Abstract
Background: We aimed to explore the still-debated association between smoking and hyperglycaemia in pregnancy (HIP). Methods: A multiethnic prospective study of 15,801 women who delivered at Jean Verdier University Hospital between 2012 and 2018. Of these, 13,943 (88.2%) were non-smokers, 624 (4.5%) former smokers, and 1234 (7.8%) current smokers. Universal HIP screening was proposed to the entire sample (IADPSG/WHO criteria). Results: A total of 13,958 women were screened for HIP. Uptake differed between non-smokers, former smokers, and current smokers (89.5%, 88.3%, and 75.7%, respectively, p < 0.0001). HIP prevalence in these groups was 19.9%, 15.4%, and 12.3%, respectively (p < 0.0001). After adjusting for age, body mass index, family history of diabetes, history of HIP, history of macrosomic baby, and ethnicity, current (odds ratio 0.790 [95% confidence interval 0.636–0.981], p < 0.05) but not former (1.017 [0.792–1.306]) smokers were less likely to have HIP than non-smokers. Furthermore, 1 h and 2 h oral plasma glucose test values were lower in current smokers than in non-smokers (p < 0.01). To exclude potential selection bias, we compared risk factors for HIP and HIP-related adverse pregnancy outcomes in current smokers according to HIP screening status. Compared with screened current smokers (n = 934), their unscreened counterparts (n = 300) were younger, less frequently employed, and more likely to be of non-European origin. Moreover, infant birthweight was lower in this group, and preterm deliveries and perinatal deaths were more likely (all p < 0.01). Conclusions: Smoking during pregnancy was independently associated with lower HIP prevalence. The low HIP screening rate in current smokers did not explain this finding.
1. Introduction
Hyperglycaemia in pregnancy (HIP) is associated with adverse pregnancy outcomes [1,2] and its prevalence is increasing worldwide [3,4]. Prevention is critical to ensure good health in mothers and their newborns [1,2]. According to the International Association of Diabetes Pregnancy Study Group (IADPSG), HIP covers two pathological conditions: gestational diabetes mellitus (GDM) and diabetes in pregnancy (DIP) [1]. DIP is defined as having test glucose values equal to or higher than the thresholds defining diabetes outside pregnancy. DIP diagnosis suggests undiagnosed type 2 diabetes before pregnancy [1,2].
Tobacco smoking outside pregnancy is still common worldwide [5]. In France, its prevalence is actually increasing [6]. Active smoking increases the risk of both pre-diabetes and diabetes [6], and these associations are dose-dependent [7]. Outside pregnancy, smoking also increases insulin resistance and has been identified as a modifiable risk factor for impaired insulin secretion, likely mediated by nicotine’s effect on beta-cell function [6].
Insulin resistance and insufficient insulin secretion are also determinants of HIP [1,2], and in theory, smoking during pregnancy encourages HIP [6]. Accordingly, smoking during pregnancy could be a modifiable risk factor for HIP. However, two recent meta-analyses did not find any significant association between current tobacco smoking and HIP [8,9]. This was also true for former smokers in one of the meta-analyses [8]. This may be due to confounding factors, such as younger age and lower body mass index (BMI) in smokers than non-smokers [6], as ageing and overweightness/obesity are risk factors for HIP [1,2,10]. Additionally, analyses in published studies may suffer from selection bias, as smokers generally tend to screen for diseases less than non-smokers [11,12,13].
In this context, using the IADPSG/World Health Organization (WHO) diagnostic criteria for HIP, we aimed to evaluate, in a large French dataset, whether smoking before and during pregnancy was associated with more prevalent HIP, independent of confounding factors, while taking into account potential recruitment bias.
2. Materials and Methods
2.1. Cohort Study
This observational prospective cohort study was conducted at Jean Verdier University Hospital in Bondy, a suburb of Paris, France. Analyses were based on data from the hospital’s routine electronic medical records of maternal and neonatal events at birth which occurred between January 2012 and December 2018 [10,14,15,16]. Data were analysed anonymously. Our database was declared to the French Data Protection Agency (Commission Nationale de l’Informatique et des Libertés, number 1704392v0).
2.2. HIP Screening and Care
We followed French recommendations for HIP screening, diagnostic criteria and care [2], and we recommended universal screening, given the high prevalence of risk factors in our hospital population. Screening is scheduled at the beginning of pregnancy; it is also performed after 24 weeks of gestation (WG) if the initial scheduled screening is not performed for some reason, or when the result of the initial screening is normal. Early screening at the beginning of pregnancy is based on the fasting plasma glucose (FPG) measurement. Women with an FPG level ≥ 5.1 mmol/L (92 mg/dL) are promptly provided care for HIP. Women not diagnosed early with HIP undergo an oral glucose tolerance test (OGTT) between 24 and 28 WG, where FPG and plasma glucose one (1h-PG) and two hours after OGTT (2h-PG) are measured.
We used the IADPSG/WHO [1] recommendations to diagnose HIP in accordance with French regulations [2]. Specifically, GDM is defined as an FPG level 5.1–6.9 mmol/L (92–125 mg/dL) and/or 1h-PG ≥ 10.0 mmol/L (180 mg/dL) and/or 2h-PG 8.5–11.0 mmol/L (153–199 mg/dL), while DIP is defined as an FPG ≥ 7.0 mmol/L (126 mg/dL) and/or a 2h-PG ≥ 11.1 mmol/L (200 mg/dL), both after 24 WG.
2.3. Selection Criteria for the Present Study Sample
The inclusion criteria for the women in the present study were as follows: delivery between January 2012 and December 2018 at Jean Verdier hospital, aged ≥18 years, no known diabetes before pregnancy, single-foetus pregnancy, no history of bariatric surgery, available smoking status, and finally, available HIP screening status (flow chart in Figure S1). Women who began smoking during pregnancy were excluded.
2.4. Data Collection
Smoking status was self-reported and categorized into three categories: women who were not smokers at the time of conception were classified as ‘non-smokers’; those who ceased smoking when they discovered they were pregnant were classified as ‘former smokers’, and those who continued smoking during their pregnancy were classified as ‘current smokers’. BMI was calculated according to self-reported weight before pregnancy and to height (measured by a professional) during pregnancy. Alcohol and drug consumption during pregnancy were self-reported. Ethnicity was also self-reported from one of six categories: European, North African, Sub-Saharan African, Indian-Pakistan-Sri Lankan, Caribbean, and Other.
2.5. Maternal and Neonatal Outcomes
We considered the following set of outcomes, using ‘maternal’ and ‘neonatal’ outcomes defined by the INSPIRED research group [10,14,15,16]. Maternal outcomes included gestational weight gain (GWG), planned and unplanned caesarean section, and hypertensive disorders. GWG was calculated as the weight measured before delivery minus the self-reported pre-pregnancy weight. Neonatal outcomes included birthweight, large-for-gestational-age (LGA) infant and small-for-gestational-age infant (defined as a birth weight greater than the 90th percentile and lower than the 10th percentile for a standard French population), gestational age at birth, and preterm delivery (any birth occurring after 22 WG + 6 days and before 37 WG), neonatal hypoglycaemia, perinatal death (death in the first 24 h of life or stillbirth), and, finally, any birth malformation. Definitions of these events are provided in previous publications [10,14,15,16].
