Impact of Ventilator Settings on Pulmonary Nodule Localization Accuracy in a Hybrid Operating Room: A Single-Center Study
Abstract
:1. Introduction
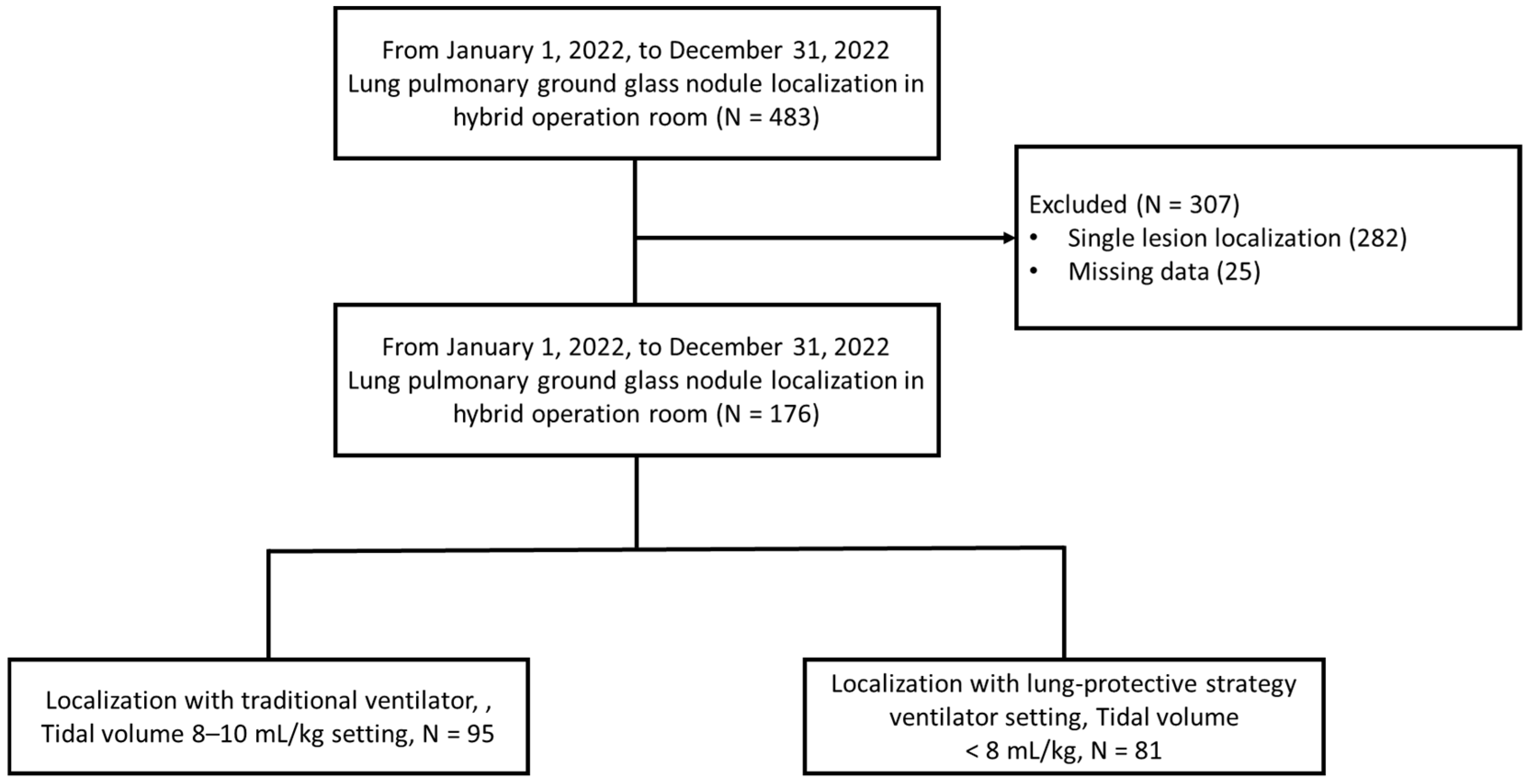
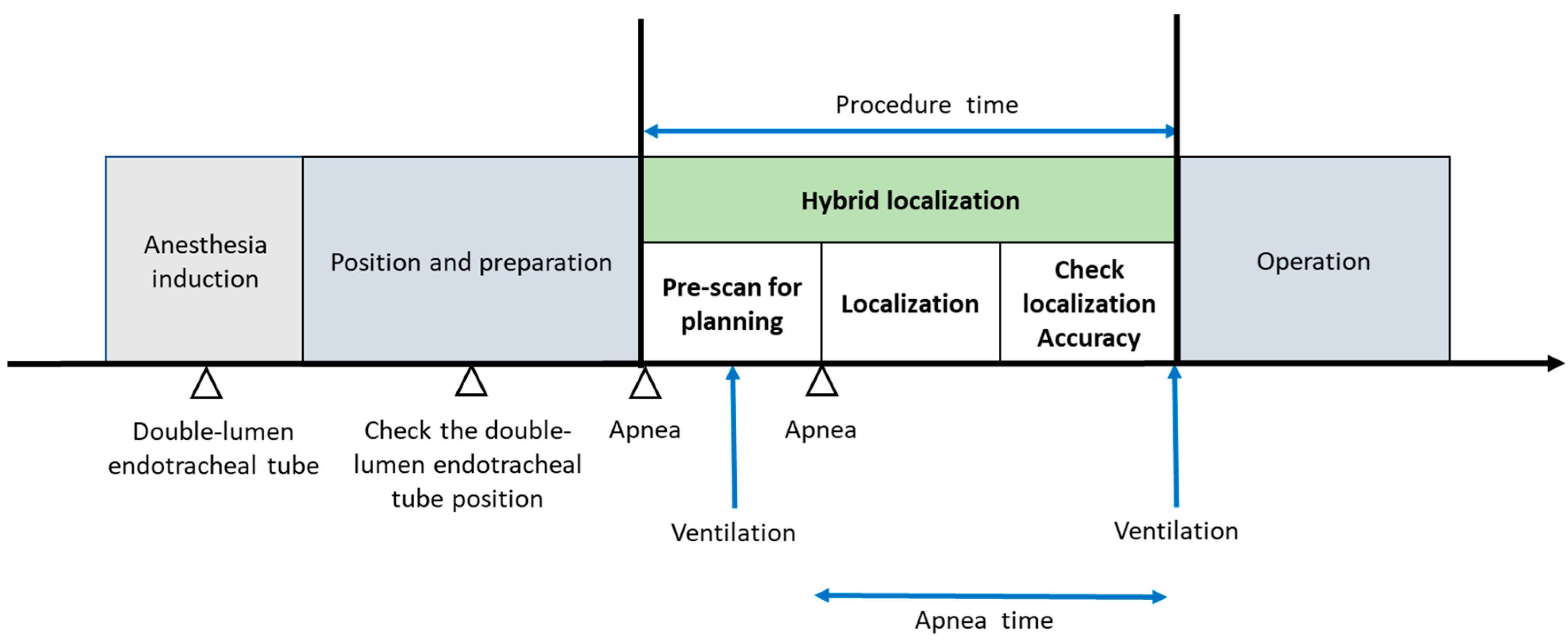
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | adenocarcinoma in situ |
| BMI | body mass index |
| BSA | body surface area |
| CT | computed tomography |
| MIA | minimally invasive adenocarcinoma |
| SpO2 | oxygen saturation |
| IOCT | intraoperative CT |
| POCT | preoperative CT |
| OR | operating room |
References
- Chang, G.-C.; Chiu, C.-H.; Yu, C.-J.; Chang, Y.-C.; Chang, Y.-H.; Hsu, K.-H.; Wu, Y.-C.; Chen, C.-Y.; Hsu, H.-H.; Wu, M.-T.; et al. Low-dose CT screening among never-smokers with or without a family history of lung cancer in Taiwan: A prospective cohort study. Lancet Respir. Med. 2024, 12, 141–152. [Google Scholar] [CrossRef]
- Cao, J.; Yuan, P.; Wang, Y.; Xu, J.; Yuan, X.; Wang, Z.; Lv, W.; Hu, J. Survival rates after lobectomy, segmentectomy, and wedge resection for non-small cell lung cancer. Ann. Thorac. Surg. 2018, 105, 1483–1491. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Tan, Z.; Zhang, T. Segmentectomy versus wedge resection for stage I non-small cell lung cancer: A meta-analysis. J. Surg. Res. 2019, 243, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Migliore, M.; Palmucci, S.; Nardini, M.; Basile, A. Imaging patterns of early stage lung cancer for the thoracic surgeon. J. Thorac. Dis. 2020, 12, 3349–3356. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Han, K.; Hur, J.; Lee, S.M.; Lee, J.W.; Hwang, S.H.; Seo, J.S.; Lee, K.H.; Kwon, W.; Kim, T.H.; et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: A systematic review and meta-analysis. Chest 2017, 151, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Cornella, K.N.; Repper, D.C.; Palafox, B.A.; Razavi, M.K.; Loh, C.T.; Markle, K.M.; Openshaw, L.E. A surgeon’s guide for various lung nodule localization techniques and the newest technologies. Innovations 2021, 16, 26–33. [Google Scholar] [CrossRef]
