Accuracy of Implant Guided Surgery in Fully Edentulous Patients: Prediction vs. Actual Outcome—Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Selection of Studies
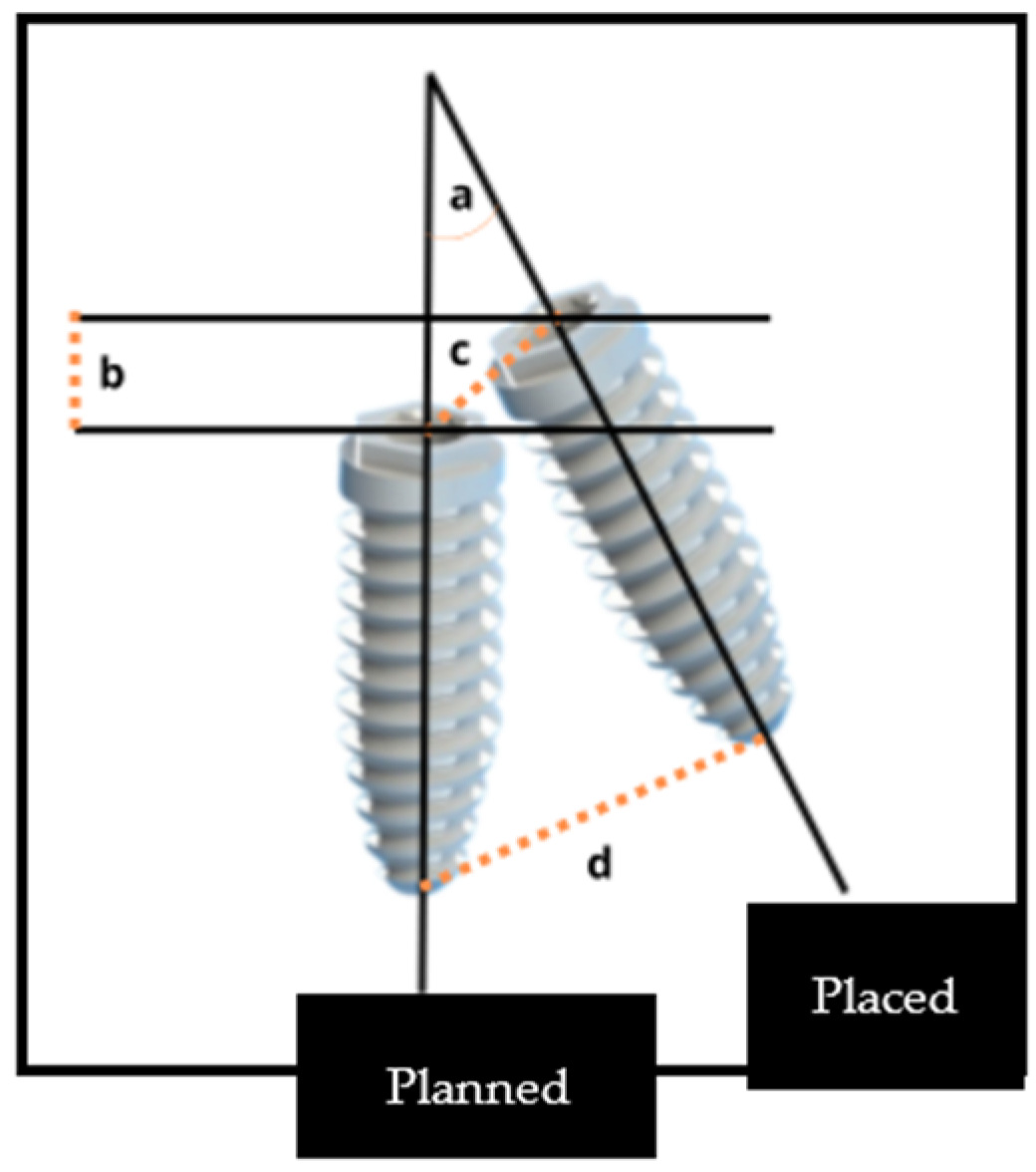
2.3. Accuracy Analysis
- Coronal disparity, assessed at the entry point center of the implant
- Apical disparity, assessed at the apex center
- Angular disparity
- Depth disparity
2.4. Data Extraction
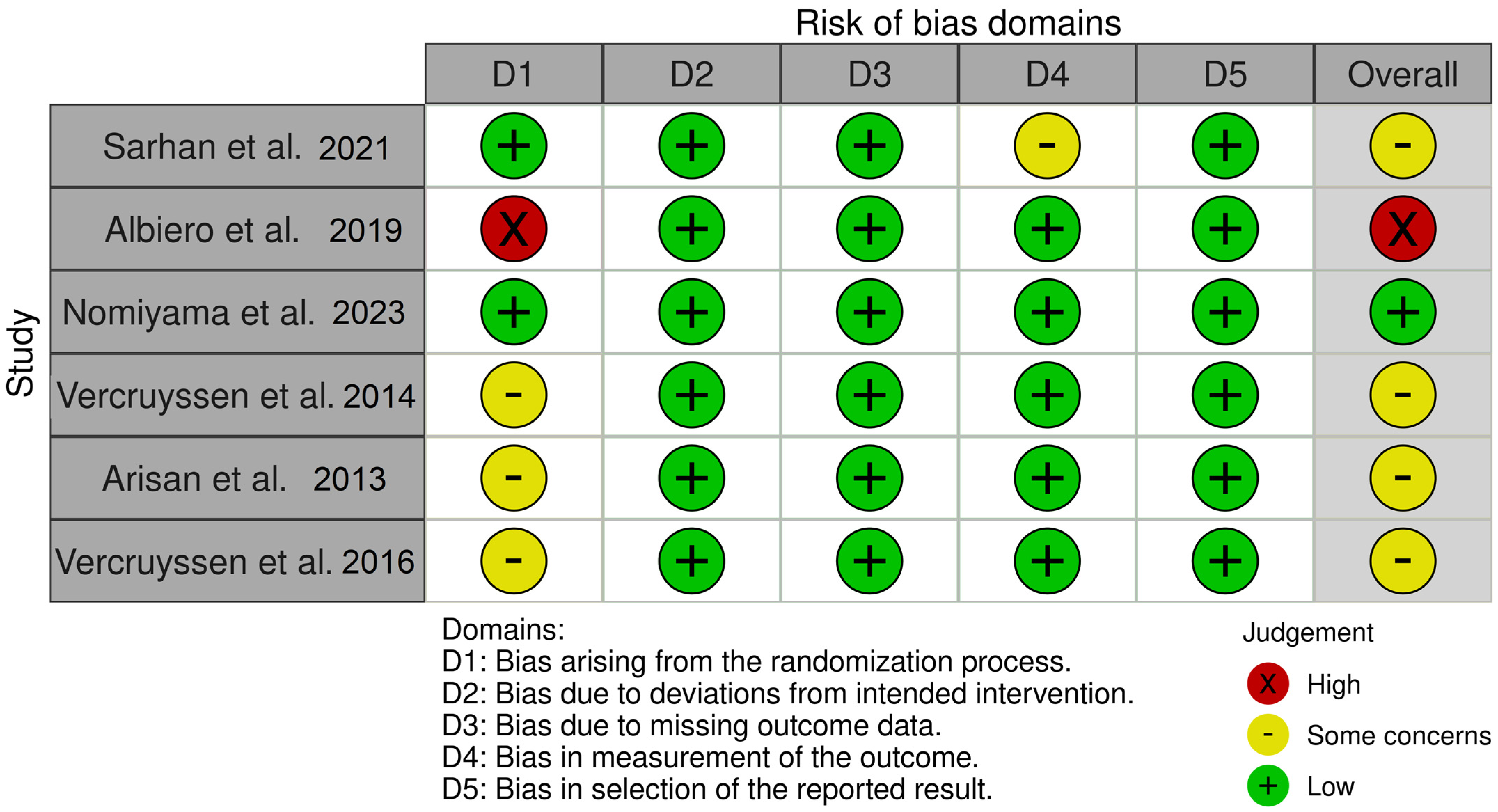
2.5. Risk of Bias
- Bias arising from the randomization process
- Bias due to deviations from intended interventions
- Bias due to missing outcome data
- Bias in measurement of the outcome
- Bias in selection of the reported result
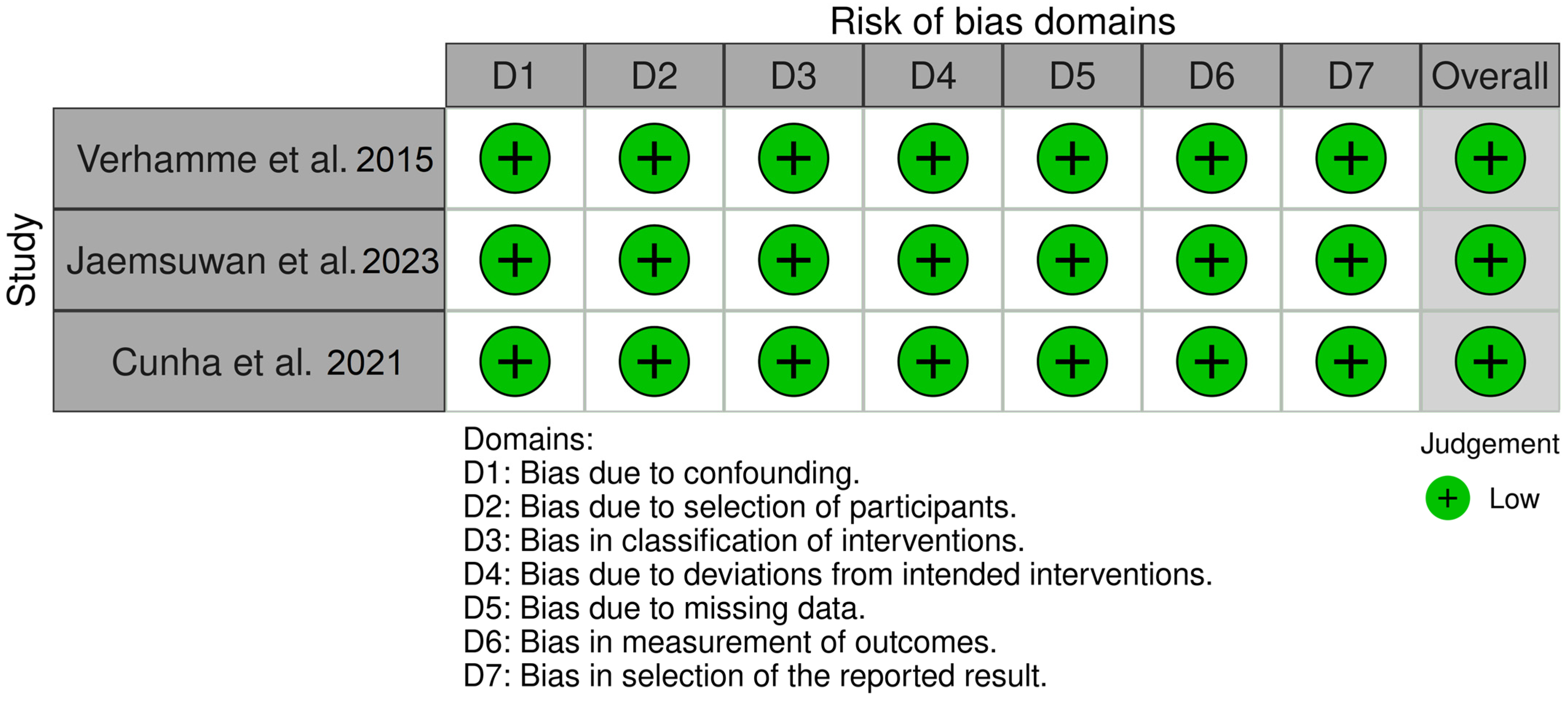
- Bias due to confounding
- Bias in selection of participants in the study
- Bias in classification of interventions
- Bias due to deviations from intended interventions
- Bias due to missing data
3. Results
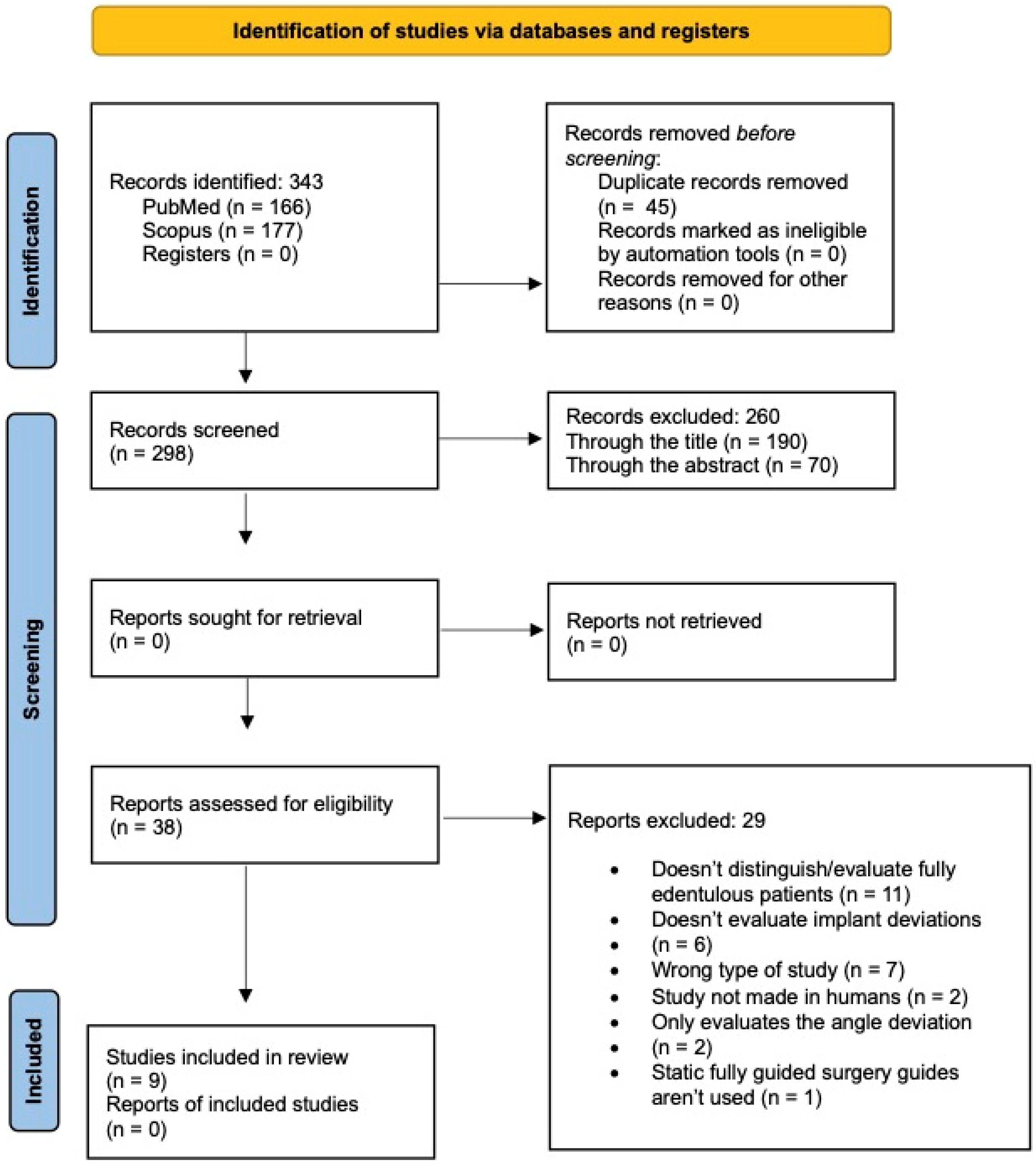
3.1. Study Selection
3.2. Study Characteristics
3.3. Study Outcomes
3.4. Risk of Bias within Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Marliere, D.A.A.; Demetrio, M.S.; Picinini, L.S.; Oliveira, R.G.; Netto, H. Accuracy of computer-guided surgery for dental implant placement in fully edentulous patients: A systematic review. Eur. J. Dent. 2018, 12, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Tahmaseb, A.; Wismeijer, D.; Coucke, W.; Derksen, W. Computer technology applications in surgical implant dentistry: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.H.; Yoda, N.; Astuti, E.R.; Sasaki, K. The accuracy of implant placement with computer-guided surgery in partially edentulous patients and possible influencing factors: A systematic review and meta-analysis. J. Prosthodont. Res. 2022, 66, 29–39. [Google Scholar] [CrossRef]
- Flugge, T.; Derksen, W.; Te Poel, J.; Hassan, B.; Nelson, K.; Wismeijer, D. Registration of cone beam computed tomography data and intraoral surface scans—A prerequisite for guided implant surgery with CAD/CAM drilling guides. Clin. Oral Implant Res. 2017, 28, 1113–1118. [Google Scholar] [CrossRef]
- Çiçekdaği İlhan, C.; Dikmen, M.; Yüzbaşioğlu, E. Accuracy And Efficiency Of Digital Implant Planning And Guided Implant Surgery. J. Exp. Clin. Med. 2021, 38, 148–156. [Google Scholar] [CrossRef]
- Kontis, P. Analysis of Digitalization Methods for Edentulous Jaws. Ph.D. Thesis, Ludwig Maximilian University of Munich, Munich, Germany, 2023. [Google Scholar] [CrossRef]