2.6. Statistical Analyses
Baseline continuous variables were expressed as the mean ± standard deviation. Categorical variables were expressed as frequencies (percentages). No data replacement procedure was used for missing data.
Patient characteristics (Table 1, study sample) were compared according to smoking status with ANOVA for continuous variables, and the Chi-squared test or Fisher’s exact test for categorical variables. We performed a post hoc analysis to conduct inter-group comparisons using Dunnett’s alpha risk correction for multiplicity.
We also compared the rates of HIP according to smoking status (reference: non-smokers) using multivariable logistic regression analyses adjusted for the following usual confounders [1,2]: age, BMI, family history of diabetes, previous pregnancies with HIP, history of macrosomic child, and ethnicity (Table 2).
The smokers’ characteristics (Table 3) and adverse pregnancy outcomes (Table 4) were compared according to HIP screening uptake status. Finally, we also compared the rates of LGA infant and preterm delivery according to HIP screening status using multivariable logistic regression analyses adjusted for the parameters differing by screening status (i.e., age, employment at the beginning of pregnancy, parity, and ethnicity (Table 3)).
All tests were two-sided. Analyses were conducted using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Study Population Characteristics
Among the 15,801 women who met the inclusion criteria (flow chart in Figure S1), 1843 (11.7%) were not screened for HIP. Of the 13,958 women who were screened, 2685 (19.2%) had HIP. Early diagnosed GDM, GDM, and DIP accounted for 4.8%, 13.3%, and 1.1% of the screened sub-population, respectively.
Table 1 describes the characteristics of the study population according to smoking status: non-smokers (n = 13,943, 88.2%), former smokers (n = 624, 4.0%), and current smokers (n = 1234, 7.8%). Compared with non-smokers (10.5%), current smokers were more likely not to screen for HIP (24.3%, odds ratio 2.73 [95% confidence interval 2.37–3.14]), while former smokers had a similar likelihood (11.7%: odds ratio 1.12 [95% confidence interval 0.88–1.44]).

Table 1.
Characteristics of the study sample according to smoking status.
Table 1.
Characteristics of the study sample according to smoking status.
| Available Data | Non-Smokers | Former Smokers | Current Smokers | Total | p-Value | |
|---|---|---|---|---|---|---|
| n = 13,943 | n = 624 | n = 1234 | n = 15,801 | |||
| Metabolic characteristics | ||||||
| Age (years) | n = 15,801 | 30.5 ± 5.6 | 29.5 ± 5.6 * | 28.7 ± 6.1 * | 30.3 ± 5.6 | <0.0001 |
| Pre-pregnancy body mass index (kg/m2) | n = 15,132 | 25.1 ± 5.0 | 24.3 ± 5.2 * | 23.6 ± 4.7 * | 25.0 ± 5.0 | <0.0001 |
| Pre-pregnancy obesity | n = 15,132 | 2259 (16.9%) | 90 (14.6%) | 142 (12.1%) * | 2491 (16.5%) | 0.0001 |
| Family history of diabetes | n = 15,801 | 3551 (25.5%) | 192 (30.8%) | 346 (28.0%) * | 4089 (25.9%) | 0.0025 |
| Employment at beginning of pregnancy | n = 15,543 | 5069 (37.0%) | 335 (54.0%) * | 492 (40.5%) * | 5896 (37.9%) | <0.0001 |
| Parity | n = 15,801 | 2.18 ± 1.28 | 1.72 ± 1.05 * | 2.08 ± 1.25 * | 2.15 ± 1.27 | <0.0001 |
| Ethnicity | n = 15,777 | <0.0001 | ||||
| Sub-Saharan African | 3081 (22.1%) | 58 (9.3%) | 72 (5.9%) | 3211 (20.4%) | ||
| North African | 4250 (30.5%) | 87 (13.9%) | 195 (15.9%) | 4532 (28.7%) | ||
| Caribbean | 754 (5.4%) | 63 (10.1%) | 46 (3.7%) | 863 (5.5%) | ||
| European | 3339 (24.0%) | 351 (56.3%) | 745 (60.6%) | 4435 (28.1%) | ||
| Indian–Pakistan–Sri Lankan | 1485 (10.7%) | 3 (0.5%) | 2 (0.2%) | 1490 (9.4%) | ||
| Other | 1014 (7.3%) | 62 (9.9%) | 170 (13.8%) | 1246 (7.9%) | ||
| Previous pregnancy history | ||||||
| History of HIP | n = 15,801 | <0.0001 § | ||||
| First child/parity = 0 | 5098 (36.6%) | 351 (56.3%) | 510 (41.3%) | 5959 (37.7%) | ||
| No | 8082 (58.0%) | 260 (41.7%) | 690 (55.9%) | 9032 (57.2%) | ||
| Yes | 763 (5.5%) | 13 (2.1%) | 34 (2.8%) * | 810 (5.1%) | ||
| History of macrosomic child | n = 15,801 | 0.0131 § | ||||
| First child/parity = 0 | 5098 (36.6%) | 351 (56.3%) | 510 (41.3%) | 5959 (37.7%) | ||
| No | 8396 (60.2%) | 260 (41.7%) | 705 (57.1%) | 9361 (59.2%) | ||
| Yes | 449 (3.2%) | 13 (2.1%) | 19 (1.5%) * | 481 (3.0%) | ||
| History of renal vascular disease in pregnancy | n = 15,801 | 0.1454 § | ||||
| First pregnancy/parity = 0 | 3364 (24.1%) | 206 (33.0%) | 239 (19.4%) | 3809 (24.1%) | ||
| No | 10,246 (73.5%) | 409 (65.5%) | 973 (78.8%) | 11,628 (73.6%) | ||
| Yes | 333 (2.4%) | 9 (1.4%) | 22 (1.8%) | 364 (2.3%) | ||
| History of perinatal death | n = 15,801 | 0.4583 § | ||||
| First pregnancy/parity = 0 | 3364 (24.1%) | 206 (33.0%) | 239 (19.4%) | 3809 (24.1%) | ||
| No | 10,258 (73.6%) | 409 (65.5%) | 969 (78.5%) | 11,636 (73.6%) | ||
| Yes | 321 (2.3%) | 9 (1.4%) | 26 (2.1%) | 356 (2.3%) | ||
| History of foetal growth restriction | n = 15,801 | <0.0001 § | ||||
| First pregnancy/parity = 0 | 3364 (24.1%) | 206 (33.0%) | 239 (19.4%) | 3809 (24.1%) | ||
| No | 10,087 (72.3%) | 404 (64.7%) | 919 (74.5%) | 11,410 (72.2%) | ||
| Yes | 492 (3.5%) | 14 (2.2%) | 76 (6.2%) * | 582 (3.7%) | ||
| Behaviours during pregnancy | ||||||
| Alcohol consumption | n = 15,801 | 10 (0.1%) | 0 (0.0%) | 11 (0.9%) * | 21 (0.1%) | <0.0001 |
| Recreational substance consumption | n = 15,801 | 22 (0.2%) | 14 (2.2%) * | 62 (5.0%) * | 98 (0.6%) | <0.0001 |
| No HIP screening | n = 15,801 | 1470 (10.5%) | 73 (11.7%) * | 300 (24.3%) * | 1843 (11.7%) | <0.0001 |
| Glycaemic status during pregnancy | n = 13,958 | <0.0001 | ||||
| Not diagnosed with HIP during screening | 9988 (80.1%) | 466 (84.6%) | 819 (87.7%) | 11,273 (80.8%) | ||
| Early diagnosed gestational diabetes mellitus | 621 (5.0%) | 22 (4.0%) | 30 (3.2%) | 673 (4.8%) | ||
| Gestational diabetes mellitus | 1712 (13.7%) | 62 (11.3%) | 78 (8.4%) | 1852 (13.3%) | ||
| Diabetes in pregnancy | 152 (1.2%) | 1 (0.2%) | 7 (0.7%) | 160 (1.1%) |
Data are shown as n (%) or mean ± standard deviation. HIP: hyperglycaemia in pregnancy; OGTT: oral glucose tolerance test; WG: weeks of gestation. *: vs. No smoking, symbol inserted only if significant (p < 0.05) after Dunnett adjustment for multiplicity. §: Yes vs. No (no history possible if first child/parity = 0).