- Ichinose, J.; Kohno, T.; Fujimori, S.; Harano, T.; Suzuki, S. Efficacy and complications of computed tomography-guided hook wire localization. Ann. Thorac. Surg. 2013, 96, 1203–1208. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, J.; Kheir, F.; Wagh, A.; Seguin-Givelet, A.; Sun, T.; Zhang, H. The disappearing hook wire: A case report. J. Thorac. Dis. 2023, 15, 7149–7154. [Google Scholar] [CrossRef]
- Chen, K.H.; Wu, C.H.; Wei, H.J.; Chan, C.W.; Hsia, J.Y. Migrating hook wire that travels to the heart via the bloodstream: A case report. Medicine 2023, 102, e33349. [Google Scholar] [CrossRef]
- Fang, H.Y.; Chang, K.W.; Chao, Y.K. Hybrid operating room for the intraoperative CT-guided localization of pulmonary nodules. Ann. Transl. Med. 2019, 7, 34. [Google Scholar] [CrossRef]
- Chao, Y.K.; Pan, K.T.; Wen, C.T.; Fang, H.Y.; Hsieh, M.J. A comparison of efficacy and safety of preoperative versus intraoperative computed tomography-guided thoracoscopic lung resection. J. Thorac. Cardiovasc. Surg. 2018, 156, 1974–1983.e1. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Constantin, J.M.; Jaber, S. Protective lung ventilation in operating room: A systematic review. Minerva Anestesiol. 2014, 80, 726–735. [Google Scholar] [PubMed]
- Marret, E.; Cinotti, R.; Berard, L.; Piriou, V.; Jobard, J.; Barrucand, B.; Radu, D.; Jaber, S.; Bonnet, F.; The PPV study group. Protective ventilation during anaesthesia reduces major postoperative complications after lung cancer surgery: A double-blind randomised controlled trial. Eur. J. Anaesthesiol. 2018, 35, 727–735. [Google Scholar] [CrossRef]
- Wang, Y.H.; Su, P.C.; Huang, H.C.; Au, K.; Lin, F.C.F.; Chen, C.Y.; Chou, M.C.; Hsia, J.Y. Pulmonary recruitment prior to intraoperative multiple pulmonary ground-glass nodule localization increases the localization accuracy—A retrospective study. J. Clin. Med. 2023, 12, 2998. [Google Scholar] [CrossRef]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Froio, S.; Chiumello, D. Protective lung ventilation during general anesthesia: Is there any evidence? Crit. Care 2014, 18, 210. [Google Scholar] [CrossRef]
