannema, S.E. Bone Development in Transgender Adolescents Treated With GnRH Analogues and Subsequent Gender-Affirming Hormones. J. Clin. Endocrinol. Metab. 2020, 105, e4252–e4263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verroken, C.; Collet, S.; Lapauw, B.; T’Sjoen, G. Osteoporosis and Bone Health in Transgender Individuals. Calcif. Tissue Int. 2022, 110, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, V.S.; Nie, T.; Zajac, J.D.; Grossmann, M.; Davey, R.A. The Utility of Preclinical Models in Understanding the Bone Health of Transgender Individuals Undergoing Gender-Affirming Hormone Therapy. Curr. Osteoporos. Rep. 2023, 21, 825–841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rothman, M.S.; Iwamoto, S.J. Bone Health in the Transgender Population. Clin. Rev. Bone Miner. Metab. 2019, 17, 77–85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosen, H.N.; Hamnvik, O.R.; Jaisamrarn, U.; Malabanan, A.O.; Safer, J.D.; Tangpricha, V.; Wattanachanya, L.; Yeap, S.S. Bone Densitometry in Transgender and Gender Non-Conforming (TGNC) Individuals: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Shuhart, C.R.; Yeap, S.S.; Anderson, P.A.; Jankowski, L.G.; Lewiecki, E.M.; Morse, L.R.; Rosen, H.N.; Weber, D.R.; Zemel, B.S.; Shepherd, J.A. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J. Clin. Densitom. 2019, 22, 453–471. [Google Scholar] [CrossRef] [PubMed]
- van Leerdam, T.R.; Zajac, J.D.; Cheung, A.S. The Effect of Gender-Affirming Hormones on Gender Dysphoria, Quality of Life, and Psychological Functioning in Transgender Individuals: A Systematic Review. Transgender Health 2023, 8, 6–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bouck, E.G.; Grinsztejn, E.; Mcnamara, M.; Stavrou, E.X.; Wolberg, A.S. Thromboembolic risk with gender-affirming hormone therapy: Potential role of global coagulation and fibrinolysis assays. Res. Pract. Thromb. Haemost. 2023, 7, 102197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aranda, G.; Halperin, I.; Gomez-Gil, E.; Hanzu, F.A.; Seguí, N.; Guillamon, A.; Mora, M. Cardiovascular Risk Associated With Gender Affirming Hormone Therapy in Transgender Population. Front. Endocrinol. 2021, 12, 718200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheung, A.S.; Nolan, B.J.; Zwickl, S. Transgender health and the impact of aging and menopause. Climacteric 2023, 26, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Herald, A.K.; Bashamboo, A. Gonadal development. Endocr. Dev. 2014, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/kunskapsstod/2023-1-8330.pdf (accessed on 2 July 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).