Age, BMI, family history of diabetes, employment, parity, ethnicity, and previous pregnancy history differed according to smoking status. Current smokers were more likely to drink alcohol than non-smokers (0.9% vs. 0.1%, respectively, odds ratio 12.5 [95% confidence interval 5.3–29.6]), and to consume recreational substances (5.0 vs. 0.2%, respectively, odds ratio 33.5 [95% confidence interval 20.5–54.6]) (Table 1).
3.2. HIP Prevalence According to Smoking Status
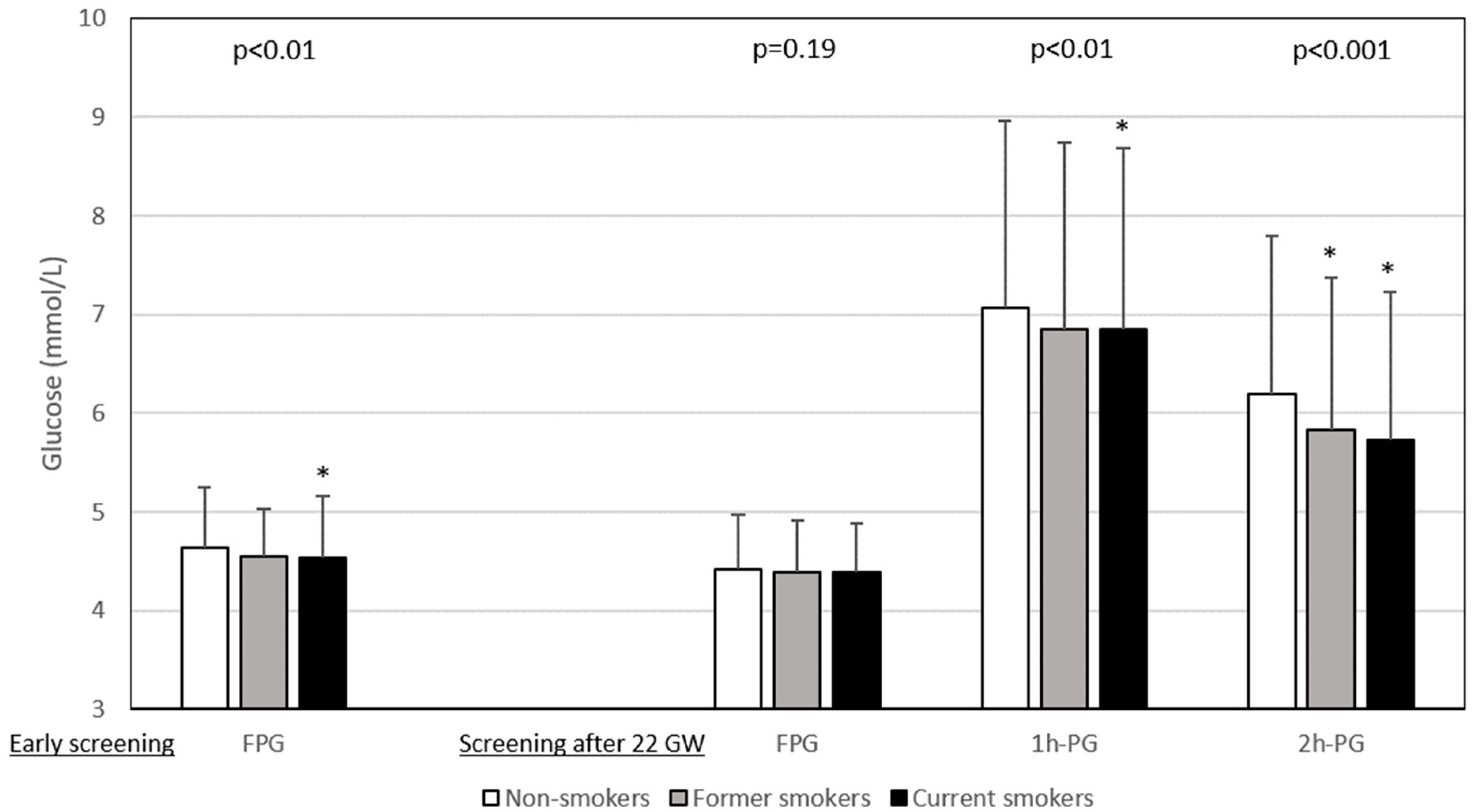
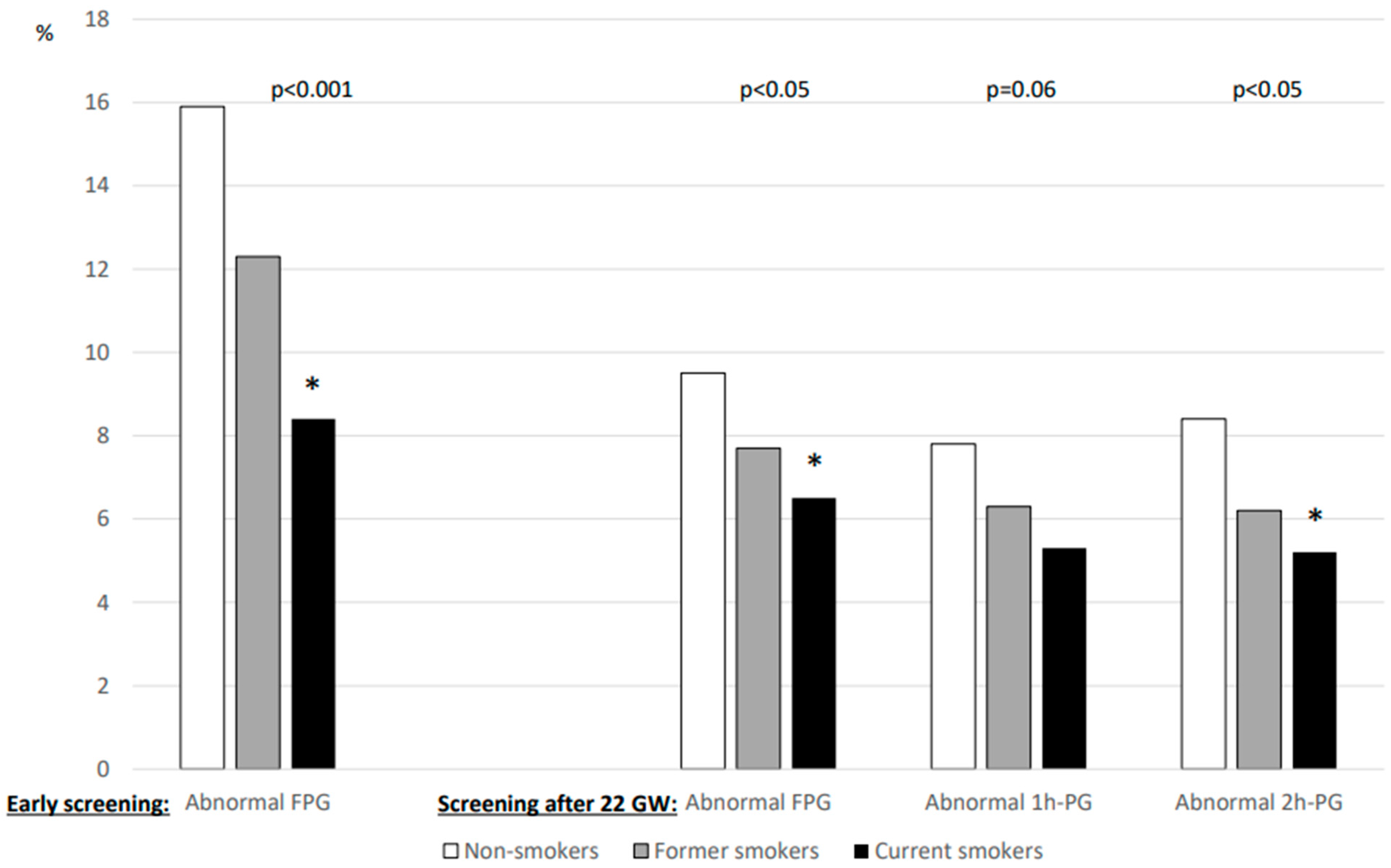
The results of glucose values from HIP screening are shown in Figure 1. FPG levels during early pregnancy were lower in current smokers than in non-smokers, whereas those after 22 GW were similar for all three smoking categories. In contrast, the 1h-PG and 2h-PG levels were lower for current smokers screened after 22 WG than for non-smokers. Figure 2 shows that the rates of abnormal plasma glucose values in persons screened for HIP differed according to smoking status.
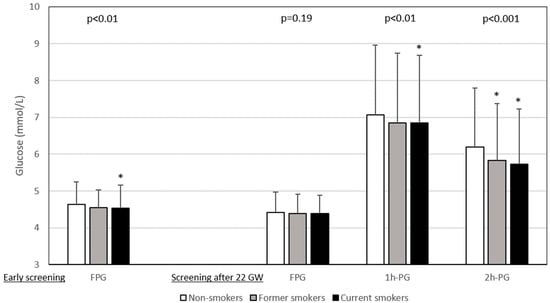
Figure 1.
Plasma glucose values during hyperglycaemia in pregnancy screening according to smoking status. Data are shown as number ± deviation standard. Left panel: early screening (before 22 weeks of gestation (WG)), only fasting plasma glucose (FPG) value was measured (n = 5495). Right panel: after 22 WG, a 75 g oral glucose tolerance test (OGTT) was performed with measurement of FPG (n = 9588) and plasma glucose 1 h (1h-PG, n = 8716) and 2 h (2h-PG, n = 8879) after OGTT. Gestational age at early screening was similar in non-smokers, former smokers and current