- Moura, G.F.; Siqueira, R.; Meirelles, L.; Maska, B.; Wang, H.L.; Mendonca, G. Denture scanning technique for computer-guided implant-supported restoration treatment of edentulous patients. J. Prosthet. Dent. 2021, 125, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Rosmaninho, A.; Vedovato, E.; Kois, J.C.; Revilla-León, M. A modified reverse impression technique for capturing and transferring soft-tissue information. J. Prosthet. Dent. 2023. [Google Scholar] [CrossRef]
- Jung, R.E.; Schneider, D.; Ganeles, J.; Wismeijer, D.; Zwahlen, M.; Hammerle, C.H.; Tahmaseb, A. Computer technology applications in surgical implant dentistry: A systematic review. Int. J. Oral Maxillofac. Implant 2009, 24, 92–109. [Google Scholar]
- Chackartchi, T.; Romanos, G.E.; Parkanyi, L.; Schwarz, F.; Sculean, A. Reducing errors in guided implant surgery to optimize treatment outcomes. Periodontol. 2000 2022, 88, 64–72. [Google Scholar] [CrossRef]
- Kalaivani, G.; Balaji, V.R.; Manikandan, D.; Rohini, G. Expectation and reality of guided implant surgery protocol using computer-assisted static and dynamic navigation system at present scenario: Evidence-based literature review. J. Indian Soc. Periodontol. 2020, 24, 398–408. [Google Scholar] [CrossRef]
- Baruffaldi, A.; Baruffaldi, A.; Baruffaldi, M.; Maiorana, C.; Poli, P.P. A suggested protocol to increase the accuracy of prosthetic phases in case of full-arch model-free fully guided computer-aided implant placement and immediate loading. Oral Maxillofac. Surg. 2020, 24, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, S.B.; Vonau, S.; Stampf, S.; Att, W. Assessing the feasibility and accuracy of digitizing edentulous jaws. J. Am. Dent. Assoc. 2013, 144, 914–920. [Google Scholar] [CrossRef]
- Divakar, T.K.; Gidean Arularasan, S.; Baskaran, M.; Packiaraj, I.; Dhineksh Kumar, N. Clinical Evaluation of Placement of Implant by Flapless Technique Over Conventional Flap Technique. J. Maxillofac. Oral Surg. 2020, 19, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Makarov, N.; Papi, P.; Santomauro, V.; Di Carlo, G.; Polimeni, A.; Di Murro, B.; Saccucci, M. In Vivo and In Vitro Accuracy Analysis of Static Computer-Assisted Implant Surgery in an Edentulous Patient. Appl. Sci. 2023, 13, 1185. [Google Scholar] [CrossRef]
- Raico Gallardo, Y.N.; da Silva-Olivio, I.R.T.; Mukai, E.; Morimoto, S.; Sesma, N.; Cordaro, L. Accuracy comparison of guided surgery for dental implants according to the tissue of support: A systematic review and meta-analysis. Clin. Oral Implants Res. 2017, 28, 602–612. [Google Scholar] [CrossRef]