- Hedenstierna, G.; Tokics, L.; Reinius, H.; Rothen, H.U.; Östberg, E.; Öhrvik, J. Higher age and obesity limit atelectasis formation during anaesthesia: An analysis of computed tomography data in 243 subjects. Br. J. Anaesth. 2020, 124, 336–344. [Google Scholar] [CrossRef]
- Reinius, H.; Jonsson, L.; Gustafsson, S.; Sundbom, M.; Duvernoy, O.; Pelosi, P.; Hedenstierna, G.; Fredén, F. Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis: A computerized tomography study. Anesthesiology 2009, 111, 979–987. [Google Scholar] [CrossRef]
- Pépin, J.L.; Timsit, J.F.; Tamisier, R.; Borel, J.C.; Lévy, P.; Jaber, S. Prevention and care of respiratory failure in obese patients. Lancet Respir. Med. 2016, 4, 407–418. [Google Scholar] [CrossRef]
- Cairo, J.M. Pilbeam’s Mechanical Ventilation: Physiological and Clinical Applications; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019; pp. 191–202. [Google Scholar]
- Hsu, P.K.; Wu, Y.C. Electromagnetic navigation-guided one-stage dual localization of small pulmonary nodules. Chest 2018, 154, 1462–1463. [Google Scholar] [CrossRef]
- Eger, E.I.; Severinghaus, J.W. The rate of rise of PaCO2 in the apneic anesthetized patient. Anesthesiology 1961, 22, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T. Effects of repeat exposure to inhalation anesthetics on liver and renal function. J. Anaesthesiol. Clin. Pharmacol. 2013, 29, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.-Y.; Chen, K.-A.; Wen, Y.-W.; Wen, C.-T.; Pan, K.-T.; Chiu, C.-H.; Hsieh, M.-J.; Chao, Y.-K. Efficacy and safety of preoperative vs. intraoperative computed tomography-guided lung tumor localization: A randomized controlled trial. Front. Surg. 2021, 8, 809908. [Google Scholar] [CrossRef] [PubMed]