smokers: 12.3 ± 4.3 WG, 12.0 ± 4.3 WG, and 12.7 ± 4.5 WG, respectively (p = 0.15). Gestational age at OGTT was highest in smokers: non-smokers 27.7 ± 3.5 WG, former smokers 27.3 ± 3.3 WG, and smokers 28.2 ± 3.8 WG, p < 0.0001. WG: weeks of gestation. *: vs. non-smokers, symbol inserted only if significant (p < 0.05) after Dunnett adjustment for multiplicity.
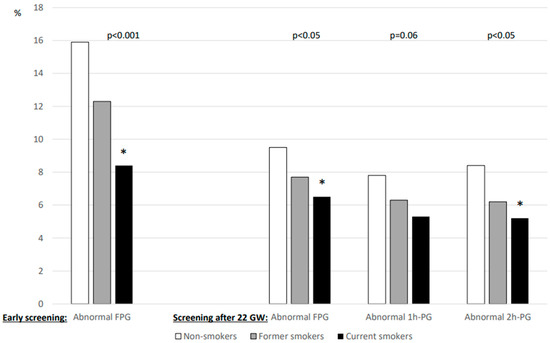
Figure 2.
Percentage of abnormal plasma glucose values during screening for hyperglycaemia in pregnancy according to smoking status. Left panel: early screening (before 22 weeks of gestation (WG)), only fasting plasma glucose (FPG) value was measured (n = 5495). Right panel: after 22 WG, a 75 g oral glucose tolerance test (OGTT) was performed with measurement of FPG (n = 9588) and plasma glucose 1 h (1h-PG, n = 8716) and 2 h (2h-PG, n = 8879) after OGTT. WG: weeks of gestation. *: vs. non-smokers, symbol inserted only if significant (p < 0.05) after Dunnett adjustment for multiplicity.
In the screened population, 19.9%, 15.4%, and 12.3% of non-smokers, former smokers, and current smokers, respectively, tested positive for HIP (p < 0.0001) (Table 1). In the multivariable analysis (Table 2), after adjusting for age, BMI, a family history of diabetes, a history of HIP, a history of macrosomic child, and ethnicity, current smokers (odds ratio 0.79 [95% confidence interval 0.64–0.98], p < 0.05), but not former smokers (odds ratio 1.02 [95% confidence interval 0.79–1.31]), were still less likely to have HIP than non-smokers.

Table 2.
Parameters associated with hyperglycaemia in pregnancy in multivariable analyses.
Table 2.