- Sunitha, R.V.; Sapthagiri, E. Flapless implant surgery: A 2-year follow-up study of 40 implants. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e237–e243. [Google Scholar] [CrossRef]
- Franchina, A.; Stefanelli, L.V.; Maltese, F.; Mandelaris, G.A.; Vantaggiato, A.; Pagliarulo, M.; Pranno, N.; Brauner, E.; De Angelis, F.; Di Carlo, S. Validation of an intra-oral scan method versus cone beam computed tomography superimposition to assess the accuracy between planned and achieved dental implants: A randomized in vitro study. Int. J. Environ. Res. Public Health 2020, 17, 9358. [Google Scholar] [CrossRef]
- D’Haese, J.; Ackhurst, J.; Wismeijer, D.; De Bruyn, H.; Tahmaseb, A. Current state of the art of computer-guided implant surgery. Periodontol. 2000 2017, 73, 121–133. [Google Scholar] [CrossRef]
- Yi, C.; Li, S.; Wen, A.; Wang, Y.; Zhao, Y.; Zhang, Y. Digital versus radiographic accuracy evaluation of guided implant surgery: An in vitro study. BMC Oral Health 2022, 22, 540. [Google Scholar] [CrossRef]
- Page, M.A.-O.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. bmj 2021, 372, 29. [Google Scholar]
- Arriola, E. Dental Implant. Available online: https://grabcad.com/library/dental-implant--1 (accessed on 13 April 2024).
- Verhamme, L.M.; Meijer, G.J.; Berge, S.J.; Soehardi, R.A.; Xi, T.; de Haan, A.F.; Schutyser, F.; Maal, T.J. An Accuracy Study of Computer-Planned Implant Placement in the Augmented Maxilla Using Mucosa-Supported Surgical Templates. Clin. Implant Dent. Relat. Res. 2015, 17, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Vercruyssen, M.; Cox, C.; Naert, I.; Jacobs, R.; Teughels, W.; Quirynen, M. Accuracy and patient-centered outcome variables in guided implant surgery: A RCT comparing immediate with delayed loading. Clin. Oral Implant Res. 2016, 27, 427–432. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Traning: London, UK, 2023. [Google Scholar]
- Sarhan, M.M.; Khamis, M.M.; El-Sharkawy, A.M. Evaluation of the accuracy of implant placement by using fully guided versus partially guided tissue-supported surgical guides with cylindrical versus C-shaped guiding holes: A split-mouth clinical study. J. Prosthet. Dent. 2021, 125, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Albiero, A.M.; Quartuccio, L.; Benato, A.; Benato, R. Accuracy of Computer-Guided Flapless Implant Surgery in Fully Edentulous Arches and in Edentulous Arches With Fresh Extraction Sockets. Implant Dent. 2019, 28, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, L.M.; Matumoto, E.K.; Corrêa, M.G.; Cirano, F.R.; Ribeiro, F.V.; Pimentel, S.P.; Casati, M.Z. Comparison between flapless-guided and conventional surgery for implant placement: A 12-month randomized clinical trial. Clin. Oral Investig. 2023, 27, 1665–1679. [Google Scholar] [CrossRef] [PubMed]