| <8 mL/kg N = 81 | 8–10 mL/kg N = 95 | p Value | ||
|---|---|---|---|---|
| Gender | Female | 62 (76.5%) | 78 (82.1%) | 0.362 † |
| Male | 19 (23.5%) | 17 (17.9%) | ||
| Age, median (IQR) | 57 (51–65) | 54 (47–65) | 0.375 | |
| Height median (IQR) | 159.0 (154.0–165.0) | 159.0 (155.0–165.0) | 0.898 | |
| Body weight, median (IQR) | 59.0 (52.0–64.0) | 58.5 (54.0–68.0) | 0.707 | |
| BMI median (IQR) | 22.4 (21.3–24.3) | 23.1 (21.4–25.3) | 0.098 | |
| PBW, median (IQR) | 54.2 (48.8–63.3) | 51.5 (48.8–58.8) | 0.132 | |
| Tidal volume median (IQR) | 450 (458–500) | 550 (500–625) | <0.001 | |
| Contralateral lung operation | No | 65 (80.2%) | 77 (81.1%) | 0.893 † |
| Yes | 16 (19.8%) | 18 (18.9%) | ||
| Localization position | 30 degree tilt | 64 (79.0%) | 72 (75.7%) | 0.611 |
| Decubitus | 17 (21.0%) | 23 (24.2%) | ||
| Lung function | FVC | 102.0 (93.0–107.0) | 101.0 (93.0–112.0) | 0.260 |
| FEV1 | 99 (88.0–107.0) | 98.0 (90.0–107.0) | 0.501 | |
| Lung function | DLCO | 95.0 (87.0–108.0) | 98.0 (87.0–105.0) | 0.547 |
| Lesions | 2 | 55 (67.9%) | 65 (68.4%) | 0.115 † |
| 3 | 13 (16.0%) | 23 (24.2%) | 0.547 | |
| Lesions | 4 | 13 (16.0%) | 7 (7.4%) | 0.115 † |
| Size (mm, median IQR) | 7.30 (6.0–8.50) | 7.20 (5.60–8.80) | 0.673 | |
| Depth (mm, median IQR) | 70.0 (55.0–80.0) | 65.0 (55.0–75.0) | 0.209 | |
| Dye or hook | Dye | 31 (38.3%) | 44 (46.3%) | 0.281 † |
| Depth (mm, median IQR) | Hook | 50 (61.7%) | 51 (53.7%) | |
| Operation | Wedge | 73 (90.1%) | 86 (90.5%) | 0.98 † |
| Segmentectomy | 3 (3.7%) | 3 (3.2%) | ||
| Operation | Lobectomy | 5 (6.2%) | 6 (6.3%) | 0.98 † |
| Pathology | AIS | 11 (13.6%) | 18 (18.9%) | 0.70 † |
| MIA | 43 (53.1%) | 46 (48.4%) | ||
| Pathology | Adenocarcinoma | 15 (18.5%) | 17 (17.9%) | 0.70 † |
| Metastasis | 1 (1.2%) | 0 | ||
| Benign | 11 (13.6%) | 14 (14.7%) | ||
| <8 mL/kg N = 81 | 8–10 mL/kg N = 95 | p Value | ||
|---|---|---|---|---|
| Apnea time | 5.25 (4.30–6.50) | 5.05 (4.25–5.80) | 0.091 | |