Parameters associated with hyperglycaemia in pregnancy in multivariable analyses.
| Odds Ratio | 95% Confidence Interval | p-Value | ||
|---|---|---|---|---|
| Minimum | Maximum | |||
| Age (for 1 year) | 1.061 | 1.052 | 1.070 | <0.001 |
| Pre-pregnancy body mass index (for 1 kg/m2) | 1.090 | 1.080 | 1.099 | <0.001 |
| Family history of diabetes | 1.388 | 1.256 | 1.533 | <0.001 |
| Previous pregnancy history | ||||
| History of hyperglycaemia in pregnancy | 4.264 | 3.628 | 5.011 | <0.001 |
| History of macrosomic child | 1.476 | 1.179 | 1.847 | <0.001 |
| Ethnicity | ||||
| European | REF | |||
| North African | 1.344 | 1.184 | 1.526 | <0.001 |
| Sub-Saharan African | 0.893 | 0.769 | 1.036 | 0.136 |
| Caribbean | 0.746 | 0.589 | 0.944 | 0.015 |
| Indian–Pakistan–Sri Lankan | 2.767 | 2.360 | 3.245 | <0.001 |
| Other | 1.388 | 1.145 | 1.682 | <0.001 |
| Smoking status | ||||
| Non-smoker | REF | |||
| Former smoker | 1.017 | 0.792 | 1.306 | 0.894 |
| Current smoker | 0.790 | 0.636 | 0.981 | 0.033 |
REF: reference.
3.3. Possible Inclusion Bias
The lower prevalence of HIP in current smokers may have been due to inclusion bias. As they were less likely to screen for HIP, there may have been a high percentage of undiagnosed HIP in this sub-population [11,12,13]. To explore this possibility, we selected the 1234 current smokers in the study sample and compared (i) the prevalence of risk factors for HIP and (ii) the incidence of adverse HIP-related pregnancy outcomes between the screened (n = 934) and unscreened groups (n = 300). Unscreened women were younger, had a higher parity, were less likely to be employed, and less likely to be of European origin (Table 3).

Table 3.
Risk factors for HIP according to HIP screening status in the 1234 women who smoked during pregnancy.
Table 3.
Risk factors for HIP according to HIP screening status in the 1234 women who smoked during pregnancy.
| Available Data | Screening | Total | p-Value | ||
|---|---|---|---|---|---|
| No n = 300 | Yes n = 934 | ||||
| Metabolic characteristics | |||||
| Age (years) | n = 1234 | 27.3 ± 6.0 | 29.2 ± 6.0 | 28.7 ± 6.1 | <0.0001 |
| Pre-pregnancy body mass index (kg/m2) | 23.2 ± 4.6 | 23.7 ± 4.8 | 23.6 ± 4.7 | 0.1510 | |
| Pre-pregnancy obesity | 26 (10.1%) | 116 (12.7%) | 142 (12.1%) | 0.2616 | |
| Family history of diabetes | n = 1234 | 75 (25.0%) | 271 (29.0%) | 346 (28.0%) | 0.1780 |
| Employment during pregnancy | n = 1234 | 71 (24.1%) | 421 (45.7%) | 492 (40.5%) | <0.0001 |
| Parity | n = 1234 | 2.3 ± 1.5 | 2.0 ± 1.1 | 2.1 ± 1.2 | <0.0001 |
| Ethnicity | n = 1234 | <0.0001 | |||
| Sub-Saharan African | 16 (5.3%) | 56 (6.0%) | 72 (5.9%) | ||
| North African | 49 (16.3%) | 146 (15.7%) | 195 (15.9%) | ||
| Caribbean | 11 (3.7%) | 35 (3.8%) | 46 (3.7%) | ||
| European | 158 (52.7%) | 587 (63.1%) | 745 (60.6%) | ||
| Indian–Pakistan–Sri Lankan | 1 (0.3%) | 1 (0.1%) | 2 (0.2%) | ||
| Other | 65 (21.7%) | 105 (11.3%) | 170 (13.8%) | ||
| Previous pregnancy history | n = 1234 | ||||
| History of HIP | 0.2052 | ||||
| First child/parity = 0 | 104 (34.7%) | 406 (43.5%) | 510 (41.3%) | ||
| No | 190 (63.3%) | 500 (53.5%) | 690 (55.9%) | ||
| Yes | 6 (2.0%) | 28 (3.0%) | 34 (2.8%) | ||
| History of macrosomic child | 0.6541 | ||||
| First child/parity = 0 | 104 (34.7%) | 406 (43.5%) | 510 (41.3%) | ||
| No | 190 (63.3%) | 515 (55.1%) | 705 (57.1%) | ||
| Yes | 6 (2.0%) | 13 (1.4%) | 19 (1.5%) | ||
Data are shown as n (%) or mean (standard deviation). HIP: hyperglycaemia in pregnancy.
Furthermore, unscreened current smokers were more likely to consume recreational substance during pregnancy and had lower GWG (Table 4). They were less likely to have a LGA infant, and their newborns had a lower birthweight. In contrast, they were more likely to experience preterm delivery and perinatal death. In multivariable analysis, after adjustment for age, ethnicity, employment during pregnancy, and parity, not screening for HIP was associated with a lower likelihood of having a LGA infant (odds ratio 0.32 [95% confidence interval 0.12–0.83], p < 0.01) and a greater likelihood of preterm delivery (odds ratio 2.14 [95% confidence interval 1.38–3.32], p < 0.001) in unscreened current smokers.

Table 4.
Behaviours during pregnancy and adverse HIP-related pregnancy outcomes, according to HIP screening status in the 1234 women who smoked during pregnancy.
Table 4.
Behaviours during pregnancy and adverse HIP-related pregnancy outcomes, according to HIP screening status in the 1234 women who smoked during pregnancy.
| Available Data | Screening | Total | Odds Ratio | 95% Confidence Interval | p-Value | ||
|---|---|---|---|---|---|---|---|
| No n = 300 | Yes n = 934 | ||||||
| Behaviours during pregnancy | n = 1234 | ||||||
| Alcohol consumption | 3 (1.0%) | 8 (0.9%) | 11 (0.9%) | 1.17 | [0.31–4.44] | 0.7343 | |
| Recreation substance consumption | 22 (7.3%) | 40 (4.3%) | 62 (5.0%) | 1.77 | [1.03–3.03] | 0.0353 | |
| Maternal outcomes | |||||||
| Gestational weight gain (kg) | n = 1047 | 11.2 ± 5.6 | 13.0 ± 6.2 | 12.6 ± 6.1 | 0.0002 | ||
| Caesarean section | n = 1234 | 50 (16.7%) | 184 (19.7%) | 234 (19.0%) | 0.82 | [0.58–1.15] | 0.2436 |
| Hypertensive disorders during pregnancy | n = 1234 | 10 (3.3%) | 38 (4.1%) | 48 (3.9%) | 0.81 | [0.40–1.65] | 0.5667 |
| Neonatal outcomes | |||||||
| Birthweight (g) | n = 1234 | 2959 ± 564 | 3117 ± 516 | 3079 ± 532 | <0.0001 | ||
| Large-for-gestational-age infant | n = 1234 | 4 (1.3%) | 43 (4.6%) | 47 (3.8%) | 0.28 | [0.10–0.79] | 0.01 |
| Small-for-gestational-age infant | n = 1234 | 45 (15.0%) | 146 (15.6%) | 191 (15.5%) | 0.95 | [0.66–1.37] | 0.7924 |
| Gestational age at birth (WG) | n = 1234 | 38.8 ± 2.6 | 39.4 ± 1.7 | 39.29 ± 1.98 | <0.0001 | ||
| Preterm delivery | n = 1234 | 41 (13.7%) | 64 (6.9%) | 105 (8.5%) | 2.15 | [1.41–3.26] | 0.0002 |
| Neonatal hypoglycemia | n = 1234 | 3 (1.9%) | 6 (1.0%) | 9 (1.2%) | 1.90 | [0.47–7.68] | 0.4062 |
| Perinatal death and stillbirth | n = 1234 | 7 (2.3%) | 4 (0.4%) | 11 (0.9%) | 5.55 | [1.61–19.11] | 0.0062 |
| Birth malformation | n = 1234 | 2 (0.7%) | 11 (1.2%) | 13 (1.1%) | 0.56 | [0.12–2.56] | 0.7451 |
Data are shown as n (%) or mean (standard deviation). HIP: hyperglycaemia in pregnancy, WG: weeks of gestation.