- Vercruyssen, M.; Cox, C.; Coucke, W.; Naert, I.; Jacobs, R.; Quirynen, M. A randomized clinical trial comparing guided implant surgery (bone- or mucosa-supported) with mental navigation or the use of a pilot-drill template. J. Clin. Periodontol. 2014, 41, 717–723. [Google Scholar] [CrossRef]
- Arisan, V.; Karabuda, Z.C.; Piskin, B.; Ozdemir, T. Conventional multi-slice computed tomography (CT) and cone-beam CT (CBCT) for computer-aided implant placement. Part II: Reliability of mucosa-supported stereolithographic guides. Clin. Implant Dent. Relat. Res. 2013, 15, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Jaemsuwan, S.; Arunjaroensuk, S.; Kaboosaya, B.; Subbalekha, K.; Mattheos, N.; Pimkhaokham, A. Comparison of the accuracy of implant position among freehand implant placement, static and dynamic computer-assisted implant surgery in fully edentulous patients: A non- randomized prospective study. Int. J. Oral Maxillofac. Surg. 2023, 52, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.M.; Souza, F.A.; Hadad, H.; Poli, P.P.; Maiorana, C.; Carvalho, P.S.P. Accuracy evaluation of computer-guided implant surgery associated with prototyped surgical guides. J. Prosthet. Dent. 2021, 125, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Bover-Ramos, F.; Viña-Almunia, J.; Cervera-Ballester, J.; Peñarrocha-Diago, M.; García-Mira, B. Accuracy of Implant Placement with Computer-Guided Surgery: A Systematic Review and Meta-Analysis Comparing Cadaver, Clinical, and In Vitro Studies. Int. J. Oral Maxillofac. Implant. 2018, 33, 101. [Google Scholar] [CrossRef]
- Noharet, R.; Pettersson, A.; Bourgeois, D. Accuracy of implant placement in the posterior maxilla as related to 2 types of surgical guides: A pilot study in the human cadaver. J. Prosthet. Dent. 2014, 112, 526–532. [Google Scholar] [CrossRef]
- Cassetta, M.; Di Mambro, A.; Giansanti, M.; Stefanelli, L.V.; Cavallini, C. The intrinsic error of a stereolithographic surgical template in implant guided surgery. Int. J. Oral Maxillofac. Surg. 2013, 42, 264–275. [Google Scholar] [CrossRef] [PubMed]
- van Steenberghe, D.; Naert, I.; Andersson, M.; Brajnovic, I.; Cleynenbreugel, J.; Suetens, P. A Custom Template and Definitive Prosthesis Allowing Immediate Implant Loading in the Maxilla: A Clinical Report. Int. J. Oral Maxillofac. Implant. 2001, 17, 663–670. [Google Scholar]