| SpO2 (%, median, IQR) | 88 (84–94) | 90 (87–97) | 0.006 | |
| Procedure time (min, IQR) | 18.0 (15.0–20.0) | 16.0 (14.0–19.0) | 0.032 | |
| Accuracy (within 5 mm) | 42 (51.9%) | 86 (90.5%) | 0.001 | |
| Accuracy with Lesions | 2 | 33 (60.0%) | 60 (92.3%) | |
| 3 | 6 (46.2%) | 21 (91.3%) | ||
| 4 | 3 (23.1%) | 5 (71.4%) | ||
| Re-inflation | No | 46 (56.8%) | 64 (67.4%) | 0.149 † |
| Yes | 35 (43.2%) | 31 (32.6%) | ||
| Pneumothorax | No | 57 (70.4%) | 76 (80%) | 0.138 † |
| Yes | 24 (29.6%) | 19 (20%) | ||
| Accuracy | Univariate Logistic Regression Analysis | Multivariate Logistic Regression Analysis | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | |
| BMI | 0.81 (0.72–0.91) | 0.000 | 0.79 (0.68–0.92) | 0.003 |
| Tidal volume/PBW 8–10 mL/kg or <8 mL/kg | 0.11 (0.05–0.25) | 0.000 | 0.08 (0.03–0.21) | 0.000 |
| Lesion number | 0.50 (0.32–0.80) | 0.003 | 0.48 (0.27–0.86) | 0.014 |
| Dye or hook | 1.19 (0.60–2.33) | 0.619 | ||
| Size (mm, median IQR) | 1.01 (0.88–1.16) | 0.875 | ||
| Depth (mm, median IQR) | 0.95 (0.93–0.98) | 0.000 | 0.96 (0.94–0.99) | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsia, J.Y.; Huang, H.C.; Au, K.-K.; Chen, C.Y.; Wang, Y.H. Impact of Ventilator Settings on Pulmonary Nodule Localization Accuracy in a Hybrid Operating Room: A Single-Center Study. J. Clin. Med. 2024, 13, 5183. https://doi.org/10.3390/jcm13175183
Hsia JY, Huang HC, Au K-K, Chen CY, Wang YH. Impact of Ventilator Settings on Pulmonary Nodule Localization Accuracy in a Hybrid Operating Room: A Single-Center Study. Journal of Clinical Medicine. 2024; 13(17):5183. https://doi.org/10.3390/jcm13175183
Chicago/Turabian StyleHsia, Jiun Yi, Hsu Chih Huang, Kwong-Kwok Au, Chih Yi Chen, and Yu Hsiang Wang. 2024. "Impact of Ventilator Settings on Pulmonary Nodule Localization Accuracy in a Hybrid Operating Room: A Single-Center Study" Journal of Clinical Medicine 13, no. 17: 5183. https://doi.org/10.3390/jcm13175183