4. Discussion
4.1. Main Results
Our results show that screened current, but not former, smokers were less likely to have HIP, independently of confounders. Furthermore, their glucose levels during the OGTT were lower than those of non-smokers. We also observed that current smokers were less likely to attend HIP screening. Selection bias did not seem to play any role in this result, as unscreened current smokers were not more likely to have HIP-related risk factors or an LGA infant than their screened counterparts.
4.2. Continued Smoking during Pregnancy
Approximately 8% of pregnant women in our series continued to smoke during pregnancy. This percentage is lower than 1986–1988 data for France [17] and lower than 2002–2010 data we collected for women who delivered in our hospital [18]. In contrast, it reflects 2000–2010 data for the United States [19].
4.3. HIP Prevalence and Smoking during Pregnancy
While meta-analyses globally show no association between smoking during pregnancy and HIP [8,9], several studies conducted worldwide—including a previous study we performed in our hospital for the 2002–2010 period—highlighted that smoking was associated with less HIP [3,4,18,20,21,22], which reflects our present findings. The odds ratio in those studies ranged from 0.47 [95% confidence interval 0.23–0.96] [22] to 0.90 [95% confidence interval 0.81–1.00] [21]. The odds ratio in the present study fell between these two limits (i.e., 0.79 [95% confidence interval 0.64–0.98]).
The association between smoking and HIP prevalence may differ (i.e., negative, neutral or positive association) because of divergences in how smoking status is assessed, the HIP diagnosis criteria used, and ethnicity-based or between-country differences in other lifestyle behaviours [8,9]. It may also differ because of a divergence in the prevalence of risk factors in HIP smokers and non-smokers. Despite this, results in all [3,4,18,20,22] but one [21] of the aforementioned studies were adjusted for several confounders. Importantly, we did not find any association between former smoking and HIP, despite the fact that smoking cessation may increase appetite and weight gain [23], two potential drivers of HIP. Outside pregnancy, smoking cessation was reported to initially increase diabetes risk [24].
4.4. Smoking and OGTT Results
FPG levels during early screening for HIP, and 1h-PG and 2h-PG levels during OGTT after 22 WG, were lower in current smokers than in non-smokers in our study sample. This explains the lower prevalence of HIP in the former group. In women with HIP, defined according to the Carpenter–Coustan criteria, Aulinas et al. also found higher plasma glucose levels at 1 h and lower levels at 3 h after 100 g OGTT in smokers [25]. Elsewhere, Konstantakou et al. found similar patterns in women with and without HIP, specifically higher FPG and lower plasma glucose levels at 3 h after 100 g OGTT in current smokers [26]. These results for current smokers during pregnancy reflect data for current smokers outside pregnancy, specifically lower 2h-PG values than those of non-smokers [27]. Values for former smokers lay between those of current smokers and non-smokers, for both pregnant and non-pregnant populations [26,27]. On the contrary, Zaren et al. reported that 12.4% of mothers who were heavy smokers (i.e., ≥10 cigarettes a day) had a 2h-PG value > 8.5 mmol/L (i.e., the definition of HIP in that study), compared with 9.2% for light smokers and 6.0% for non-smokers [28]. However, in their study, the OGTT was performed late during pregnancy, at 37 WG, which could explain these different OGTT patterns [28].
4.5. Why Might Smoking Be Negatively Associated with HIP?
The reason why current smoking may influence OGTT results and ‘protect’ against HIP is still unclear. First, smoking is known to increase insulin resistance and therefore hepatic glucose production [6]. This may increase FPG values in some women [26,27]. However, there is no specific data about the role of smoking in insulin resistance during pregnancy. Furthermore, if smoking actually increases insulin resistance during pregnancy, such an increase might be of minor significance compared with pregnancy-induced insulin resistance [29]. Second, smokers appear to have accelerated gastric emptying during OGTT, leading to earlier glucose absorption, and lower plasma glucose values after OGTT [27]. Third, nicotine-induced insulin release might be involved [30]. Finally, current smokers may have fewer HIP risk factors than non-smokers in general. However, our results were similar after adjusting for most risk factors (we did not have data on BMI at screening time).
4.6. Could the Lower Prevalence of HIP in Smokers Be Explained by Less HIP Screening Uptake?
The current smokers in our study sample were half as likely to screen for HIP compared with non-smokers. This reflects previous findings highlighting that current smokers are less likely to undertake preventive screening, irrespective of the disease [11,12,13].
We hypothesized that the rate of HIP we found in current smokers might have been higher if more of this sub-population in our sample had decided to undertake screening. Having said that, unscreened current smokers did not have a higher risk of HIP than their screened counterparts. Specifically, they were less likely to have certain HIP risk factors, such as younger age, but more likely to have a higher prevalence of non-European ethnicity (reflecting previous findings [18]) and a higher parity. Furthermore, given the higher percentages of persons who were unemployed and individuals of non-European origin in this group, they were likely to have greater psychosocial vulnerability (as found elsewhere [3]).
Furthermore, screened current smokers were less likely to have an LGA infant; had the opposite been true, it could have been an indicator of undiagnosed (and therefore untreated) HIP. Psychosocial vulnerability did not appear to be a driver of LGA infants in our sample [16]. Finally, unscreened current smokers were at a higher risk of experiencing preterm delivery and perinatal death than their screened counterparts. This highlights the importance of targeted care during pregnancy for this sub-population.
4.7. Strengths and Limitations
Our study has important strengths. First, it involved a large multi-ethnic prospective cohort recruited over a decade. Second, it confirmed results in a similar study of women who delivered at our hospital in 2002–2010 [18]. Third, there was a high rate of HIP screening, we adjusted for several confounders, and we considered former and current smokers.
This study also has limitations. First, weight gain until HIP screening was unavailable. Second, data collected on smoking, alcohol, and recreational substances consumption were self-reported; having said that, previous studies found good validity of self-reported tobacco use when compared with measured plasma cotinine levels [31]. Third, we did not formally assess insulin resistance in the women of our series. Finally, we were not able to evaluate the impact of smoking at different gestational time points and we had no quantitative data on cigarette smoking.