- Arisan, V.; Karabuda, C.Z.; Ozdemir, T. Implant surgery using bone- and mucosa-supported stereolithographic guides in totally edentulous jaws: Surgical and post-operative outcomes of computer-aided vs. standard techniques. Clin. Oral Implant Res. 2010, 21, 980–988. [Google Scholar] [CrossRef]
- Stübinger, S.; Buitrago-Tellez, C.; Cantelmi, G. Deviations between placed and planned implant positions: An accuracy pilot study of skeletally supported stereolithographic surgical templates. Clin. Implant. Dent. Relat. Res. 2014, 16, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, P.; Rau, A.; Engelke, W.; Troeltzsch, M.; Brockmeyer, P.; Dagmar, L.S.; Cordesmeyer, R. Accuracy of Navigation-Guided Dental Implant Placement with Screw Versus Hand Template Fixation in the Edentulous Mandible. Clin. Implant. Dent. Relat. Res. 2012, 16, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, M.; Di Mambro, A.; Giansanti, M.; Stefanelli, L.V.; Barbato, E. How does an error in positioning the template affect the accuracy of implants inserted using a single fixed mucosa-supported stereolithographic surgical guide? Int. J. Oral Maxillofac. Surg. 2014, 43, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J.; Zuabi, O.; Machtei, E.E. Accuracy of a computerized tomography-guided template-assisted implant placement system: An in vitro study. Clin. Oral Implant Res. 2009, 20, 1156–1162. [Google Scholar] [CrossRef]
- Koop, R.; Vercruyssen, M.; Vermeulen, K.; Quirynen, M. Tolerance within the sleeve inserts of different surgical guides for guided. Clin. Oral Implant Res. 2012, 24, 630–634. [Google Scholar] [CrossRef]
- El Kholy, K.; Janner, S.F.M.; Schimmel, M.; Buser, D. The influence of guided sleeve height, drilling distance, and drilling key length on the accuracy of static Computer-Assisted Implant Surgery. Clin. Implant Dent. Relat. Res. 2019, 21, 101–107. [Google Scholar] [CrossRef]
- Cassetta, M.; Pompa, G.; Di Carlo, S.; Piccoli, L.; Pacifici, A.; Pacifici, L. The influence of smoking and surgical technique on the accuracy of mucosa-supported stereolithographic surgical guide in complete edentulous upper jaws. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1546–1553. [Google Scholar] [PubMed]
- D’Haese, J.; De Bruyn, H. Effect of smoking habits on accuracy of implant placement using mucosally supported stereolithographic surgical guides. Clin. Implant Dent. Relat. Res. 2013, 15, 402–411. [Google Scholar] [CrossRef]