4.8. Perspectives
Despite confirming data from a previous study in our hospital [18], the lower prevalence of HIP we found in current smokers in our present study was unexpected. Of course, this result does not advocate smoking to prevent HIP. The higher risk of adverse pregnancy outcomes in current smokers during both pregnancy [32] and the postpartum period [33] neutralizes any apparent protective effect. It is essential to promote smoking cessation during pregnancy.
We plan to complement the work described in the present study with the analyses of the rates of different adverse pregnancy outcomes according to smoking status and HIP status. Smoking may act through nicotine exposure [34]; and altered endometrial maturation [35], immune response [36], and endothelial function [8]. The current study’s results already indicate the need for targeted care for current smokers who do not screen for HIP, given the higher risk of preterm delivery and perinatal death than in their screened counterparts.
5. Conclusions
The present study confirmed our previous findings [18] of lower HIP prevalence in current smokers compared with non-smokers, even after adjustment for confounders. Current smokers were less likely to screen for HIP, and selection bias was excluded as a possible reason for this. The pathophysiology explaining this lower prevalence of HIP in current smokers is still unclear and warrants further research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13175149/s1, Figure S1: Flow chart of this study.
Author Contributions
Conceptualization: E.C., S.T., E.V., J.-J.P. and A.B.; Methodology: E.C., E.V. and J.-J.P.; Software, J.-J.P.; Validation: E.C., S.T., E.V., J.-J.P. and A.B.; Formal Analysis, J.-J.P.; Investigation: all authors; Resources: E.C., E.V. and L.C.; Data Curation: E.C. and J.J.P.; Writing—Original Draft Preparation, E.C.; Writing—Review and Editing: all authors; Supervision: E.C. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Avicenne University Hospital (CLEA-2023-n°340, 15/12/2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study. In Jean Verdier Hospital, as in all Public Assistance Hospitals in Paris, patients are informed at admission that their medical records may be used for research unless they indicate their opposition.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to ongoing studies.
Acknowledgments
Our thanks to Jude Sweeney (Milan, Italy) for the English editing and revision of the manuscript. The authors thank the experts of the French-Speaking Society on Tobacco (Société Francophone de Tabacologie) and the French-Speaking Society of Diabetes (Societé Francophone du Diabète), please see [6], for their help in interpreting the results of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Vambergue, A. Expert Consensus on Gestational Diabetes Mellitus. Diabetes Metab. 2010, 36, 511. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.; Abraham, E.C.; Armstrong, J.; Godwin, J.; Monteath, K.; Lindsay, R. Reported Prevalence of Gestational Diabetes in Scotland: The Relationship with Obesity, Age, Socioeconomic Status, Smoking and Macrosomia, and How Many Are We Missing? J. Diabetes Investig. 2017, 8, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, M.L.; Mondschein, S.; Montiel, B.; Kusanovic, J.P. Trends and Predictors of Gestational Diabetes Mellitus in Chile. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2020, 148, 210–218. [Google Scholar] [CrossRef]
- Roderick, P.; Turner, V.; Readshaw, A.; Dogar, O.; Siddiqi, K. The Global Prevalence of Tobacco Use in Type 2 Diabetes Mellitus Patients: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pract. 2019, 154, 52–65. [Google Scholar] [CrossRef]
- Durlach, V.; Vergès, B.; Al-Salameh, A.; Bahougne, T.; Benzerouk, F.; Berlin, I.; Clair, C.; Mansourati, J.; Rouland, A.; Thomas, D.; et al. Smoking and Diabetes Interplay: A Comprehensive Review and Joint Statement. Diabetes Metab. 2022, 48, 101370. [Google Scholar] [CrossRef]
- Willi, C.; Bodenmann, P.; Ghali, W.A.; Faris, P.D.; Cornuz, J. Active Smoking and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. JAMA 2007, 298, 2654–2664. [Google Scholar] [CrossRef]
- Wang, J.-W.; Cao, S.-S.; Hu, R.-Y.; Wang, M. Association between Cigarette Smoking during Pregnancy and Gestational Diabetes Mellitus: A Meta-Analysis. J. Matern.-Fetal Neonatal Med. 2020, 33, 758–767. [Google Scholar] [CrossRef]
- Athanasiadou, K.I.; Paschou, S.A.; Papakonstantinou, E.; Vasileiou, V.; Kanouta, F.; Kazakou, P.; Stefanaki, K.; Kassi, G.N.; Psaltopoulou, T.; Goulis, D.G.; et al. Smoking during Pregnancy and Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Endocrine 2023, 82, 250–262. [Google Scholar] [CrossRef]
- Cosson, E.; Vicaut, E.; Sandre-Banon, D.; Gary, F.; Pharisien, I.; Portal, J.-J. Performance of a Selective Screening Strategy for Diagnosis of Hyperglycaemia in Pregnancy as Defined by IADPSG/WHO Criteria. Diabetes Metab. 2019, 46, 311–318. [Google Scholar] [CrossRef]
- Sharpe, T.; Alsahlanee, A.; Ward, K.D.; Doyle, F. Systematic Review of Clinician-Reported Barriers to Provision of Smoking Cessation Interventions in Hospital Inpatient Settings. J. Smok. Cessat. 2018, 13, 233–243. [Google Scholar] [CrossRef]
- Bihan, H.; Cosson, E.; Khiter, C.; Vittaz, L.; Faghfouri, F.; Leboeuf, D.; Carbillon, L.; Dauphin, H.; Reach, G.; Valensi, P. Factors Associated with Screening for Glucose Abnormalities after Gestational Diabetes Mellitus: Baseline Cohort of the Interventional IMPACT Study. Diabetes Metab. 2014, 40, 151–157. [Google Scholar] [CrossRef]
- Eng, V.A.; David, S.P.; Li, S.; Ally, M.S.; Stefanick, M.; Tang, J.Y. The Association between Cigarette Smoking, Cancer Screening, and Cancer Stage: A Prospective Study of the Women’s Health Initiative Observational Cohort. BMJ Open 2020, 10, e037945. [Google Scholar] [CrossRef] [PubMed]
- Cosson, E.; Nachtergaele, C.; Vicaut, E.; Tatulashvili, S.; Sal, M.; Berkane, N.; Pinto, S.; Fabre, E.; Benbara, A.; Fermaut, M.; et al. Metabolic Characteristics and Adverse Pregnancy Outcomes for Women with Hyperglycaemia in Pregnancy as a Function of Insulin Resistance. Diabetes Metab. 2022, 48, 101330. [Google Scholar] [CrossRef]