- Ersoy, A.E.; Turkyilmaz, I.; Ozan, O.; McGlumphy, E. Reliability of implant placement with stereolithographic surgical guides generated from computed tomography: Clinical data from 94 implants. J. Periodontol. 2008, 78, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Tahmaseb, A.; Wu, V.; Wismeijer, D.; Coucke, W.; Evans, C. The accuracy of static computer-aided implant surgery: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Vasak, C.; Strbac, G.D.; Huber, C.D.; Lettner, S.; Gahleitner, A.; Zechner, W. Evaluation of three different validation procedures regarding the accuracy of template-guided implant placement: An In Vitro study. Clin. Implant. Dent. Relat. Res. 2013, 17, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, G.A.; da Silva, J.V.; da Silva, A.M.; Paschoal, G.H.; Cury, P.R.; Szarf, G. Accuracy and complications of computer-designed selective laser sintering surgical guides for flapless dental implant placement and immediate definitive prosthesis installation. J. Periodontol. 2012, 83, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, M.; Stefanelli, L.V.; Giansanti, M.; Di Mambro, A.; Calasso, S. Depth deviation and occurrence of early surgical complications or unexpected events using a single stereolithographic surgi-guide. Int. J. Oral Maxillofac. Surg. 2011, 40, 1377–1387. [Google Scholar] [CrossRef]
- Verhamme, L.M.; Meijer, G.J.; Boumans, T.; de Haan, A.F.; Bergé, S.J.; Maal, T.J. A clinically relevant accuracy study of computer-planned implant placement in the edentulous maxilla using mucosa-supported surgical templates. Clin. Implant Dent. Relat. Res. 2015, 17, 343–352. [Google Scholar] [CrossRef]
- Vercruyssen, M.; Coucke, W.; Naert, I.; Jacobs, R.; Teughels, W.; Quirynen, M. Depth and lateral deviations in guided implant surgery: An RCT comparing guided surgery with mental navigation or the use of a pilot-drill template. Clin. Oral Implant Res. 2015, 26, 1315–1320. [Google Scholar] [CrossRef]
- Moraschini, V.; Velloso, G.; Luz, D.; Barboza, E.P. Implant survival rates, marginal bone level changes, and complications in full-mouth rehabilitation with flapless computer-guided surgery: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2015, 44, 892–901. [Google Scholar] [CrossRef]
- Vohra, F.; Al-Kheraif, A.A.; Almas, K.; Javed, F. Comparison of crestal bone loss around dental implants placed in healed sites using flapped and flapless techniques: A systematic review. J. Periodontol. 2015, 86, 185–191. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Type of Study | Patients (n) | Mean Age | CAD-CAM Software | Implant Brand | Follow-Up |
|---|---|---|---|---|---|---|---|