- Cosson, E.; Vicaut, E.; Sandre-Banon, D.; Gary, F.; Pharisien, I.; Portal, J.-J. Early Screening for Gestational Diabetes Mellitus Is Not Associated with Improved Pregnancy Outcomes: An Observational Study Including 9795 Women. Diabetes Metab. 2019, 45, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Bihan, H.; Nachtargeale, C.; Vicaud, E.; Sal, M.; Berkane, N.; Pinto, S.; Tatulashvili, S.; Fermaut, M.; Carbillon, L.; Cosson, E. Impact of Experiencing Multiple Vulnerabilities on Fetal Growth and Complications in Women with Hyperglycemia in Pregnancy. BMC Pregnancy Childbirth 2023, 23, 740. [Google Scholar] [CrossRef]
- Gestation and Diabetes in France Study Group. Multicenter Survey of Diabetic Pregnancy in France. Diabetes Care 1991, 14, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Cosson, E.; Cussac-Pillegand, C.; Benbara, A.; Pharisien, I.; Jaber, Y.; Banu, I.; Nguyen, M.T.; Valensi, P.; Carbillon, L. The Diagnostic and Prognostic Performance of a Selective Screening Strategy for Gestational Diabetes Mellitus According to Ethnicity in Europe. J. Clin. Endocrinol. Metab. 2014, 99, 996–1005. [Google Scholar] [CrossRef]
- Tong, V.T.; Dietz, P.M.; Morrow, B.; D’Angelo, D.V.; Farr, S.L.; Rockhill, K.M.; England, L.J.; Centers for Disease Control and Prevention (CDC). Trends in Smoking before, during, and after Pregnancy—Pregnancy Risk Assessment Monitoring System, United States, 40 Sites, 2000–2010. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2013, 62, 1–19. [Google Scholar]
- Wendland, E.M.D.R.; Duncan, B.B.; Belizán, J.M.; Vigo, A.; Schmidt, M.I. Gestational Diabetes and Pre-Eclampsia: Common Antecedents? Arq. Bras. Endocrinol. Metabol. 2008, 52, 975–984. [Google Scholar] [CrossRef]
- Roelands, J.; Jamison, M.G.; Lyerly, A.D.; James, A.H. Consequences of Smoking during Pregnancy on Maternal Health. J. Womens Health 2009, 18, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Moore Simas, T.A.; Szegda, K.L.; Liao, X.; Pekow, P.; Markenson, G.; Chasan-Taber, L. Cigarette Smoking and Gestational Diabetes Mellitus in Hispanic Woman. Diabetes Res. Clin. Pract. 2014, 105, 126–134. [Google Scholar] [CrossRef]
- Tian, J.; Venn, A.; Otahal, P.; Gall, S. The Association between Quitting Smoking and Weight Gain: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2015, 16, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.-E.; Lee, S.-J.; Lee, M.-Y.; Rhee, E.-J.; Sung, K.-C. Longitudinal Analysis of Diabetes Mellitus Risk: Smoking Status and Smoking Cessation. J. Clin. Med. 2024, 13, 3927. [Google Scholar] [CrossRef]
- Aulinas, A.; Colom, C.; García Patterson, A.; Ubeda, J.; María, M.A.; Orellana, I.; Adelantado, J.M.; de Leiva, A.; Corcoy, R. Smoking Affects the Oral Glucose Tolerance Test Profile and the Relationship between Glucose and HbA1c in Gestational Diabetes Mellitus. Diabet. Med. J. Br. Diabet. Assoc. 2016, 33, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Konstantakou, P.; Paschou, S.A.; Patinioti, I.; Vogiatzi, E.; Sarantopoulou, V.; Anastasiou, E. The Effect of Smoking on the Risk of Gestational Diabetes Mellitus and the OGTT Profile during Pregnancy. Diabetes Res. Clin. Pract. 2019, 158, 107901. [Google Scholar] [CrossRef]
- Soulimane, S.; Simon, D.; Herman, W.H.; Lange, C.; Lee, C.M.Y.; Colagiuri, S.; Shaw, J.E.; Zimmet, P.Z.; Magliano, D.; Ferreira, S.R.G.; et al. HbA1c, Fasting and 2 h Plasma Glucose in Current, Ex- and Never-Smokers: A Meta-Analysis. Diabetologia 2014, 57, 30–39. [Google Scholar] [CrossRef]
- Zarén, B.; Lindmark, G.; Wibell, L.; Følling, I. The Effect of Smoking on Glucose Homeostasis and Fetal Growth in Pregnant Women. Ups. J. Med. Sci. 2000, 105, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, K.; Głuchowska, M.; Garnysz, K.; Horzelski, W.; Grzesiak, M.; Lewiński, A. High Prevalence of Early (1st Trimester) Gestational Diabetes Mellitus in Polish Women Is Accompanied by Marked Insulin Resistance—Comparison to PCOS Model. Endokrynol. Pol. 2022, 73, 1–7. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.-A.; Kenny, P.J. Central and Peripheral Actions of Nicotine That Influence Blood Glucose Homeostasis and the Development of Diabetes. Pharmacol. Res. 2023, 194, 106860. [Google Scholar] [CrossRef]
- Kvalvik, L.G.; Nilsen, R.M.; Skjærven, R.; Vollset, S.E.; Midttun, O.; Ueland, P.M.; Haug, K. Self-Reported Smoking Status and Plasma Cotinine Concentrations among Pregnant Women in the Norwegian Mother and Child Cohort Study. Pediatr. Res. 2012, 72, 101–107. [Google Scholar] [CrossRef] [PubMed]
- McElwee, E.R.; Oliver, E.A.; McFarling, K.; Haney, A.; Cuff, R.; Head, B.; Karanchi, H.; Loftley, A.; Finneran, M.M. Risk of Stillbirth in Pregnancies Complicated by Diabetes, Stratified by Fetal Growth. Obstet. Gynecol. 2023, 141, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Blacher, J.; Lailler, G.; Gabet, A.; Grave, C.; Regnault, N.; Deneux-Tharaux, C.; Kretz, S.; Tsatsaris, V.; Plu-Bureau, G.; Olié, V. Acute Coronary Syndrome during Pregnancy and Postpartum in France: The Nationwide CONCEPTION Study. Am. J. Obstet. Gynecol. MFM 2023, 5, 100781. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Castillo, S.; Bravo, K.; Eugenín, J. Impact of Prenatal Nicotine Exposure on Placental Function and Respiratory Neural Network Development. Adv. Exp. Med. Biol. 2023, 1428, 233–244. [Google Scholar] [CrossRef]
- Kida, N.; Nishigaki, A.; Kakita-Kobayashi, M.; Tsubokura, H.; Hashimoto, Y.; Yoshida, A.; Hisamatsu, Y.; Tsuzuki-Nakao, T.; Murata, H.; Okada, H. Exposure to Cigarette Smoke Affects Endometrial Maturation Including Angiogenesis and Decidualization. Reprod. Med. Biol. 2021, 20, 108–118. [Google Scholar] [CrossRef]
- Corleis, B.; Tzouanas, C.N.; Wadsworth, M.H.; Cho, J.L.; Linder, A.H.; Schiff, A.E.; Zessin, B.; Stei, F.; Dorhoi, A.; Dickey, A.K.; et al. Tobacco Smoke Exposure Recruits Inflammatory Airspace Monocytes That Establish Permissive Lung Niches for Mycobacterium Tuberculosis. Sci. Transl. Med. 2023, 15, eadg3451. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).