| Sarhan et al. [26] | 2021 | Randomized Clinical Trial | 12 | NR | Blue Sky Bio® | Dentium® | Same day |
| Albiero et al. [27] | 2019 | Randomized Clinical Trial | 20 | 58.9 (43–86) | SimPlant® | Dentsply® | 1 week |
| Nomiyama et al. [28] | 2023 | Randomized Clinical Trial | 29 | 62 (18–76) | Dental Slice Virtual Navigation® | Implacil® | 10 days |
| Vercruyssen et al. [29] | 2014 | Randomized Clinical Trial | 59 | 58 (NR) | SimPlant® | Astra Tech® | 10 days |
| Verhamme et al. [23] | 2015 | Clinical Trial | 25 | 59 (45–79) | Procera Clinical Design® | Nobel BioCare® | 2 weeks |
| Jaemsuwan et al. [31] | 2022 | Clinical Trial | 13 | 66 (51–75) | coDiagnostiX® | Straumann® | 1 week |
| Cunha et al. [32] | 2021 | Clinical Trial | 8 | NR | P3Dental® | Easy Implant® | 30 days |
| Arisan et al. [30] | 2013 | Randomized Clinical Trial | 11 | NR | SimPlant® | Thommen SPI Element® | 2 months-mandible 3 months-maxilla |
| Vercruyssen et al. [24] | 2016 | Randomized Clinical Trial | 15 | 60 (NR) | SimPlant® | Dentsply® | Same day |
| Authors | Inclusion Criteria | Exclusion Criteria | Type of Deviation |
|---|---|---|---|
| Sarhan et al. [26] |
|
| 3D Deviations |
| Albiero et al. [27] |
| NR | 3D Deviations |
| Nomiyama et al. [28] |
|
| 3D Deviations |
| Vercruyssen et al. [29] |
|
| 3D Deviations |
| Verhamme et al. [23] |
| NR | 3D Deviations MD and BL Deviations |
| Jaemsuwan et al. [31] |
|
| 3D Deviations |
| Cunha et al. [32] |
| NR | 3D Deviations |
| Arisan et al. [30] |
|
| 3D Deviations |
| Vercruyssen et al. [24] |
|
| 3D Deviations MD and BL Deviations |
| Primary Deviations | Secondary Deviations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Jaw | Implants * (n) (Analyzed) (n) | Flapless | Type of Surgical Guide | Cervical (mm) | Apical (mm) | Angular (°) | Depth (mm) | MD | BL |
| Sarhan et al. [26] | Mand | 24 (24) | Yes | Mucosa-supported with fixation | Cyl: = 1.65 (0.91–4.54) Csh: = 1.27 (0.84–2.16) | Cyl: = 1.91 (0.77–6.68) Csh: = 2.46 (1.10–3.95) | Cyl: = 11.11 (4.22–14.76) Csh: = 11.04 (4.05–20.55) | Cyl: = 1.01 (0.28–2.54) Csh: = 1.69 (0.53–3.43) | NR | NR |
| Albiero et al. [27] | Both | 114 (114) | Yes | Mucosa-supported with fixation | = 1.20 ± 0.56 EA: = 1.12 ± 0.52 FS: = 1.28 ± 0.59 | = 1.51 ± 0.71 EA: = 1.36 ± 0.68 FS: = 1.65 ± 0.71 | = 3.30 ± 1.65 EA: = 3.16 ± 1.79 FS: =3.42 ± 1.52 | = 0.52 ± 0.85 EA: = 0.51 ± 0.74 FS: = 0.53 ± 0.94 | NR | NR |
| Nomiyama et al. [28] | Max | 87 (86) | Yes | Mucosa-supported with fixation | = 2.01 ± 0.77 | = 2.41 ± 1.45 | =2.39 ± 0.79 | =1.67 ± 0.82 | NR | NR |
| Vercruyssen et al. [29] | Both | 212 (209) | No | Mucosa/Bone-supported with fixation | MatMu: = 1.23 ± 0.06 * MatBo: = 1.60 ± 0.92 FacMu: = 1.38 ± 0.64 * FacBo: = 1.33 ± 0.82 | MatMu: = 1.57 ± 0.71 MatBo: = 1.65 ± 0.82 FacMu: = 1.60 ± 0.70 * FacBo: = 1.50 ± 0.72 | MatMu: = 2.86 ± 1.60 MatBo: = 3.79 ± 2.36 FacMu: = 2.71 ± 1.36 * FacBo: = 3.20 ± 2.70 | NR | NR | NR |
| Verhamme et al. [23] | Max | 150 (150) | Yes | Mucosa-supported with and without fixation | = 1.963 ± 0.232 | = 2.288 ± 0.269 | = 3.926 ± 0.414 | = −0.584 ± 0.155 | Cervical: = 1.270 ± 0425 Apical: = 1.494 ± 0.466 Angular: = 2.504 ± 0.573 Depth: − = 0.602 ± 0.315 | Cervical: = 0.757 ± 0.180 Apical: = 0.987 ± 0.279 Angular: = 2.484 ± 0.568 Depth: − = 0.571 ± 0.291 |
| Jaemsuwan et al. [31] | Both | 20 (20) | No | Mucosa-supported with fixation | = 1.40 ± 0.72 | = 1.66 ± 0.61 | = 4.98 ± 2.16 | NR | NR | NR |
| Cunha et al. [32] | Both | 60 (60) | Yes | Mucosa-supported with fixation | = 0.68 ± 0.36 Mand: = 0.79 ± 0.42 Max: = 0.61 ± 0.30 | = 0.82 ± 0.39 Mand: = 0.92 ± 0.39 Max: = 0.75 ± 0.37 | = 2.04 ± 1.21 Mand: = 2.44 ± 1.43 Max: = 1.77 ± 0.0.95 | NR | NR | NR |
| Arisan et al. [30] | Both | 108 (102) | Yes | Mucosa-supported with fixation | CBCT: =0.81 ± 0.32 MSCT: = 0.75 ± 0.32 | CBCT: = 0.87 ± 0.32 MSCT: = 0.80 ± 0.35 | CBCT: = 3.47 ± 1.144 MSCT: = 3.30 ± 1.08 | NR | NR | NR |
| Vercruyssen et al. [24] | Max | 90 (90) | Yes | Mucosa-supported with fixation | = 0.9 (0.1–4.5) | = 1.2 (0.2–4.9) | = 2.7 (0.6–6) | = 0.11 (−3.18–1.79) | = −0.12 (−2.25–1.33) | = −0.2 (−2.17–1.65) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, M.; Correia, F.; Faria Almeida, R. Accuracy of Implant Guided Surgery in Fully Edentulous Patients: Prediction vs. Actual Outcome—Systematic Review. J. Clin. Med. 2024, 13, 5178. https://doi.org/10.3390/jcm13175178
Azevedo M, Correia F, Faria Almeida R. Accuracy of Implant Guided Surgery in Fully Edentulous Patients: Prediction vs. Actual Outcome—Systematic Review. Journal of Clinical Medicine. 2024; 13(17):5178. https://doi.org/10.3390/jcm13175178
Chicago/Turabian StyleAzevedo, Mafalda, Francisco Correia, and Ricardo Faria Almeida. 2024. "Accuracy of Implant Guided Surgery in Fully Edentulous Patients: Prediction vs. Actual Outcome—Systematic Review" Journal of Clinical Medicine 13, no. 17: 5178. https://doi.org/10.3390/jcm13175178
APA StyleAzevedo, M., Correia, F., & Faria Almeida, R. (2024). Accuracy of Implant Guided Surgery in Fully Edentulous Patients: Prediction vs. Actual Outcome—Systematic Review. Journal of Clinical Medicine, 13(17), 5178. https://doi.org/10.3390/jcm13175178







